Abstract
Intra-axial pumps are increasingly used to support cardiogenic shock. The occurrence of electrical storms in this setting is a rising issue, and data remain scarce about optimal management. We report the feasibility of ventricular tachycardia ablation in the presence of a recent surgically inserted Impella 5.0 device (Abiomed, Danvers, Massachusetts). (Level of Difficulty: Intermediate.)
Key Words: case report, cardiogenic shock, electrical storm, Impella 5.0, radiofrequency ablation
Abbreviations and Acronyms: ECLS, extracorporeal life support; ES, electrical storm; LV, left ventricular; LVAD, left ventricular assist device; VT, ventricular tachycardia
Graphical abstract
History of presentation
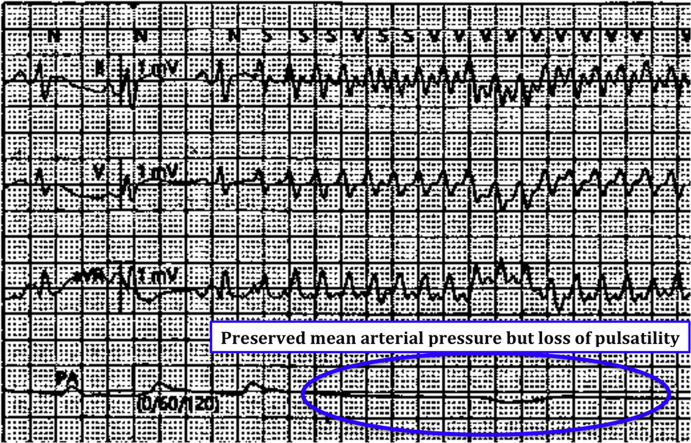
A 47-year-old man, assisted by an Impella 5.0 device (Abiomed, Danvers, Massachusetts) for cardiogenic shock in the setting of a recent myocardial infarction, presented with recurrent episodes of pulseless rhythm. This condition was related to incessant monomorphic ventricular tachycardia (VT) (Figure 1), which caused hemodynamic instability defined as low mean arterial pressure (60 mm Hg) and decreased urine output (20 ml/h).
Learning Objectives
-
•
To evaluate treatment options for patients with implanted Impella 5.0 devices and presenting with an electrical storm.
-
•
To understand the interest of Impella 5.0 in supporting patients undergoing radiofrequency ablation for sustained VT.
Figure 1.
Electrocardiogram Leads and Invasive Pressure Monitoring Showing the Beginning of Ventricular Tachycardia and Its Hemodynamic Consequences
Past Medical History
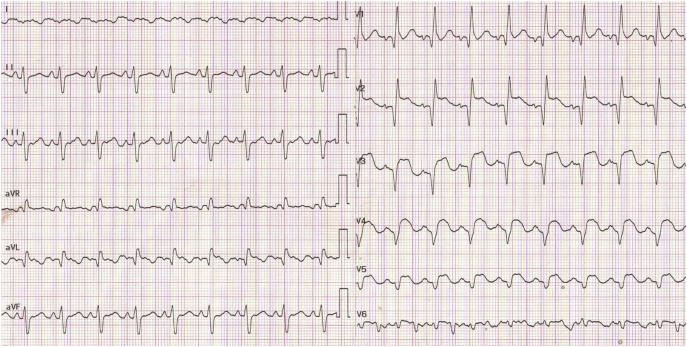
The patient was a heavy smoker with no history of other cardiovascular risk factor or known cardiac disease. He was admitted 10 days earlier for dyspnea, chest pain, and syncope. The electrocardiogram showed a Q-wave and ST-segment elevation in the anterolateral leads associated with troponin elevation up to 7,500 ng/l (reference range 0 to 14 ng/l), with mild liver and kidney dysfunction (Figure 2). The left ventricular (LV) ejection fraction was 15%, with low cardiac output of 2.8 l/min (Video 1). Coronary angiography showed semirecent occlusion of the proximal left anterior descending artery, not revascularized because of delayed presentation. Cardiac arrest during coronary angiography led to the implantation of peripheral femorofemoral extracorporeal life support (ECLS), combined with an intra-aortic balloon pump to unload the left ventricle. Later transthoracic echocardiographic examinations attested to the excellent state of the right ventricle but the lack of recovery of LV function. On the basis of these findings, a transaortic intra-axial pump 5.0 was surgically inserted through a vascular graft in the left axillary artery by the cardiac surgery team. After 1 h of ECLS interruption, pulmonary capillary wedge pressure and cardiac output remained stable on 20 mm Hg and 3.8 l/min, respectively, thus permitting immediate weaning from ECLS.
Figure 2.
12-Lead Electrocardiogram Revealing Q Waves and Persistent ST-Segment Elevations in the Anterolateral Territory
Investigations
A blood sample showed no electrolyte disturbances. Transthoracic echocardiography showed a stable reduced LV ejection fraction related to anteroseptal akinesia, associated with moderate functional mitral regurgitation. The Impella 5.0 inlet was located 4.8 cm below the aortic annulus. No right ventricular failure or pericardial effusion was noted.
Management
Because the patient was still conscious thanks to the cardiac output generated by the intra-axial pump, the initial medical strategy was to administer full doses of amiodarone and lidocaine (Xylocaine), followed by intravenous potassium and magnesium. We suggested that this was a triggered arrhythmia induced by mechanical irritation of the ventricular assistance. In consequence, we decided to reposition the Impella device 1.5 cm higher (Video 2). Given the persistence of the VT, the patient was administered general anesthesia and received multiple electrical shocks. The cardioversions were efficient, but the arrhythmia always recurred. After heart team discussion, the patient was considered noneligible for LV assist device (LVAD) implantation. Therefore, catheter ablation appeared to be the only reliable treatment for such a drug-refractory electrical storm.
The procedure lasted 4 h and 50 min (fluoroscopy time, 7 min and 54 s; total kerma-area product, 0.887 mGy/m2) and was performed using general anesthesia. Clinical VT was initially drug induced (isoproterenol) and persisted throughout the procedure (Figure 3). Hemodynamic indices remained stable in sustained VT; blood pressure was nonpulsatile, but mean arterial pressure was maintained in 65 mm Hg with low doses of vasopressor, and cardiac output was 3.8 l/min. After transeptal puncture, the first step of the procedure consisted of carry out a mapping substrate using a SMARTTOUCH SF catheter (Biosense Webster, Inc., Diamond Bar, California) that identified a large, low-voltage zone corresponding to the myocardial scar (Figure 4). Pace mapping showed a possible exit in the apicolateral area, but only with an 11/12 electrocardiographic similarity. In VT, we rapidly noticed diastolic potentials consistent with critical isthmus depolarization located next to the apicolateral region previously identified (Figure 5). Radiofrequency ablation at this point immediately restored sinus rhythm. Noninducibility of the VT attested to the success of the procedure.
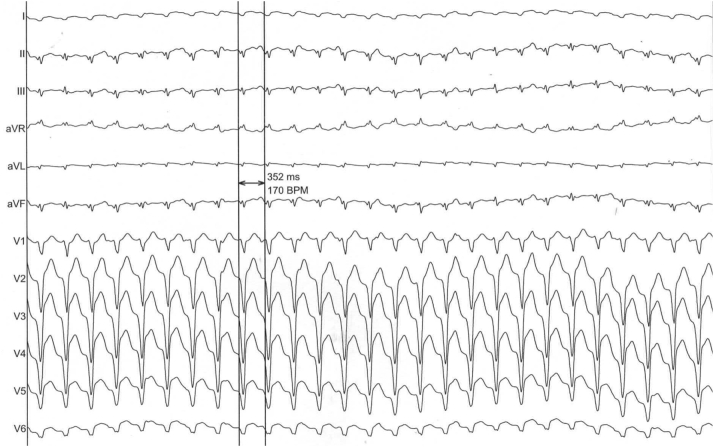
Figure 3.
Electrocardiogram in Arrhythmia
The electrocardiogram showed wide QRS complex monomorphic tachycardia (cycle, 350 ms), with a negative concordance pattern in the precordial leads associated with extreme axis deviation and a negative aspect in the inferior territory, consistent with ventricular tachycardia emerging from the apical region.
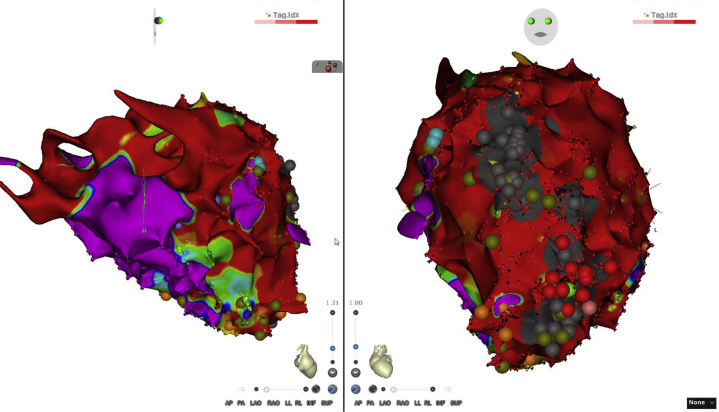
Figure 4.
Voltage Mapping
The voltage mapping (septal incidence on the left, apical upper view on the right) identified a large anteroseptolateral zone of low–electrical voltage regions corresponding to the red color code (contrary to the purple color for normal conduction areas). The radiofrequency points are represented by the red dots on the right.
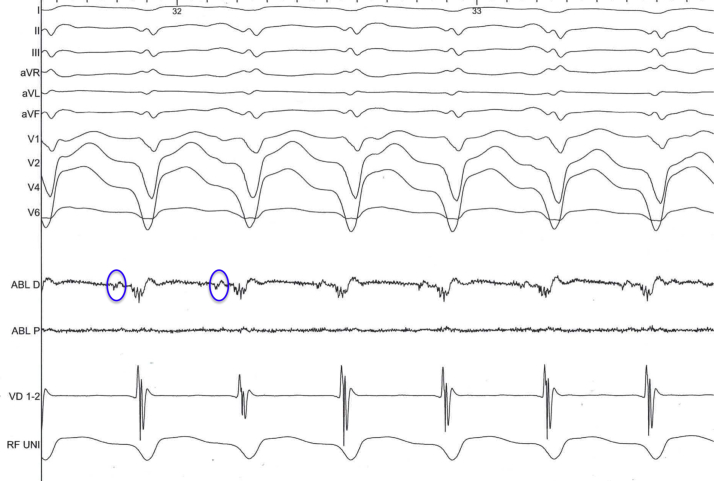
Figure 5.
Intracardiac Electrogram
Diastolic potentials preceding every ventricular depolarization are circled. This pattern is highly suggestive of being the exit site of the re-entry circuit.
Discussion
An Impella 5.0 device implanted surgically through the axillary artery is currently a novel approach to intra-axial flow mechanical circulatory support (1). It is worth noting that the hemodynamic instability justifying Impella device implantation carries a high risk of life-threatening arrhythmias potentially requiring ablation. Because of the proximity between the radiofrequency catheter and the distal pump, the procedure may be challenging no matter which type of cardiac assistance is used (2,3). Data are particularly scarce for the Impella 5.0 device (4).
Percutaneous Impella devices have shown an interesting profile to support hemodynamically prolonged ablation procedures for poorly tolerated VT (5). Several studies demonstrated technical limitations of various devices working at the same time, specifically because of magnetic interferences (6). This could be problematic when mapping using magnet-based systems (CARTO3, Biosense Webster). In this case of CARTO3-guided intervention, although the ablation site was located in the anterolateral region, close to the left assistance device distal tip set on P4, we did not report interference precluding accurate mapping. We were even able to characterize low-voltage diastolic potentials that were crucial to define radiofrequency target points.
In comparison with percutaneous pumps, the larger diameter of the Impella 5.0 device (21 F), did not compromise the effectiveness of the ablation; in addition, the higher flow rate ensured hemodynamic stability. The singular transaxillary approach is appropriate for patients presenting with severe peripheral arterial disease, and unlike transfemoral devices, it allows higher levels of mobility for patients requiring prolonged assistance.
What makes this case original is that the mechanical circulatory support was initially placed for severe LV dysfunction that was subsequently complicated by refractory VT. The only reliable treatment at this point was the radiofrequency intervention (7,8). Indeed, specific data on ablation in the setting of cardiogenic shock requiring ventricular assistance are limited (9). Despite the high mortality reported in patients with implanted LVADs who present with electrical storm, this case suggests that monomorphic ventricular arrhythmia can be successfully treated in the presence of an Impella 5.0 device, thus allowing subsequent implantation of long-term mechanical cardiac support (10).
Follow-Up
The LVAD was successfully implanted 12 days after catheter ablation. Three months later, the patient was still free from VT recurrence while awaiting a cardiac transplant.
Conclusions
The Impella 5.0 device is becoming a useful temporary circulatory support for cardiogenic shock related to isolated LV dysfunction. Because of severe hemodynamic instability, such patients may have life-threatening arrhythmia. Radiofrequency ablation of refractory VT is feasible in patients supported by an Impella 5.0 device and may provide good clinical outcomes.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank all the members of Nantes shock team who actively collaborate in daily practice to make possible the multidisciplinary management of critically ill patients. Interventions and laboratory testing were done first in the Breton Atlantic Hospital Center in Vannes and then in the Nantes University Hospital Center.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Echocardiogram Showing a Severely Reduced Left Ventricular Ejection Fraction
Echocardiogram Showing the Impella 5.0 Device After Being Repositioned
References
- 1.Lima B., Kale P., Gonzalez-Stawinski G. Effectiveness and safety of the Impella 5.0 as a bridge to cardiac transplantation or durable left ventricular assist device. Am J Cardiol. 2016;117:1622–1628. doi: 10.1016/j.amjcard.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 2.Miller M.A., Dukkipati S.R., Koruth J.S., d'Avila A., Reddy V.Y. How to perform ventricular tachycardia ablation with a percutaneous left ventricular assist device. Heart Rhythm. 2012;9:1168–1176. doi: 10.1016/j.hrthm.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Turagam M.K., Vuddanda V., Koerber S. Percutaneous ventricular assist device in ventricular tachycardia ablation: a systematic review and meta-analysis. J Interv Card Electrophysiol. 2019;55:197–205. doi: 10.1007/s10840-018-0477-1. [DOI] [PubMed] [Google Scholar]
- 4.Castelein T., Balthazar T., Adriaenssens T. Impella to resist the storm. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.119.006698. [DOI] [PubMed] [Google Scholar]
- 5.Miller M.A., Dukkipati S.R., Chinitz J.S. Percutaneous hemodynamic support with Impella 2.5 during scar-related ventricular tachycardia ablation (PERMIT 1) Circ Arrhythm Electrophysiol. 2013;6:151–159. doi: 10.1161/CIRCEP.112.975888. [DOI] [PubMed] [Google Scholar]
- 6.Vaidya V.R., Desimone C.V., Madhavan M. Compatibility of electroanatomical mapping systems with a concurrent percutaneous axial flow ventricular assist device. J Cardiovasc Electrophysiol. 2014;25:781–786. doi: 10.1111/jce.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santangeli P., Muser D., Maeda S. Comparative effectiveness of antiarrhythmic drugs and catheter ablation for the prevention of recurrent ventricular tachycardia in patients with implantable cardioverter-defibrillators: a systematic review and meta-analysis of randomized controlled trials. Heart Rhythm. 2016;13:1552–1559. doi: 10.1016/j.hrthm.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Sapp J.L., Wells G.A., Parkash R. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med. 2016;375:111–121. doi: 10.1056/NEJMoa1513614. [DOI] [PubMed] [Google Scholar]
- 9.Ballout J.A., Wazni O.M., Tarakji K.G. Catheter ablation in patients with cardiogenic shock and refractory ventricular tachycardia. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.119.007669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martins R.P., Leclercq C., Bourenane H. Incidence, predictors, and clinical impact of electrical storm in patients with left ventricular assist devices: new insights from the ASSIST-ICD study. Heart Rhythm. 2019;16:1506–1512. doi: 10.1016/j.hrthm.2019.06.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Echocardiogram Showing a Severely Reduced Left Ventricular Ejection Fraction
Echocardiogram Showing the Impella 5.0 Device After Being Repositioned