Abstract
After a 20-year-old woman suddenly died, autopsy showed characteristic findings of biventricular arrhythmogenic cardiomyopathy. Screening of her family members revealed the same desmoplakin gene mutation and imaging abnormalities predominantly involving the left ventricle. We describe the variable phenotypic expression in a family that shares a common gene variant. (Level of Difficulty: Advanced.)
Key Words: cardiac magnetic resonance, cardiomyopathy, echocardiography, genetic disorders, phenotype
Abbreviations and Acronyms: AVC, arrhythmogenic ventricular cardiomyopathy; CMR, cardiac magnetic resonance; DSP, desmosomal protein desmoplakin; LGE, late gadolinium enhancement; LV, left ventricular; RV, right ventricular; SCD, sudden cardiac death
Graphical abstract
Case 1
A 20-year-old White woman with no significant past medical history suddenly collapsed and died while watching a baseball game at home. Her mother reported that she was asymptomatic with the exception of mild chest discomfort days before the event and a vague feeling of intermittent pre-syncope. She did not seek medical attention for either episode. Her autopsy showed significant biventricular arrhythmogenic ventricular cardiomyopathy (AVC) (Figures 1A to 1L). She became the proband of this study, prompting several family members to seek screening.
Learning Objectives
-
•
To recognize LV involvement in AVC, even in isolation, and especially in the setting of an established family history and positive genetic screening results.
-
•
To recognize the utility of CMR in screening individuals because the screened family members in our case were all asymptomatic, had normal echocardiograms, and unremarkable electrocardiograms.
-
•
To recognize the variance in phenotypic expression of AVC within the same family.
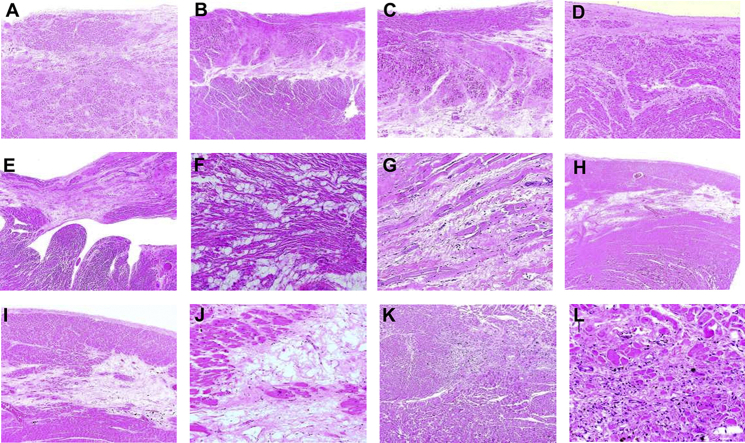
Figure 1.
Histologic Findings During Autopsy
(A to D) Areas of mature (i.e., older) scarring in left ventricular subendocardial areas. (E to G) Accumulation of mature adipocytes and fibrosis in the right ventricle. (H to J) Subepicardial and midmyocardial bands of fibrosis and adipose tissue in the lateral and posterolateral free walls of the left ventricle, a feature characteristic of disease in patients with desmosomal protein desmoplakin variants. (K, L) Recent (and ongoing) myocardial injury in the left ventricle.
Case 2
The proband’s mother is a 50-year-old woman with a history of breast cancer who received chemotherapy (with doxorubicin) and radiation 15 years earlier. She reported 2 family members on her maternal side who had sudden cardiac death (SCD) around age 30s to 40s, and 1 family member underwent cardiac transplantation. She denied symptoms of palpitations, syncope, or dizziness and was screened with cardiac magnetic resonance (CMR) and underwent genetic panel testing. On CMR, she had normal right ventricular (RV) size with mildly reduced systolic function. A focal aneurysmal segment was seen in the RV free wall that enhanced with gadolinium, thus indicating scar formation and myocardial fibrosis (Figure 2.2a). There was also nonspecific subepicardial late gadolinium enhancement (LGE) involving the anteroseptal, inferior, and inferoseptal wall of the left ventricle, indicating regional scar formation and myocardial fibrosis (Figures 2.2b to 2.2e). The left ventricle had normal size and systolic function. A targeted genetic panel for cardiomyopathy revealed a variant, p.T356k, in the gene for the desmosomal protein desmoplakin (DSP). In addition to the DSP mutation, she also had a variant in JUP (p.S730G), another desmosomal protein that encodes plakoglobin. Both findings were defined as variants of undetermined significance by the diagnostic laboratory.
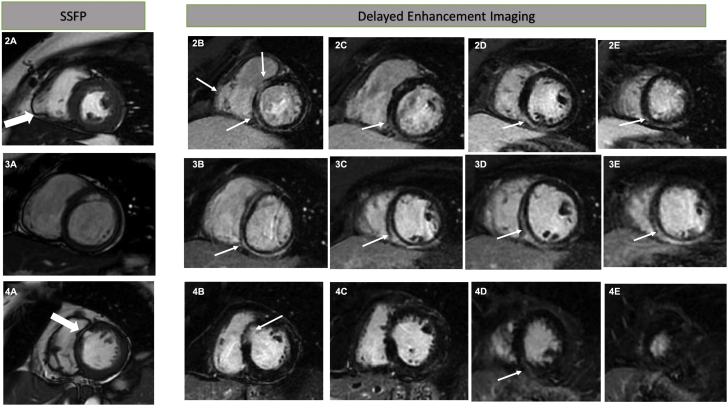
Figure 2.
Cardiac Magnetic Resonance Findings of Screened Family Members
(2A) Cine steady-state free precession (SSFP) short-axis imaging shows a right ventricular free wall segmental aneurysm (large arrow). (2B to 2E) Late gadolinium enhancement seen in the left ventricle predominantly involving subepicardium and the right ventricular free wall where an aneurysm was seen on the previous image (small arrows). (3A) Cine SSFP short-axis image showing a normal right ventricular free wall. (3B to 3E) Late gadolinium enhancement seen predominantly involving the left ventricular subepicardium (arrows). (4A) SSFP imaging in short-axis view showing thinning and fatty infiltration of the basal anteroseptal segment (large arrow). (4B) Delayed enhancement Imaging in short axis view showing thinning of the anteroseptal segment with subendocardial late gadolinium enhancement (thin arrow pointing at the late gadolinium enhancement). (4C) A short-axis delayed enhancement image showing thinning of the anteroseptal segment of the left ventricle with no late gadolinium enhancement. (4D) Delayed enhancement image showing mid myocardial late gadolinium enhancement in the inferoseptal segment (thin arrow pointing at the late gadolinium enhancement). (4E) Delayed enhancement image with short axis view at the level of the distal left ventricle showing no late gadolinium enhancement.
Case 3
On screening the proband’s asymptomatic 18-year-old sister, the same mutation of the DSP gene, p.T356K, was discovered. CMR revealed extensive LGE involving the anteroseptal, inferoseptal, inferior, and inferolateral wall of the left ventricle only, sparing the right ventricle entirely (Figures 2.3b to 2.3e). Both ventricles had normal size and systolic function.
Case 4
CMR of the 72-year-old maternal grandmother of the proband revealed mildly reduced LV systolic function with thinning and severe hypokinesis of the distal and apical inferior, inferolateral, and basal anteroseptal wall. A small area of basal anteroseptal wall was thin and replaced by fatty tissue and demonstrated subendocardial delayed gadolinium enhancement in that segment (Figures 2.4a to 2.4e). The right ventricle had normal size and function. She was also found to have the same mutation of the DSP gene, p. T356K. Although the proband’s grandmother, mother, and younger sister shared the mutation, her older sister remained asymptomatic, with normal test results, including CMR and testing for genetic mutation.
Discussion
AVC is classically an autosomal dominant genetic disease that leads to fibrofatty infiltration of the myocardium. It is a known cause of heart failure, syncope, arrhythmia, and SCD. Clinical presentation may vary from vague symptoms of palpitations, chest pain, and dyspnea to devastating consequences such as ventricular arrhythmias and SCD. The age of presentation is generally between 10 and 50 years, and patients with long-standing disease often present with more severe symptoms.
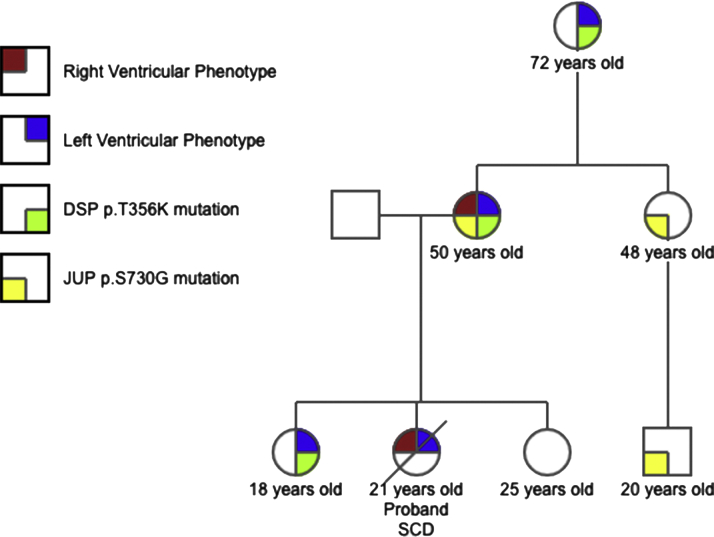
The proband’s autopsy findings were consistent with AVC, with a notable feature of fibrofatty infiltration of both ventricles. Three family members shared the mutation of the DSP gene, p.T356K, whereas the proband’s older sister remained free of genetic mutation and disease (Figure 3).The tissue obtained at autopsy from the proband was compromised because of the time elapsed from the autopsy and could not have genetic testing performed. CMR imaging of the family members showed minor RV cardiac abnormalities that did not fulfill the AVC task force major criteria. There was an isolated aneurysm of the RV free wall in the mother, with abnormal LGE mostly involving the left ventricle. The younger sister had a normal right ventricle with subepicardial LGE seen extensively in the left ventricle. The grandmother had subtle findings of thinning and fatty replacement of the basal anteroseptal segment with subendocardial enhancement of the left ventricle.
Figure 3.
Family Pedigree
Family pedigree illustrating the varying phenotypic expression of the disease and associated genetic variants. DSP = desmosomal protein desmoplakin; JUP = another desmosomal protein that encodes plakoglobin.
Revised diagnostic criteria were established in 2010 to increase sensitivity in the diagnosis of AVC by the inclusion of quantitative imaging and electrocardiographic diagnostic criteria and genetics (1). However, the criteria do not currently include left ventricular findings of LGE. Although biventricular involvement is known to occur commonly in advanced AVC (2), the clinical implication of LV involvement within current diagnostic criteria and guidelines is not well established. LV disease, both in isolation and in combination with the RV disease, is being identified with increased frequency (3, 4, 5, 6, 7). More specifically, isolated LV disease has also been noted in mutations of DSP and other desmosomal genes (8); however, the DSP p.T356K variant has not been proven to be pathogenic and is extremely rare.
Genes associated with inheritable cardiac disorders have been shown to have pleiotropic effects, which could help explain why family members with the same mutation have different phenotypes (9), such as the family presented. A recent cohort of 202 patients with SCD and histologically confirmed AVC at autopsy found isolated LV involvement in 17% of patients, and 87% of patients had some degree of LV involvement (10). The study also found that LV involvement did not correlate with the age of death and that LV involvement can occur early in the disease course (10). In this family, the shared DSP variant segregated within the family and correlated with the presence of abnormal findings on CMR. The significance of the plakoglobin variant is unclear because it did not segregate within the family and was present in only 2 family members who did not have worse disease.
Management
Given the combination of imaging findings, genetic testing, and history of SCD in a relative, all 3 patients decided to undergo implantable cardioverter-defibrillator placement for primary prevention of fatal arrhythmias and SCD. All 3 have recovered well after implantable cardioverter-defibrillator placement and have not had any significant cardiac complications.
Conclusions
A sudden cardiac death in a 20-year-old resulted in cascade screening of multiple first-degree relatives leading to the diagnosis of AVC with varying biventricular involvement within the family. It highlights the importance of recognizing variance in phenotype even within the same family in this often heterogenous disease.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.McCrohon J.A., John A.S., Lorenz C.H., Davies S.W., Pennell D.J. Images in cardiovascular medicine. Left ventricular involvement in arrhythmogenic right ventricular cardiomyopathy. Circulation. 2002;105:1394. doi: 10.1161/hc1102.104521. [DOI] [PubMed] [Google Scholar]
- 2.Corrado D., Basso C., Thiene G. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol. 1997;30:1512–1520. doi: 10.1016/s0735-1097(97)00332-x. [DOI] [PubMed] [Google Scholar]
- 3.Nava A., Bauce B., Basso C. Clinical profile and long-term follow-up of 37 families with arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2000;36:2226–2233. doi: 10.1016/s0735-1097(00)00997-9. [DOI] [PubMed] [Google Scholar]
- 4.Sen-Chowdhry S., Syrris P., Prasad S.K. Left-dominant arrhythmogenic cardiomyopathy: an under-recognized clinical entity. J Am Coll Cardiol. 2008;52:2175–2187. doi: 10.1016/j.jacc.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Sen-Chowdhry S., Syrris P., Ward D., Asimaki A., Sevdalis E., McKenna W.J. Clinical and genetic characterization of families with arrhythmogenic right ventricular dysplasia/cardiomyopathy provides novel insights into patterns of disease expression. Circulation. 2007;115:1710–1720. doi: 10.1161/CIRCULATIONAHA.106.660241. [DOI] [PubMed] [Google Scholar]
- 6.El Ghannudi S., Nghiem A., Germain P., Jeung M.Y., Gangi A., Roy C. Left ventricular involvement in arrhythmogenic right ventricular cardiomyopathy - a cardiac magnetic resonance imaging study. Clin Med Insights Cardiol. 2014;8:27–36. doi: 10.4137/CMC.S18770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rastegar N., Zimmerman S.L., Te Riele A. Spectrum of biventricular involvement on CMR among carriers of ARVD/C-associated mutations. J Am Coll Cardiol Img. 2015;8:863–864. doi: 10.1016/j.jcmg.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Towbin J., McKenna W., Abrams D. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019;16:e301–e372. doi: 10.1016/j.hrthm.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Cerrone M., Remme C., Tadros R., Bezzina C., Delmar M. Beyond the one gene-one disease paradigm. Circulation. 2019;140:595–610. doi: 10.1161/CIRCULATIONAHA.118.035954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miles C., Finocchiaro G., Papadakis M. Sudden death and left ventricular involvement in arrhythmogenic cardiomyopathy. Circulation. 2019;139:1786–1797. doi: 10.1161/CIRCULATIONAHA.118.037230. [DOI] [PMC free article] [PubMed] [Google Scholar]