Abstract
Injury of the circumflex artery is an uncommon but dangerous complication during mitral valve surgery. We report the case of a patient who presented an occlusion of the circumflex artery after a minimally invasive mitral valve repair, which was treated with angioplasty in the immediate post-operative period. (Level of Difficulty: Intermediate.)
Key Words: intravascular ultrasound, mitral valve, myocardial revascularization, percutaneous coronary intervention, valve repair
Abbreviations and Acronyms: LCx, circumflex artery; PCI, percutaneous coronary intervention
Graphical abstract
A 51-year-old man with severe mitral valve regurgitation was admitted to our department for elective mitral valve repair. He had a past medical history of Barlow disease with a dilated left ventricle but a preserved ejection fraction. Both mitral leaflets were thickened, with myxomatous appearance, mild calcification, and a central jet regurgitation. The preoperative angiogram showed normal coronary arteries, with a right dominance anatomy (Figure 1A, Video 1). The patient underwent mitral valve repair with minimally invasive surgery, using the da Vinci Xi surgical system: then with posterior leaflet plication and closing indentation between P2 and P3, posterior commissurotomy, and annuloplasty with COSGROVE Annuloplasty Band (Edwards LifeSciences, Irvine, California) implantation. After weaning from extracorporeal circulation, transesophageal echocardiography showed normal ventricular function without segmental wall motion abnormalities and mitral valve without residual regurgitation. The patient was transferred to the intensive care unit and extubated 6 h after surgery was finished. Then he presented oppressive chest pain, without any other accompanying sign or symptom. The vital signs remained stable, and the symptom improved with nitroglycerine.
Learning Objectives
-
•
To recognize circumflex artery injury as a potential complication of mitral valve repair with annuloplasty.
-
•
To show that in selected cases the percutaneous approach can be safe and effective in treating this complication.
-
•
To highlight the importance of intravascular imaging guidance for the optimization of the percutaneous coronary stenting.
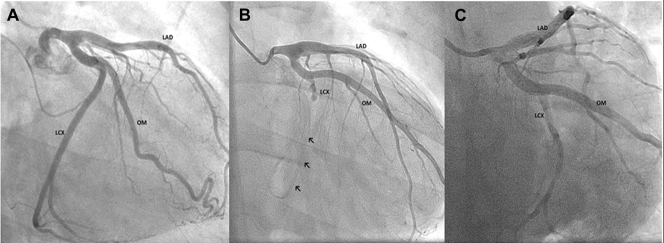
Figure 1.
Left Coronary Artery Angiogram
(A) Pre-operative angiogram shows the left coronary network without any obstructive lesion. (B) Post-operative angiogram shows circumflex artery occlusion and the small distance from this artery to the COSGROVE Annuloplasty Band (arrows). (C) Final angiogram shows the reinstatement of the blood flow to the distal circumflex. LAD = left anterior descending artery; LCX = circumflex artery; OM = obtuse marginal artery.
Differential Diagnosis
The differential diagnosis included transient coronary vasospasm, air embolism, or a mechanical injury of a coronary artery.
Investigations
The electrocardiogram showed a transient ST-segment elevation in inferior leads. There was an elevation in the high-sensitivity troponin I levels with a peak of 11,642.5 ng/l (Siemens Healthineers high-sensitivity troponin I assay). A transthoracic echocardiogram was performed, with hypokinesia on the basal inferolateral wall segment. Patient underwent cardiac catheterization, which revealed an occlusion of the circumflex artery (LCx) after the first obtuse marginal artery's origin close to the mitral ring's suture line (Figure 1B, Video 2).
Management
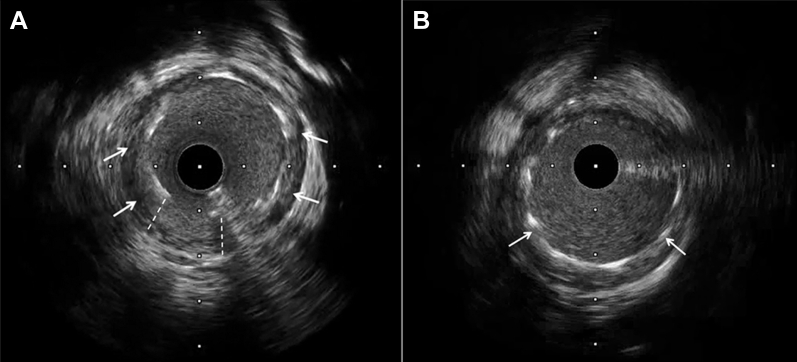
After a dedicated heart team evaluation, percutaneous coronary intervention (PCI) was decided. Percutaneous treatment of the LCx was performed approximately 12 h after the beginning of chest pain (18 h after surgery). The lesion was crossed with a 0.014-inch BMW wire by using a Finecross microcatheter, which was exchanged for a BHW guide to improve support. The coronary stenosis was predilated with 2 × 8 mm and a 3 × 15 mm noncompliant balloons (16 atm), re-establishing coronary flow. A 3 × 15 mm everolimus-eluting stent (Xience Sierra, Abbott Vascular, Santa Clara, California) was implanted to 14 atm. Intravascular imaging was performed for stent optimization. Because optical coherence tomography did not cross the lesion, intravascular ultrasound was eventually done. The intravascular ultrasound assessment showed a stent under expansion in the midportion, in the absence of an atherosclerotic plaque, in correspondence of a probable external compression of the suture (Figure 2A, Video 3). The stent was post-dilated with a 3.5 × 10 mm noncompliant balloon (14 atm). The final intravascular ultrasound and angiogram assessment showed an optimal stent expansion (Figures 1C and 2B, Videos 4 and 5). Before hospital discharge, the patient was re-evaluated with transthoracic echocardiography, showing preserved ejection fraction but persistent hypokinesia on the basal inferolateral wall segment, without any evidence of a paravalvular leak or other mechanical complication.
Figure 2.
Intravascular Ultrasound
(A) Intravascular ultrasound performed previously to post-dilation showing underexpansion of the stent. (B) Final intravascular ultrasound evidence of optimization of the stent apposition. Arrows show stent struts. Dashed lines show bad apposition of the stent.
Discussion
The iatrogenic lesion of the LCx is an uncommon complication of a mitral valve repair (1). The close course of the LCx to the anterolateral commissure and the posterior mitral valve leaflet's annulus explains its potential injury in this surgical procedure. Moreover, it has been described that left coronary dominance and codominance are associated with a smaller distance between the LCx and the mitral annulus. Therefore, there is a greater risk of the proximal LCx injury during mitral valve repair (2,3). However, extreme dilation and hypermobility of Barlow disease makes it more difficult to predict the position of the LCx, and the remodeling of the annuloplasty is greater (4), increasing the risk of distortion of the vessel.
The possible injury mechanisms are either caused by a suture that passes through and completely obliterates the artery or the vessel's laceration, which can lead to either hemorrhagic complications or thrombotic occlusion because of an injury of the endothelium. Furthermore, another possible mechanism of coronary occlusion reported in this type of valve repair is embolization during surgery (5).
In our case, the LCx occlusion was probably caused by a vessel distortion from suture tension in the vessel plane rather than a vascular puncture and obliteration. In this latter hypothesis, vessel puncture with a suture needle would have been associated with intraoperative bleeding. Moreover, angioplasty of a vessel with a transecting suture may have resulted in a large perforation and complete disjunction of the vessel.
The decision for treatment (either PCI or surgical revascularization) depends on the clinical circumstances. If the circumflex injury is diagnosed intraoperatively, an emerging coronary bypass grafting can be performed (6). However, if the lesion is diagnosed in the post-operative period, coronary angiography and subsequent PCI may be the first option (5,7,8). Angioplasty with a balloon alone can be enough if a good expansion of the vessel with <30% of residual diameter stenosis is achieved. Nevertheless, if the diameter stenosis remains >30% after balloon treatment, coronary stenting may be necessary. Of note, in case of technical difficulties in performing PCI (difficulty to cross the lesion, incomplete balloon expansion, absence of flow recovery), surgical treatment should be considered.
Intravascular imaging can help to better evaluate the lesion, mostly by distinguishing between atherosclerosis and a suture injury. Also, it helps to achieve optimal stent expansion and apposition of the stent (5,6).
The final echocardiography evaluation was performed to exclude some possible mechanical complications after PCI. Somekh et al. (9) reported a perforation of the LCx and dehiscence of the mitral annuloplasty ring with a severe paravalvular mitral regurgitation after PCI of an occluded circumflex following a mitral annuloplasty.
Follow-Up
The patient was discharged 6 days after the surgery, with optimal functional class and without any persistent symptom. A new coronary angiography was scheduled in 6 months to re-evaluate the results of the PCI. The probable influence of the dynamic movement of the tissues, the annuloplasty, and the traction generated by the suture in this setting are unknown, so we planned this angiography for a better follow-up of this patient.
Conclusions
LCx injury after mitral valve repair, especially in left dominance or codominance cases, is a potentially severe complication to take into account. Percutaneous treatment of this complication seems safe and feasible and should always be guided by intravascular imaging.
Funding Support and Author Disclosures
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Pre-operative angiogram.
Post-operative angiogram.
IVUS performed before post-dilation of the stent.
Final control angiogram after PCI
Final control IVUS
References
- 1.Grande A.M., Fiore A., Massetti M., Viganò M. Iatrogenic circumflex coronary lesion in mitral valve surgery: case report and review of the literature. Tex Heart Inst J. 2008;35:179–183. [PMC free article] [PubMed] [Google Scholar]
- 2.Meursing D.F., Boonswang N.A., Dobrilovic N., Wait M.A. Perioperative myocardial infarction: secondary to dynamic circumflex coronary artery occlusion after mitral valve repair. Tex Heart Inst J. 2006;33:85–87. [PMC free article] [PubMed] [Google Scholar]
- 3.Tavilla G., Pacini D. Damage to the circumflex coronary artery during mitral valve repair with sliding leaflet technique. Ann Thorac Surg. 1998;66:2091–2093. doi: 10.1016/s0003-4975(98)01071-6. [DOI] [PubMed] [Google Scholar]
- 4.Anyanwu A.C., Adams D.H. Etiologic classification of degenerative mitral valve disease: Barlow’s disease and fibroelastic deficiency. Semin Thorac Cardiovasc Surg. 2007;19:90–96. doi: 10.1053/j.semtcvs.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Busu T., Alqahtani F., Kawsara A., Hijazi M., Alkhouli M. Iatrogenic circumflex artery stenosis following mitral valve repair. Cureus. 2017;9:1–4. doi: 10.7759/cureus.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sangha R., Hui P. Intravascular ultrasound imaging and percutaneous intervention in a patient with post-mitral valve replacement circumflex coronary artery occlusion. J Invasive Cardiol. 2004;16:351–352. [PubMed] [Google Scholar]
- 7.Aybek T., Risteski P., Miskovic A. Seven years’ experience with suture annuloplasty for mitral valve repair. J Thorac Cardiovasc Surg. 2006;131:99–106. doi: 10.1016/j.jtcvs.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 8.Mantilla R., Legarra J.J., Pradas G., Bravo M., Sanmartín M. Percutaneous coronary intervention for iatrogenic occlusion of the circumflex artery after mitral annuloplasty. Tex Heart Inst J. 2004;57:2003–2005. [PubMed] [Google Scholar]
- 9.Somekh N.N., Haider A., Makaryus A.N., Katz S., Bello S., Hartman A. Left circumflex coronary artery occlusion after mitral valve annuloplasty: “a stitch in time. Tex Hear Inst J. 2012;39:104–107. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pre-operative angiogram.
Post-operative angiogram.
IVUS performed before post-dilation of the stent.
Final control angiogram after PCI
Final control IVUS