Abstract
A 3-month-old infant who developed persistent junctional reciprocating tachycardia (PJRT)–induced cardiomyopathy that was successfully treated with radiofrequency ablation. To our knowledge this is the youngest reported patient with a successful epicardial lesion placed in a diverticulum off the coronary sinus and also the first report of a PJRT connection located at an epicardial site distinct from the mitral and tricuspid valve annulus. We use this case to highlight how low-power lesions in the coronary sinus in the youngest of patients can achieve results safely. (Level of Difficulty: Advanced.)
Key Words: epicardial ablation, incessant SVT, infant ablation, PJRT, tachycardia-induced cardiomyopathy
Abbreviations and Acronyms: AV, atrioventricular; BNP, B-type natriuretic peptide; CS, coronary sinus; ECG, electrocardiogram; EP, electrophysiology; PJRT, persistent junctional reciprocating tachycardia; RF, radiofrequency; SVT, supraventricular tachycardia
Graphical abstract
Introduction
Persistent junctional reciprocating tachycardia (PJRT) is a rare form of refractory supraventricular tachycardia (SVT) occurring in infants and children, and it accounts for approximately 1% of all SVTs in this group (1). Because of the incessant nature of PJRT, if left untreated, this arrhythmia may lead to tachycardia-induced cardiomyopathy. The circuit of PJRT is an orthodromic atrioventricular (AV) re-entry with a concealed, slow conducting accessory pathway as the retrograde limb of the circuit (2, 3, 4, 5). Because both limbs in a PJRT circuit are decremental, these tachycardias typically tend to occur at lower heart rates than other SVTs and can be undetected until patients present with heart failure. Electrocardiograms (ECGs) during SVT typically show negative P waves in the inferior leads with a long RP interval (2,3).
Learning Objectives
-
•
To understand that tachycardia-induced cardiomyopathy can be reversed early after successful ablation in PJRT.
-
•
To understand the risks of lesions in the CS and to take necessary precautions to minimize risk such as delivery of low-power lesions and delineation of coronary anatomy before RF ablation.
Here, we report the case of a 3-month-old infant who developed tachycardia-induced cardiomyopathy and was successfully treated with radiofrequency (RF) ablation. To our knowledge, this is the youngest reported patient with a successful epicardial lesion placed in a diverticulum off of the coronary sinus (CS).
History of Presentation
A 3-month-old, 5.5-kg, male infant with incessant SVT evident since fetal life presented to the emergency department with grunting, respiratory distress, and decreased feeding for 2 days. He had a low-grade fever, runny nose, and a cough 2 weeks before presentation. There were other ill contacts in the household. On examination, he had signs and symptoms of heart failure, with a heart rate of 150 beats/min, a respiratory rate 50 breaths/min, and the liver 3 cm below the costal margin.
Investigations
An ECG showed a long RP tachycardia and echocardiogram revealed an ejection fraction of 22%. Laboratory work was significant for a B-type natriuretic peptide (BNP) level of 10,000 ng/ml and a viral respiratory panel positive for parainfluenza and coronavirus NL63 but negative for all the other coronaviruses, including severe acute respiratory syndrome coronavirus type-2, the agent of coronavirus disease-2019 (COVID-19).
Past Medical History
The infant was well known to the electrophysiology (EP) service for incessant SVT present since fetal life. SVT was first diagnosed at 32 weeks of gestation on a fetal ultrasound examination, with heart rates in the 180s to 200s (beats/min). Treatment during fetal life had been attempted with flecainide and digoxin, and the baby was delivered early, at 35 weeks, for mild fetal hydrops. In the postnatal period, SVT rates were in the 200s (beats/min) (Figure 1), and rate but not rhythm control (heart rate 140s to 150s [beats/min]) was achieved with high doses of amiodarone (20 mg/kg/day) and procainamide (60 μg/kg/min) infusions and oral sotalol. The infant was transitioned to oral flecainide, and amiodarone, and sotalol was switched to a beta-blocker in view of concerns about combining QTc interval–prolonging drugs. The SVT continued to be incessant but at lower rates of 120 to 130 beats/min as an outpatient, and the patient was monitored closely for the first 2 months with a 30-day remote telemetry monitor (Biotel, Malvern, Pennsylvania) and an Owlet sock (Owlet, Lehi, Utah). Serial outpatient echocardiograms showed that at the 2-month checkup the infant’s ejection fraction had dropped from 60% on discharge at 3 weeks of age to 50%. The infant did not return for follow-up for the 2.5-month check because of intercurrent viral respiratory illnesses in the family, and the home monitors showed that the average heart rates were increasing from the 120s to the 140s (beats/min). At 3 months, he presented to the emergency department with the foregoing picture.
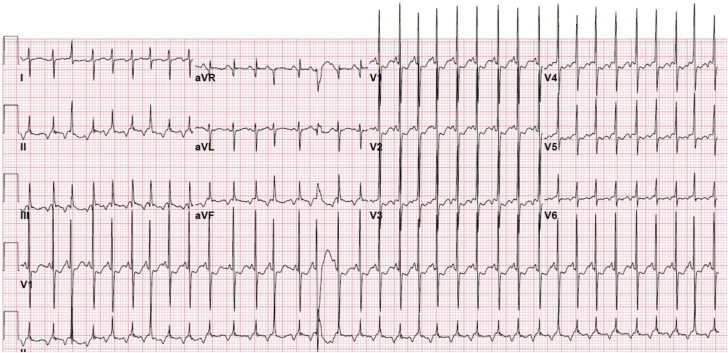
Figure 1.
12-Lead Electrocardiogram With Negative P Waves in Leads ll, III, aVF, and V3 to V6 Characteristic of Persistent Junctional Reciprocating Tachycardia
Management
He was started on milrinone and furosemide, and from an antiarrhythmic standpoint, he was loaded with digoxin and another short load of oral amiodarone followed by maintenance amiodarone, and his beta-blockers increased. Flecainide was discontinued in view of the deterioration in cardiac function. On this regimen, he had an unresponsive staring episode that lasted a few seconds. His heart rates were low, in the 70s (beats/min), and his digoxin level was elevated at 4.1 ng/ml. A decision was made to take him to the EP laboratory for catheter ablation. At 72 h before the planned ablation, the oral amiodarone, digoxin, and beta-blockers were stopped, and he was placed on an esmolol infusion to maintain heart rates lower than 130 beats/min. The esmolol was titrated up to 150 μg/kg/min and was stopped 30 min before the ablation.
Electrophysiological Study
The EP study was performed with the infant under general anesthesia and receiving inotropic support. The infant was in incessant SVT in the EP laboratory. A 3-catheter study was performed with access through the right and left femoral veins. A 3-dimensional electroanatomic mapping procedure was performed with the EnSite Precision mapping system (Abbott Medical, Inc., St. Paul, Minnesota). A 4-F decapolar (Inquiry, Abbott Medical, Inc.) was placed in the CS, and a 4-F quadripolar catheter (JSN, Abbott Medical, Inc.) was placed in the right ventricular apex. Mapping was performed with a 5-F Mariner small curve catheter (Medtronic, Inc., Minneapolis, Minnesota). Of note, this is the smallest RF ablation catheter available on the market. Atrial extrastimuli resulted in transient interruption followed by resumption of SVT after 2 to 3 sinus beats. His bundle-refractory premature ventricular contractions during tachycardia delayed atrial activation. Entrainment during SVT was performed with pacing in the right ventricular apex, and the atrial activation sequence during pacing was identical to the tachycardia. It was clear early on in the case that the pathway location was close to the CS dipoles 7.8 within the CS. Detailed mapping of the right septum and posteroseptal space was performed, and the Mariner catheter was inserted into the CS alongside the decapolar catheter. Earliest atrial activation during SVT was noted in a diverticulum off the os of the CS (Figure 2). An aortic root angiogram was performed through a 4-F pigtail catheter in the right femoral artery delineate coronary anatomy. The catheter tip was distinct from the main coronary arteries and branches (Figure 3). Current delivery with a low-power 7-W lesion resulted in an immediate temperature increase to 50°C, impedance of 115 ohms, and cessation of the SVT within 1.3 s. This was evidenced by a change in heart rate and CS activation pattern. The lesion was applied for a full 40 s. No insurance burns were applied. A full EP study was then performed in sinus rhythm, and results were normal. Repeat electrical stimulation was performed with atrial and ventricular singles and doubles at coupling intervals down to 180 ms on and off isoproterenol therapy and during the washout phase of the drug with no evidence of SVT. At 60 min post-ablation, the catheters were removed.
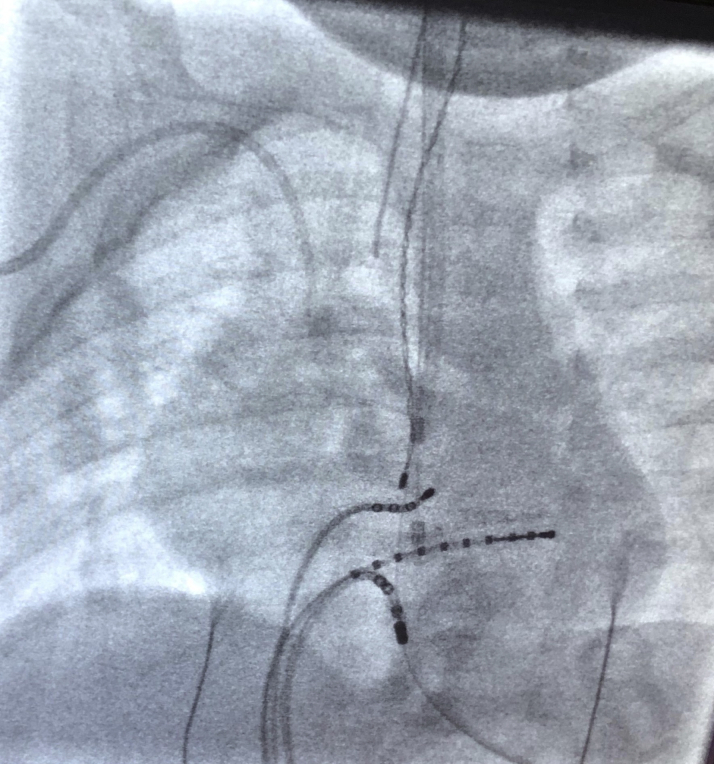
Figure 2.
Location of Catheter at Successful Ablation Site in a Diverticulum Close to the Os of the Coronary Sinus
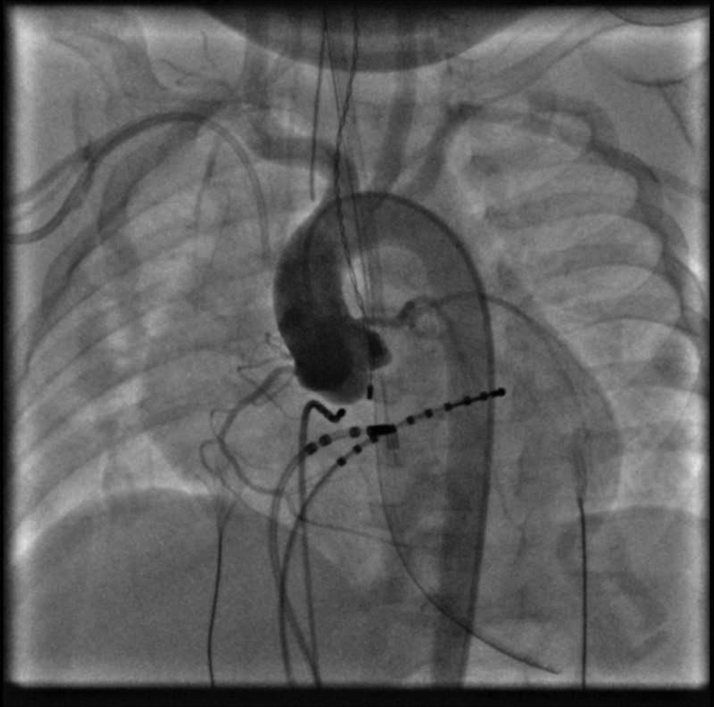
Figure 3.
Aortic Root Angiogram Performed Before Ablation Delineating Coronary Anatomy Confirmed Catheter Tip Distinct From the Main Coronary Arteries and Branches
Both right and left coronary arteries take off normally from the corresponding sinuses. Of note, the ablation catheter bounced back into the main coronary sinus during the angiogram and was repositioned shortly afterward.
Follow-Up
The patient was transferred back to the intensive care unit with continuous telemetry monitoring. Milrinone was stopped 12 h post-ablation, and an echocardiogram 48 h later showed an improvement in ejection fraction to 45%. Normal flow was demonstrated in both coronary artery braches post-procedure. The BNP 48 h post-ablation was 145 ng/ml. It is not our routine practice to obtain troponins post-ablation; however, given the high-risk location of the lesion, a troponin level was obtained 18 h post-ablation and was normal. Post-operative ECGs obtained daily showed no evidence of ST-segment changes. The patient continued to be in sinus rhythm 3 days later and was discharged home on a home telemetry monitor. The discharge heart rate was 118 beats/min, and the ejection fraction was 50%. The patient was discharged on furosemide, which was tapered and stopped within 2 weeks of discharge. Results of a repeat set of laboratory tests, including BNP, were normal. A follow-up coronary computed tomography angiogram obtained at 6 months post-ablation showed normal coronary arteries with no evidence of stenosis. For the first 6 months, we also obtained frequent external cardiac monitors, and the patient demonstrated normal heart rate ranges for age and no evidence of SVT. Nine month later, the patient is doing very well, with completely recovered cardiac function (ejection fraction 70%) off all medications.
Discussion
PJRT is an uncommon but important cause of refractory SVT in infants and children (5,6). These accessory pathway typically have only retrograde conduction properties and are slow and decremental. The pathway characteristics are similar to AV nodal tissue, and therefore the circuit has 2 decrementally conducting limbs, thus making for stable re-entrant SVT at varying cycle lengths (2, 3, 4).
Medical management can be difficult, and poor rhythm and or rate control can lead to cardiac decompensation and tachycardia-induced cardiomyopathy. These patients may be managed medically, despite being in SVT, if meticulous rhythm control is achieved. We surmise that in our patient, a combination of inadequate rate control seen as an increase in average heart rates from the 120s to the 140s (beats/min) during the period when he had not returned for follow-up combined with the intercurrent viral illness likely led to the cardiac decompensation. These pathways are most commonly located in the right septal area, more commonly near the posterior septum, but middle or anterior septal locations have also been described. The right posteroseptal pathways may be close to the ostium of the CS, and locations have been described in almost any position along the AV groove (3,5,6). Epicardial connections distant from the mitral and tricuspid valve annulus within a diverticulum of the CS are unusual, and to the best of our knowledge, both: 1) PJRT pathways in this location; and 2) successful RF ablation in an infant of an epicardial pathway have not been previously described.
Epicardial lesions can be achieved with an endocardial catheter placed through a transvenous approach. We opted to perform an aortic root angiogram in our patient before RF application because ablation of accessory pathways from within the CS may lead to damage to nearby coronary arteries and their branches. Specifically, the posterolateral (inferolateral) ventricular branch of the right coronary artery and the left circumflex artery run inferiorly and in close proximity to the CS. Use of RF catheter ablation within the CS has been reported to cause ischemia and infarction secondary to stenosis or complete occlusion. Symptoms may be acute and catastrophic or may manifest several weeks later (7).
Other complications described with RF lesions within the CS include cardiac tamponade, AV block, and pericarditis, and our patient went home on a 30-day event recorder to monitor for arrhythmia recurrence, as well as late onset heart block (8).
In our patient, we chose to deliver a low-power RF burn at 10 W, to avoid perforation of this tiny CS branch. However, before the set power was delivered, given the low-flow nature of this branch of the CS, we achieved our temperature cutoff before the power rose to more than 7 W. Kusano et al. have described low-power 10-W lesions in the CS achieving temperatures of 60°C (9).
Finally, we would like to mention that ablations in infants are rare and should be approached with extreme caution. Children weighing <15 kg represent only 6% of ablations performed in pediatrics, and for good reason (10). Factors such as technical issues with catheters in small hearts, ease of perforation, and the unknown long-term effects of RF in the maturing myocardium contribute to challenges associated with ablation procedures in the very young.
Conclusions
Tachycardia-induced cardiomyopathy can be effectively reversed by appropriate treatment of the substrate with RF ablation. Low-power burns in the CS were effective in achieving adequate temperatures in our case. Lesions in the CS are high risk because they are low flow structures and pose a high risk for perforation. Additionally, given the proximity to other epicardial structures such as coronary artery branches that run alongside veins, extreme care must be taken to delineate coronary anatomy before placing such lesions.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Josephson M.E. Supraventricular tachycardias. In: Josephson M.E., editor. Josephson’s Clinical Cardiac Electrophysiology: Techniques and Interpretations. 5th edition. Wolters Kluwer; Philadelphia, PA: 2016. pp. 171–280. [Google Scholar]
- 2.Coumel P., Cabrol C., Fabiato A. Tachycardie permanente par rythme réciproque. Preuves du diagnostic par stimulation auriculaire et ventriculaire. Arch Mal Coeur. 1967;60:1830–1864. [Google Scholar]
- 3.Coumel P. Junctional reciprocating tachycardias. The permanent and paroxysmal forms of A-V nodal reciprocating tachycardias. J Electrocardiol. 1975;8:79–90. doi: 10.1016/s0022-0736(75)80043-4. [DOI] [PubMed] [Google Scholar]
- 4.Critelli G. Recognizing and managing permanent junctional reciprocating tachycardia in the catheter ablation era. J Cardiovasc Electrophysiol. 1997;8:226–236. doi: 10.1111/j.1540-8167.1997.tb00784.x. [DOI] [PubMed] [Google Scholar]
- 5.Vaksmann G., D'Hoinne C., Lucet V. Permanent junctional reciprocating tachycardia in children: a multicenter study on clinical profile and outcome. Heart. 2006;92:101–104. doi: 10.1136/hrt.2004.054163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang K.T., Potts J.E., Radbill A.E. Permanent junctional reciprocating tachycardia in children: a multicenter experience. Heart Rhythm. 2014;11:1426–1432. doi: 10.1016/j.hrthm.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 7.Schneider H.E., Kriebel T., Gravenhorst V.D., Paul T. Incidence of coronary artery injury immediately after catheter ablation for supraventricular tachycardias in infants and children. Heart Rhythm. 2009;6:461–467. doi: 10.1016/j.hrthm.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Jackman W.M., Wang X., Friday K.J. Catheter ablation of accessory atrioventricular pathways (Wolff-Parkinson-White syndrome) by radiofrequency current. N Engl J Med. 1991;324:1605–1611. doi: 10.1056/NEJM199106063242301. [DOI] [PubMed] [Google Scholar]
- 9.Kusano K.F., Morita H., Fujimoto Y., Hirose E., Ohe T. Catheter ablation of an epicardial accessory pathway via the middle cardiac vein guided by monophasic action potential recordings. Europace. 2001;3:164–167. doi: 10.1053/eupc.2001.0161. [DOI] [PubMed] [Google Scholar]
- 10.Blaufox A.D. Catheter ablation of tachyarrhythmias in small children. Indian Pacing Electrophysiol J. 2005;5:51–62. [PMC free article] [PubMed] [Google Scholar]