Abstract
Rationale
Heart failure (HF) management in chronic obstructive pulmonary disease (COPD) is often delayed or suboptimal.
Objectives
To examine the effect of HF and HF medication use on moderate-to-severe COPD exacerbations.
Methods and measurements
Retrospective cohort studies from 2006 to 2016 using nationally representative English primary care electronic healthcare records linked to national hospital and mortality data. Patients with COPD with diagnosed and possible HF were identified. Possible HF was defined as continuous loop diuretic use in the absence of a non-cardiac indication. Incident exposure to HF medications was defined as ≥2 prescriptions within 90 days with no gaps >90 days during ≤6 months of continuous use; prevalent exposure as 6+ months of continuous use. HF medications investigated were angiotensin receptor blockers, ACE inhibitors, beta-blockers, loop diuretics and mineralocorticoid receptor antagonists. Cox regression, stratified by sex and age, further adjusted for patient characteristics, was used to determine the association of HF with exacerbation risk.
Main results
86 795 patients with COPD were categorised as no evidence of HF (n=60 047), possible HF (n=8476) and newly diagnosed HF (n=2066). Newly diagnosed HF (adjusted HR (aHR): 1.45, 95% CI: 1.30 to 1.62) and possible HF (aHR: 1.65, 95% CI: 1.58 to 1.72) similarly increased exacerbation risk. Incident and prevalent use of all HF medications were associated with increased exacerbation risk. Prevalent use was associated with reduced exacerbation risk compared with incident use.
Conclusions
Earlier opportunities to improve the diagnosis and management of HF in the COPD population are missed. Managing HF may reduce exacerbation risk in the long term.
Keywords: COPD epidemiology, COPD exacerbations, clinical epidemiology
Key messages.
What is the key question?
What is the relationship of heart failure (HF) and HF medication use with risk of moderate-to-severe chronic obstructive pulmonary disease (COPD) exacerbations?
What is the bottom line?
Earlier opportunities to improve the diagnosis and management of heart failure in UK primary care patients with COPD could reduce exacerbation risk.
Why read on?
We find evidence that managing HF may reduce risk of acute exacerbations of COPD in the long term.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterised by persistent, irreversible airflow limitation resulting from exposure to harmful particulates and is often accompanied by progressive inflammatory disease of the airways. Patients with COPD experience periods of acute symptom worsening or exacerbations.1 Heart failure (HF) is a common comorbidity in people with COPD, with an estimated global prevalence of HF comorbidity in patients with COPD at over 14.8 million2; however, HF remains under-recognised in the COPD population.3
The diagnosis of HF in the presence of COPD is difficult due to shared risk factors and symptomatology.4 5 HF diagnosis and management are significantly delayed in patients with COPD compared with patients without COPD.6
There is underuse of cardiovascular medications, particularly beta-blockers (BB), in patients with COPD with cardiovascular indications.7–9 Studies have also investigated the effect of cardiovascular medications on patients with COPD with respect to survival, with mixed results10–13; however, studies examining the effect on risk of COPD exacerbations are rarer. One observational study found that angiotensin receptor blocker (ARB) use was associated with fewer severe exacerbations than ACE inhibitor (ACEi) use.14 A systematic review of BB use on exacerbation risk concluded that most evidence supported no change or reduced exacerbation risk in BB users compared with non-users in patients with COPD with, or at risk of, cardiovascular disease.15 Long-term effects of HF medications on exacerbation risk in patients with COPD with HF have not been assessed.
We investigated the relationship between having HF diagnosed and exacerbation risk and the effect of possible HF diagnosis on exacerbation risk. In addition, we investigated the effect of incident and prevalent use of HF medications on exacerbation risk in patients with COPD with diagnosed HF.
Methods
Data source
Pseudonymised primary care electronic records from the Clinical Practice Research Datalink (CPRD) Global Initiative for Chronic Obstructive Lung Disease (GOLD) database were obtained. CPRD represents 6.9% of the UK population and is representative in terms of sex, age, body mass index (BMI) and ethnicity.16 Linked mortality data from the Office for National Statistics, socioeconomic data from the Index of Multiple Deprivation (IMD) and secondary care data from Hospital Episode Statistics (HES) were provided for this study by CPRD for patients in England.
General inclusion and exclusion criteria
Patients with COPD were identified using a validated algorithm17 between 1 January 2006 and 31 December 2016. Briefly, patients with COPD diagnosed at age ≥35 years with validated primary care codes and a history of smoking were eligible for inclusion.17 18 HF was identified in CPRD using a previously published Read code list (see online supplemental material).19
thoraxjnl-2020-216390supp001.pdf (3.2MB, pdf)
Outcome
Moderate acute exacerbations of COPD (AECOPD) (those managed in primary care) were identified from CPRD using a validated algorithm identifying AECOPD Read codes, lower respiratory tract infection, antibiotic prescription and oral corticosteroid prescription.20 Severe AECOPD (those requiring hospitalisation) were identified from HES using validated International Classification of Diseases, 10th Revision codes.21 The outcome was time-to-first moderate-or-severe AECOPD.
Covariates
Age groups were 35–64 years, 65–74 years, 75–84 years and 85+years. Patients were categorised as current or former smokers. BMI (kg/m2) was categorised as ‘underweight’ (<18.5), ‘healthy weight’ (18.5–24.9), ‘overweight’ (25–29.9) and ‘obese’ (≥30). Socioeconomic status was evaluated using IMD 2010 divided into quintiles. The severity of airflow limitation was assessed using the GOLD guidelines and grouped as GOLD1 (forced expiratory volume in 1 s (FEV1) % predicted >80%, mild), GOLD2 (FEV1 % predicted 50%–79%, moderate), GOLD3 (FEV1 % predicted 30%–49%, severe) and GOLD4 (FEV1 % predicted less than 30%, very severe).1 The number of moderate-to-severe exacerbations in the year prior to the start of follow-up was considered as baseline exacerbation history. History of cardiovascular disease included prior diagnosis of ischaemic heart disease, peripheral artery disease, atrial fibrillation, hypertension and/or stroke. Baseline use of short-acting beta agonists, short-acting muscarinic antagonists, long-acting beta agonists (LABA), long-acting muscarinic antagonist (LAMA), inhaled corticosteroids (ICS) or combination was defined as at least two prescriptions for a given drug class in the year prior to the start of follow-up. Baseline cardiovascular medication use, including ACEi, ARB, BB, calcium channel blockers, loop diuretics, mineralocorticoid receptor antagonists (MRA), statins and vasodilators, was defined as at least two prescriptions for a given drug class in the year prior to the start of follow-up.
HF hospitalisation and investigation
History of HF hospitalisation was identified as those with an HF diagnostic code (I11.0, I13.0, I13.2, I50.0, I50.1 or I50.9) as the primary or secondary condition related to a hospitalisation.22
History of echocardiography was identified in CPRD using the echocardiography Read codes from the Quality and Outcomes Framework.23 Tests for B-type natriuretic peptide (BNP) levels24 were identified using the following Read codes: 44AN.00, 44AR.00, 44AP.00, 44AF.00 or 44AF.00. Cardiology outpatient visits were identified in HES Outpatient data using the main or treatment specialty code ‘320—Cardiology’.
Analyses of the effect of possible and diagnosed HF on AECOPD risk
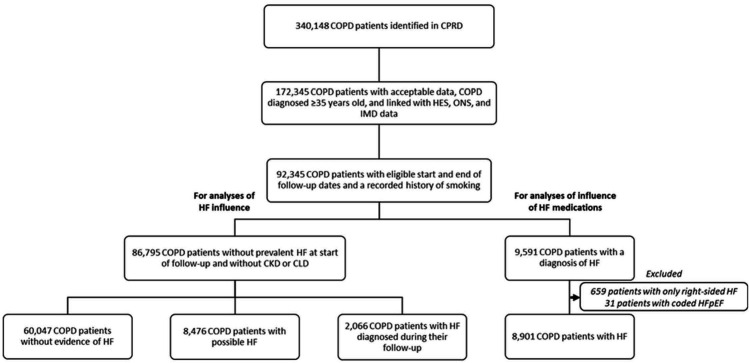
Identification of the patient populations for analyses of the effect of possible and diagnosed HF on exacerbation risk is outlined in figure 1, following the left pathway. To be included, a patient’s COPD diagnosis must have preceded his or her HF diagnosis, and his or her HF diagnosis had to occur in primary care when a patient met the other eligibility criteria for follow-up and between 1 January 2006 and 31 December 2016. Patients with non-cardiac indications for loop diuretic use (chronic kidney disease (CLD) stages 3–5, nephrotic syndrome and chronic liver disease (CLD)) were excluded. ‘Possible HF’ was defined as continuous loop diuretic use, in the absence of other indications and in the absence of an HF diagnosis in primary care.25 Continuous use was defined as three consecutive prescriptions within 100 days.
Figure 1.
Derivation of the study populations. For analyses of the influences of HF and HF medications on exacerbation risk from the CPRD. CKD, chronic kidney disease; CLD, chronic liver disease; COPD, chronic obstructive pulmonary disease; CPRD, Clinical Practice Research Datalink; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HES, Hospital Episode Statistics; IMD, Index of Multiple Deprivation; ONS, Office for National Statistics.
Patients with COPD with possible HF or diagnosed HF were matched 1:2 on sex and age (±1.0 year) to patients with COPD without evidence of HF with replacement. Matching and analyses were performed separately for each exposure group. Start of follow-up was defined as the latest of (1) date data were deemed to be acceptable for research as determined by CPRD (ie, had passed a number of quality checks), (2) date from which the patient has continuous data available, (3) start of the study (1 January 2006) or (4) date of COPD diagnosis, date of HF diagnosis or date of first loop diuretic prescription for possible HF. Patients were censored on (1) transferring from the practice, (2) last date of data collection from the practice, (3) last date of linked data collection, (4) death or (5) end of study (31 December 2016).
Stratified Cox regression was used to assess the hazard for moderate-to-severe exacerbation. Each regression was stratified by matched set, to account for matching on sex and age.26 Robust variance estimates were used to account for clustering on general practice. Regression was adjusted for smoking status, BMI, IMD, exacerbation history, inhaler use, severity of airflow limitation, a history of cardiovascular disease and use of cardiovascular medications. Adjusted HRs (aHRs) were used to assess exacerbation risk comparing patients with COPD with possible or diagnosed HF with patients with COPD without evidence of HF.
Analyses of the effect of HF medications on AECOPD risk
Identification of the patient population for analyses of the effect of HF medications on exacerbation is outlined in figure 1, following the right pathway. To be included, a patient must have diagnoses for COPD and HF in primary care, regardless of which condition came first. Patients with incident and prevalent comorbidity were eligible for inclusion, as were patients with CKD or CLD because loop diuretic use was not used to define HF status in these analyses. Patients with right-sided HF codes only (n=659) and patients with explicitly coded HF with preserved ejection fraction (HFpEF; n=31) were excluded from these analyses as we were interested in left-sided HF specifically, and HFpEF does not have the same treatment recommendations and outcomes as HFrEF.27
Cardiovascular medications of interest were those commonly used in the management of HF,27 including ACEi, ARB, BB, loop diuretics and MRA, and were assessed in separate analyses. A patient was considered to be exposed to a given medication providing they had at least two prescriptions within 90 days. Continuous use was defined as having regular prescriptions for a given medication with gaps between prescriptions being no more than 90 days. Incident use was defined as <6 months of continuous exposure. Prevalent use was defined as ≥6 months of continuous exposure. A period of 6 months was chosen to represent prevalent use to minimise effects of drugs being trialled and then discontinued. Start and end of follow-up were defined as above, except that start of follow-up could begin only on comorbidity diagnosis. Exposed patients were additionally censored 90 days after their last prescription for a given medication as longer gaps between prescriptions make determining continuous exposure less accurate. Exposure was defined using person-time, meaning that patients could have multiple exposure statuses throughout follow-up, whereby patients whose exposure status changed during the study period were censored from their original exposure cohort and would begin contributing person-time to their new exposure cohort. (online supplemental figure E1).
The hazard for moderate-to-severe exacerbation in incident and prevalent users of a given medication was compared with non-users using Cox proportional hazards regression with robust variance estimates, adjusted for smoking status, BMI, IMD, exacerbation history, inhaler use, severity of airflow limitation, a history of cardiovascular disease and use of other cardiovascular medications.
Sensitivity analyses
First, to assess whether removing patients with CKD or CLD noticeably changed the exacerbation risk of our study populations, we assessed exacerbation risk comparing patients with COPD with a diagnosis of HF (combined patients with newly diagnosed HF with and without CKD or CLD) with patients with COPD without a diagnosis of HF (combined patients with no evidence of HF, possible HF and patients with COPD with CKD or CLD without a diagnosis of HF in primary care).
Second, we identified possible patients with HF with evidence of being investigated for HF, defined as a cardiology outpatient visit or echocardiography or BNP test. We compared exacerbation risk in patients with COPD with possible HF who had evidence of being investigated for HF with those without evidence of HF investigations.
Third, we included patients with a history of HF hospitalisation without a primary care diagnosis as having newly diagnosed HF, as opposed to possible HF.
Results
Description of patients with COPD without evidence of HF, with possible HF and with newly diagnosed HF
We identified 86 795 patients with COPD without prevalent HF at the start of follow-up, of whom 60 047 patients had no evidence of HF, 8476 patients had possible HF and 2066 patients had HF diagnosed during follow-up (figure 1). Compared with patients with COPD without evidence of HF, patients with COPD with possible or diagnosed HF were older, former smokers, obese, with severe-to-very-severe airflow limitation (GOLD3–4), greater history of exacerbations, more use of triple (LABA+LAMA+ICS) inhaler therapy and with a greater history of cardiovascular disease (table 1). The proportion of newly diagnosed and possible patients with HF with a history of HF investigations and the median time since the most recent investigation are summarised in online supplemental table E1.
Table 1.
Descriptive statistics for patients with COPD without evidence of HF, possible HF and newly diagnosed HF
| COPD patients without evidence of HF | COPD patients with possible HF | COPD patients with diagnosed HF | COPD patients without evidence of HF | COPD patients with possible HF | COPD patients with diagnosed HF | ||
| Number of patients (N) | 60 047 | 8476 | 2066 | Exacerbation history* | 1.52 (0–15) | 2.17 (0–13) | 2.70 (0–14) |
| Female | 27 155 (45.2) | 4247 (50.1) | 743 (36.0) | COPD medications* | |||
| SABA/SAMA | 37 990 (63.3) | 6222 (73.4) | 1489 (72.1) | ||||
| Age, years (IQR) | 66 (59, 74) | 73 (66, 80) | 74 (67, 81) | LABA alone | 1453 (2.4) | 250 (3.0) | 31 (1.5) |
| LAMA alone | 4744 (7.9) | 537 (6.3) | 163 (7.9) | ||||
| Smoking status | ICS alone | 5969 (9.9) | 947 (11.2) | 92 (4.5) | |||
| Current Smoker | 26 291 (43.8) | 2566 (30.3) | 497 (24.1) | LABA+LAMA | 631 (1.1) | 81 (1.0) | 29 (1.4) |
| Former Smoker | 33 756 (56.2) | 5910 (69.7) | 1569 (75.9) | LABA+ICS | 14 685 (24.5) | 2435 (28.7) | 476 (23.0) |
| LAMA+ICS | 1121 (1.9) | 206 (2.4) | 27 (1.3) | ||||
| Body mass index | Triple | 10 239 (17.1) | 1925 (22.7) | 823 (39.8) | |||
| Underweight (<18.5) | 3295 (5.49) | 356 (4.20) | 113 (5.47) | No long-acting inhaler | 21 205 (35.3) | 2095 (24.7) | 425 (20.6) |
| Healthy weight (18.5–24.9) | 21 463 (35.7) | 2253 (26.6) | 640 (31.0) | ||||
| Overweight (25.0–29.9) | 18 982 (31.6) | 2339 (27.6) | 595 (28.8) | History of cardiovascular disease† | 29 504 (49.1) | 6527 (77.0) | 1687 (81.7) |
| Obese (≥30) | 14 581 (24.3) | 3183 (37.6) | 667 (32.3) | Atrial fibrillation | 2120 (3.5) | 999 (11.8) | 671 (32.5) |
| Missing data | 1726 (2.9) | 345 (4.1) | 51 (2.5) | Hypertension | 24 759 (41.2) | 5474 (64.6) | 1033 (50.0) |
| Ischaemic heart disease | 7343 (12.2) | 2231 (26.3) | 838 (40.6) | ||||
| Index of Multiple Deprivation | Peripheral artery disease | 3023 (5.0) | 654 (7.7) | 222 (10.8) | |||
| 1: Most deprived | 8581 (14.3) | 1144 (13.5) | 268 (13.0) | Stroke | 3195 (5.3) | 788 (9.3) | 231 (11.2) |
| 2 | 11 540 (19.2) | 1718 (20.3) | 403 (19.5) | ||||
| 3 | 11 698 (19.5) | 1613 (19.0) | 406 (19.7) | Diabetes mellitus | 5794 (9.7) | 1358 (16.0) | 459 (22.2) |
| 4 | 13 978 (23.3) | 1972 (23.3) | 473 (22.9) | ||||
| 5: Least deprived | 14 250 (23.7) | 2029 (23.9) | 516 (25.0) | CVD medications* | |||
| ACEi | 9789 (16.3) | 2446 (28.7) | 1230 (59.5) | ||||
| GOLD Stage | ARB | 3538 (5.9) | 900 (10.6) | 332 (16.1) | |||
| 1: Mild | 23 860 (39.7) | 2998 (35.4) | 703 (34.0) | Beta-blockers | 4491 (7.5) | 1007 (11.9) | 737 (35.7) |
| 2: Moderate | 15 336 (25.5) | 1762 (20.8) | 486 (23.5) | Calcium channel blockers | 5054 (8.4) | 1464 (17.3) | 329 (15.9) |
| 3: Severe | 7767 (12.9) | 1449 (17.1) | 426 (20.6) | MRA | 248 (0.4) | 276 (3.3) | 479 (23.2) |
| 4: Very Severe | 1776 (3.0) | 401 (4.7) | 166 (8.0) | Statins | 17 000 (28.3) | 3499 (41.3) | 1152 (55.8) |
| Missing | 11 308 (18.8) | 1866 (22.0) | 285 (13.8) | Vasodilators | 3504 (5.8) | 1257 (14.8) | 320 (15.5) |
Severity of airflow limitation using the GOLD guidelines.1
*At least two prescriptions >15 days apart in the year prior to the start of follow-up.
†Prior diagnosis of ischaemic heart disease, peripheral artery disease, atrial fibrillation, hypertension and/or stroke.
‡Average number of exacerbations per patient (range) in the year prior to the start of follow-up.
ACEi, ACE inhibitor; ARB, angiotensin receptor blockers; BB, beta-blockers; CCB, calcium channel blockers; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HF, heart failure; ICS, inhaled corticosteroids; LABA, long-acting beta agonists; LAMA, long-acting muscarinic antagonists; MRA, mineralocorticoid receptor antagonists; SABA, short-acting beta agonists; SAMA, short-acting muscarinic antagonists.
Relationship between newly diagnosed HF and AECOPD risk
We matched 2066 patients (100%) with newly diagnosed HF to 4132 patients without evidence of HF (online supplemental table E2). Patients with COPD with newly diagnosed HF experienced greater risk of moderate-to-severe exacerbation than patients with COPD without evidence of HF (figure 2A; aHR: 1.45; 95% CI: 1.30 to 1.62).
Figure 2.
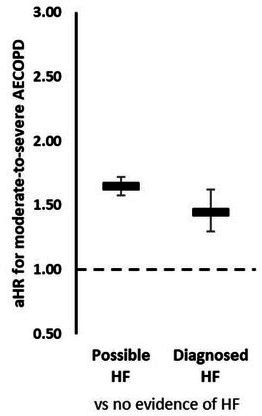
Effect of newly diagnosed and possible HF on AECOPD risk. aHR comparing risk of moderate-to-severe AECOPD in patients with COPD with possible HF and newly diagnosed HF compared with patients with COPD without evidence of HF. Estimates from stratified Cox regression stratified by matched set (sex and age) and adjusted for smoking status, body mass index, index of multiple deprivation, exacerbation history, severity of airflow limitation, inhaler use, a history of cardiovascular disease and cardiovascular medication use. AECOPD, acute exacerbations of chronic obstructive pulmonary disease; aHR, adjusted HR; COPD, chronic obstructive pulmonary disease; HF, heart failure.
Relationship between possible HF and AECOPD risk
We matched 8423 patients (99.4%) with possible HF to 16 792 patients without evidence of HF (online supplemental table E3). Patients with COPD with possible HF experienced greater risk of moderate-to-severe exacerbation than patients with COPD without evidence of HF (figure 2A; aHR: 1.65; 95% CI: 1.58 to 1.72).
Description of patients with COPD with diagnosed HF for analysis of HF medications
For analysis of the effect of HF medications on exacerbation risk, we identified 8901 patients with COPD with diagnosed HF (figure 1). Patient characteristics are summarised in online supplemental table E4). Patient characteristics stratified for non-users, incident users and prevalent users of ACEi, ARB, BB, loop diuretic and MRA are detailed in online supplemental tables E5–E9. Most patients were non-users or prevalent users of HF medications at the start of follow-up and did not change status (online supplemental figure E2).
Relationship between incident and prevalent HF medication use and AECOPD risk
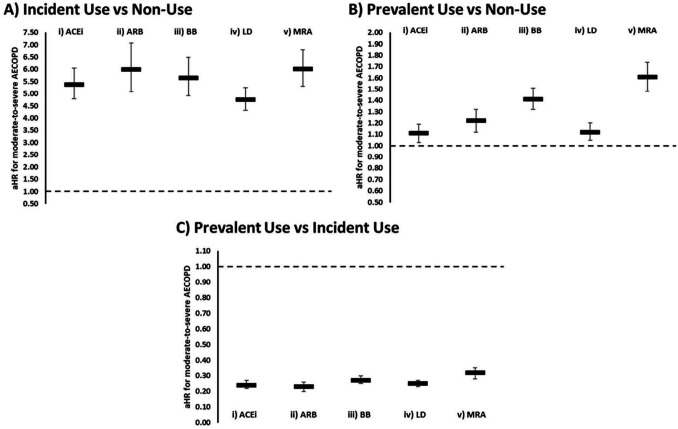
Incident use of ACEi, ARB, BB, loop diuretics and MRA was associated with higher risk of moderate-to-severe exacerbation compared with non-use (figure 3A; online supplemental table E10). Prevalent use of ACEi, ARB, BB, loop diuretics and MRA was also associated with higher risk of moderate-to-severe exacerbation compared with non-use (figure 3B; online supplemental table E11). Prevalent use of ACEi, ARB, BB, loop diuretics and MRA was associated with lower risk of moderate-to-severe exacerbation compared with incident use (figure 3C; online supplemental table E12).
Figure 3.
Effect of HF medication use on AECOPD risk. aHR for the risk of moderate-to-severe AECOPD in patients with diagnosed HF comparing incident medication use (<6 months) with non-use, (B) prevalent medication use (≥6 months) with non-use and (C) prevalent medication use with incident medication use for (I) ACEi, (II) ARB, (III) BB, (IV) LD and (v) MRA. Estimates with 95% CIs from Cox regression adjusted for age, sex, smoking status, body mass index, Index of Multiple Deprivation, exacerbation history, severity of airflow limitation, inhaler use, a history of cardiovascular disease and cardiovascular medication use. ACEi, ACE inhibitor; AECOPD, acute exacerbations of chronic obstructive pulmonary disease; aHR, adjusted HR; ARB, angiotensin receptor blockers; BB, beta-blockers; COPD, chronic obstructive pulmonary disease; HF, heart failure; LD, loop diuretics; MRA, mineralocorticoid receptor antagonists.
Sensitivity analyses
We conducted three sensitivity analyses; results were similar to those in the main analysis (see online supplemental material).
Discussion
Patients with COPD with newly diagnosed HF experienced a significantly higher risk of moderate and severe exacerbations compared with patients with COPD without evidence of HF diagnosis. Patients with COPD with possible HF also experienced a similarly increased risk. Patients with COPD with possible HF who had evidence of investigation but no HF diagnosed still experienced a higher risk of exacerbation than patients with COPD without evidence of HF.
Both incident (<6 months) and prevalent (≥6 months) use of HF medications were associated with a higher risk of exacerbation compared with non-use, for each of the medication investigated; however, exacerbation risk was lower with prevalent use compared with incident use. The similarity in effects suggests that it is not the effect of individual medications on exacerbation risk that is being seen, but rather the effect of symptomatic HF indicative for medication use on exacerbation risk that is being seen—confounding by indication.28 Patients with COPD with diagnosed HF who were prescribed medication may be more likely to be symptomatic than those not prescribed medication. With prevalent use, BB and MRA were associated with slightly greater exacerbation risk compared with non-use than seen in the other drug types. BB and MRA use in patients with COPD may indicate more severe HF, with a greater symptom burden and therefore higher risk of exacerbation compared with non-users of these medications.
Prevalent use (≥6 months) of all HF medications was associated with lower exacerbation risk compared with incident use. The effect was similar for all drug types, suggesting again that it is not the individual medication affecting exacerbation risk but appropriate HF management. Previously, Matamis et al hypothesised that identification and subsequent management of HF in patients admitted to intensive care for exacerbation were responsible for better outcomes following exacerbations, even in the short term.29 Our study also suggests that HF contributes to exacerbation risk and that early diagnosis and management of HF in the COPD population may reduce that risk.
These results suggest there may be opportunity for earlier diagnosis of HF in primary care in the COPD population and that early diagnosis and optimal management of HF may reduce COPD exacerbation risk in these patients. There are a number of possible reasons by which patients with COPD with newly diagnosed and possible HF experience greater exacerbation risk than patients with COPD without HF. Failure to optimally manage HF may hasten the progression of HF or general patient decline, both of which may increase exacerbation risk. Patients with COPD with HF are more likely to have additional comorbidities than patients with only COPD.30 31 Higher comorbidity burden is associated with increased risk of death in patients with COPD32 and increased systemic inflammation.31 33 Previous cohort studies have found that up to 26% of exacerbations may be triggered by HF or other cardiovascular conditions, such as arrhythmia.34–36 Underlying cardiac problems have been seen in around a quarter of exacerbation episodes when echocardiography has been performed.37 38 In addition, there is the potential for misclassification whereby events are labelled as COPD exacerbation but may be acute HF.39 40 Pathophysiologically, there are a number of mechanisms by which underlying HF, particularly uncontrolled HF, may increase exacerbation risk. Chronic congestion may lead to reduced airflow in some patients, and airflow obstruction can be reversed with proper management of congestion.41 42 Pulmonary oedema, and impaired oxygen transport due to HF, may intensify dyspnoea and reduced exercise capacity already present due to lung hyperinflation in COPD.43 Cardiomegaly may contribute to worsening alveolar gas diffusion, resulting in a restrictive lung pattern and reduced alveolar volume.44 Obesity and diabetes, risk factors for HF and more common in our patients with COPD with HF, are associated with reduced pulmonary function and airway hyperactivity.45
These results suggest that HF may be an underlying factor triggering exacerbation of COPD. There are a number of challenges facing clinicians in the management of patients with COPD and HF. COPD exacerbation is a diagnosis of exclusion, with no biomarkers for exacerbation; the overlapping symptoms with HF make misdiagnosis possible.40 Similarly, the signs and symptoms of HF are non-specific and many patients with HF are initially treated for exacerbation of other conditions.46 COPD and HF share common risk factors (eg, smoking) and symptoms (eg, dyspnoea, fatigue, exercise intolerance) that can make diagnosing one in the presence of the other difficult.4 5 Air trapping due to pulmonary disease can affect echocardiogram acoustic windows, leading to unsatisfactory imaging quality and making diagnosis more difficult.47 The severity of COPD may be overestimated in patients with comorbid HF as part of the reduction in lung function may be caused by HF.48 In patients with COPD, the median time between symptom presentation and HF diagnosis in UK primary care has been shown to be over 3 years, compared with only 2.4 years in patients without COPD.6 HF treatment is also delayed, with patients with COPD waiting 2.9 years compared with only 1.9 years in patients without COPD.6 There are currently no bespoke guidelines available for the management of patients with COPD and HF, despite the ongoing debate around the use of beta-agonists49 50 and the lingering hesitancy around BB use, which appears to be largely unfounded.51 52
There is increasing recognition of the multimorbid patient,53 54 but the translation of this into clinical practice has been slow. These results highlight the need for more collaborative management of patients with chronic heart and lung conditions, increased awareness and screening for conditions beyond the first one to be recognised, and the development of bespoke guidelines for diagnosis and management of patients with coexisting COPD and HF. Increasing patient-centred care over specialty-focused care could reduce symptom burden in patients with COPD and HF.
Strengths and limitations
A strength of this study is the use of one of the largest longitudinal, nationally representative databases in the world, CPRD.16 A validated definition for COPD was used,17 and exacerbations were identified using validated algorithms.20 21 There is no validated case definition for the identification of HF in CPRD. HF was identified using a previously published code list19 that was created by clinicians, and unfortunately, explicit coding of HF type and severity is not common in CPRD. Duration of prescription is not always recorded in CPRD, and the issuing of a prescription does not mean the patient used the prescription, which limits the accuracy of determining continuous medication exposure; however, stratification of medication use into incident and prevalent use allowed for a more nuanced evaluation of the effect of HF medication use on exacerbation risk.
Possible patients with HF had characteristics similar to newly diagnosed patients with HF and a similarly increased risk of exacerbation, suggesting internal validity. Similar techniques have been used in previous research to identify possible HF.25 However, at best, the definition requiring long-term loop diuretic use for a probable cardiac indication would only identify possible patients with HF with fluid congestion, which is not experienced in all cases. The exclusion of patients with CKD or CLD was designed to restrict cases to those patients taking loop diuretics for a cardiac indication; however, these conditions were more common in patients with diagnosed HF than in those without an HF diagnosis. We conducted a sensitivity analysis by looking at a less selected population that included patients with CKD or CLD. Comparing patients with COPD with an HF diagnosis with those without an HF diagnosis demonstrated similarly increased risk of exacerbation as did our more selected comparison of patients with COPD with newly diagnosed HF and patients with COPD without evidence of HF that excluded patients with CKD or CLD. Nevertheless, the patients with COPD in our population with long-term loop diuretic use in the absence of non-cardiac indications experienced greater risk of exacerbation than patients without this characteristic, suggesting that, whether or not they have HF or develop HF in the future, they are a high-risk patient group that should be recognised and for whom special consideration regarding treatment and management should be taken. Finally, we did consider time-varying covariates in the model; however, from previous COPD studies, and given the relatively short follow-up period and similarity in characteristics between exposure groups, we do not think this would have significantly changed our findings.
Conclusions
Newly diagnosed HF and possible HF were associated with similar, higher exacerbation risk compared with patients without evidence of HF. All HF medications examined were associated with higher exacerbation risk with both incident and prevalent use compared with non-use. Importantly, prevalent use of all HF medications examined was associated with lower exacerbation risk compared with incident use. There appears to be opportunity for earlier recognition and management of HF in the COPD population in primary care, which may reduce COPD exacerbation risk.
Acknowledgments
The authors would like to thank Dr Rosita Zakeri (King’s College London) for crafting this specific definition of possible HF and Dr Chloe Bloom (Imperial College London) for commenting on an early draft of the manuscript.
Footnotes
Contributors: ELA conducted the analyses and drafted the manuscript. AB, MRC and JKQ contributed to the design of the study and revision of the manuscript. ELA and JKQ had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This research was supported by the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre (BRC).
Disclaimer: The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Competing interests: ELA has nothing to disclose. AB reports grants from Dr Foster, during the conduct of the study, and grants from Medtronic, outside the submitted work. MRC reports receiving research funding and speaker fees from ResMed, Boston Scientific, Medtronic and Abbott, and consultancy and speaker fees from Servier, Novartis, Vifor, LivaNova, Pfizer, Roche Diagnostics and Amgen, outside the submitted work. JKQ reports grants from MRC, grants from BLF, grants from The Health Foundation, grants and personal fees from AZ, grants and personal fees from BI, grants from Chiesi, grants and personal fees from Bayer, and grants and personal fees from GSK, outside the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data may be obtained from a third party and are not publicly available. Linked pseudonymised mortality data from the Office for National Statistics (ONS), socioeconomic data from the Index of Multiple Deprivation (IMD) and secondary care data from Hospital Episode Statistics (HES) were provided for this study by CPRD for patients in England. Data are linked by NHS Digital, the statutory trusted third party for linking data, using identifiable data held only by NHS Digital. Select general practices consent to this process at a practice level, with individual patients having the right to opt-out. Use of HES and ONS data is Copyright © (2018), reused with the permission of The Health & Social Care Information Centre, all rights reserved. Data are available on request from the CPRD. Their provision requires the purchase of a license, and this license does not permit the authors to make them publicly available to all. This work used data from the version collected in January 2018 and have clearly specified the data selected in each Methods section. To allow identical data to be obtained by others, via the purchase of a license, the code lists will be provided upon request. Licenses are available from the CPRD (http://www.cprd.com): The Clinical Practice Research Datalink Group, The Medicines and Healthcare products Regulatory Agency, 10 South Colonnade, Canary Wharf, London E14 4PU.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This work is based in part on data from the Clinical Practice Research Datalink (CPRD) obtained under license from the UK Medicines and Healthcare products Regulatory Agency (MHRA). The data are provided by patients and collected by the National Health Service (NHS) as part of their care and support. The interpretation and conclusions contained in this study are those of the authors alone. A protocol for this research were approved by the Independent Scientific Advisory Committee (ISAC) for MHRA Database Research (protocol number 18_074RARA2), and the approved protocol was made available to the journal in which this research is published and to the reviewers during peer review. Generic ethical approval for observational research using the CPRD with approval from ISAC has been granted by a Health Research Authority (HRA) Research Ethics Committee (East Midlands—Derby, REC reference number 05/MRE04/87).
References
- 1. GOLD . Global strategy for the diagnosis, management and prevention of COPD, global initiative for chronic obstructive lung disease (GOLD) 2017 [Webpage], 2017. Available: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/ [Accessed 10 Jan 2018].
- 2. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018;392:1789–858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rutten FH, Cramer M-JM, Lammers J-WJ, et al. Heart failure and chronic obstructive pulmonary disease: an ignored combination? Eur J Heart Fail 2006;8:706–11. 10.1016/j.ejheart.2006.01.010 [DOI] [PubMed] [Google Scholar]
- 4. Hawkins NM, Petrie MC, Jhund PS, et al. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail 2009;11:130–9. 10.1093/eurjhf/hfn013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rutten FH. Diagnosis and mangement of heart failure in COPD. In: Rabe KF, Wedzicha JA, Wouters EF, eds. Eur respir monogr, 2013: 50–63. [Google Scholar]
- 6. Hayhoe B, Kim D, Aylin PP, et al. Adherence to guidelines in management of symptoms suggestive of heart failure in primary care. Heart 2019;105:678–85. 10.1136/heartjnl-2018-313971 [DOI] [PubMed] [Google Scholar]
- 7. Hawkins NM, Jhund PS, Simpson CR, et al. Primary care burden and treatment of patients with heart failure and chronic obstructive pulmonary disease in Scotland. Eur J Heart Fail 2010;12:17–24. 10.1093/eurjhf/hfp160 [DOI] [PubMed] [Google Scholar]
- 8. Rasmussen D, Bodtger U, Lamberts M. Beta-blocker, aspirin and statin usage after myocardial infarction in patients with and without COPD. A nationwide analysis from 1995 to 2015 in Denmark. Eur Respir J 2018;52:1933. [DOI] [PubMed] [Google Scholar]
- 9. Pirina P, Martinetti M, Spada C, et al. Prevalence and management of COPD and heart failure comorbidity in the general practitioner setting. Respir Med 2017;131:1–5. 10.1016/j.rmed.2017.07.059 [DOI] [PubMed] [Google Scholar]
- 10. Ellingsen J, Johansson G, Larsson K, et al. Impact of Comorbidities and Commonly Used Drugs on Mortality in COPD - Real-World Data from a Primary Care Setting. Int J Chron Obstruct Pulmon Dis 2020;15:235–45. 10.2147/COPD.S231296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Su VY-F, Yang Y-H, Perng D-W, et al. Correction for: real-world effectiveness of medications on survival in patients with COPD-heart failure overlap. Aging 2019;11:8728–9. 10.18632/aging.102363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liao K-M, Lin T-Y, Huang Y-B, et al. The evaluation of β-adrenoceptor blocking agents in patients with COPD and congestive heart failure: a nationwide study. Int J Chron Obstruct Pulmon Dis 2017;12:2573–81. 10.2147/COPD.S141694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ekström MP, Hermansson AB, Ström KE. Effects of cardiovascular drugs on mortality in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;187:715–20. 10.1164/rccm.201208-1565OC [DOI] [PubMed] [Google Scholar]
- 14. Lai C-C, Wang Y-H, Wang C-Y, et al. Comparative effects of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on the risk of pneumonia and severe exacerbations in patients with COPD. Int J Chron Obstruct Pulmon Dis 2018;13:867–74. 10.2147/COPD.S158634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mersfelder TL, Shiltz DL. β-blockers and the rate of chronic obstructive pulmonary disease exacerbations. Ann Pharmacother 2019;53:1249–58. 10.1177/1060028019862322 [DOI] [PubMed] [Google Scholar]
- 16. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research Datalink (CPRD). Int J Epidemiol 2015;44:827–36. 10.1093/ije/dyv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quint JK, Müllerova H, DiSantostefano RL, et al. Validation of chronic obstructive pulmonary disease recording in the clinical practice research Datalink (CPRD-GOLD). BMJ Open 2014;4:e005540. 10.1136/bmjopen-2014-005540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Institute for Health and Care Excellence (NICE) . Chronic obstructive pulmonary disease in over 16S: diagnosis and management, 2018. [PubMed] [Google Scholar]
- 19. Axson EL, Sundaram V, Bloom CI, et al. Temporal trends in the incidence of heart failure among patients with chronic obstructive pulmonary disease and its association with mortality. Ann Am Thorac Soc 2020;17:939–48. 10.1513/AnnalsATS.201911-820OC [DOI] [PubMed] [Google Scholar]
- 20. Rothnie KJ, Müllerová H, Hurst JR, et al. Validation of the recording of acute exacerbations of COPD in UK primary care electronic healthcare records. PLoS One 2016;11:e0151357. 10.1371/journal.pone.0151357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rothnie KJ, Müllerová H, Thomas SL, et al. Recording of hospitalizations for acute exacerbations of COPD in UK electronic health care records. Clin Epidemiol 2016;8:771–82. 10.2147/CLEP.S117867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bottle A, Kim D, Aylin P, et al. Routes to diagnosis of heart failure: observational study using linked data in England. Heart 2018;104:600–5. 10.1136/heartjnl-2017-312183 [DOI] [PubMed] [Google Scholar]
- 23. Business . Rules for quality and outcomes framework (QOF) 2017/18. 36.0 edn. (SDS) NDPCDSDS, 2018. [Google Scholar]
- 24. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of cardiology (ESC). developed with the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 25. de Giuli F, Khaw K-T, Cowie MR, et al. Incidence and outcome of persons with a clinical diagnosis of heart failure in a general practice population of 696,884 in the United Kingdom. Eur J Heart Fail 2005;7:295–302. 10.1016/j.ejheart.2004.10.017 [DOI] [PubMed] [Google Scholar]
- 26. Matthews A, Langan SM, Douglas IJ, et al. Phosphodiesterase type 5 inhibitors and risk of malignant melanoma: matched cohort study using primary care data from the UK clinical practice research Datalink. PLoS Med 2016;13:e1002037. 10.1371/journal.pmed.1002037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Institute for Health and Care Excellence (NICE) . Chronic heart failure in adults: diagnosis and management, 2018. [PubMed] [Google Scholar]
- 28. Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA 2016;316:1818–9. 10.1001/jama.2016.16435 [DOI] [PubMed] [Google Scholar]
- 29. Matamis D, Tsagourias M, Papathanasiou A, et al. Targeting occult heart failure in intensive care unit patients with acute chronic obstructive pulmonary disease exacerbation: effect on outcome and quality of life. J Crit Care 2014;29:315.e7–315.e14. 10.1016/j.jcrc.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 30. Kaszuba E, Odeberg H, Råstam L, et al. Heart failure and levels of other comorbidities in patients with chronic obstructive pulmonary disease in a Swedish population: a register-based study. BMC Res Notes 2016;9:215. 10.1186/s13104-016-2008-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fabbri LM, Luppi F, Beghé B, et al. Complex chronic comorbidities of COPD. Eur Respir J 2008;31:204–12. 10.1183/09031936.00114307 [DOI] [PubMed] [Google Scholar]
- 32. Kaszuba E, Odeberg H, Råstam L, et al. Impact of heart failure and other comorbidities on mortality in patients with chronic obstructive pulmonary disease: a register-based, prospective cohort study. BMC Fam Pract 2018;19:178. 10.1186/s12875-018-0865-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morgan AD, Zakeri R, Quint JK. Defining the relationship between COPD and CVD: what are the implications for clinical practice? Ther Adv Respir Dis 2018;12:1753465817750524. 10.1177/1753465817750524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Connors AF, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The support Investigators (study to understand prognoses and preferences for outcomes and risks of treatments). Am J Respir Crit Care Med 1996;154:959–67. 10.1164/ajrccm.154.4.8887592 [DOI] [PubMed] [Google Scholar]
- 35. Fuso L, Incalzi RA, Pistelli R, et al. Predicting mortality of patients hospitalized for acutely exacerbated chronic obstructive pulmonary disease. Am J Med 1995;98:272–7. 10.1016/S0002-9343(99)80374-X [DOI] [PubMed] [Google Scholar]
- 36. Wang CS, FitzGerald JM, Schulzer M, et al. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA 2005;294:1944–56. 10.1001/jama.294.15.1944 [DOI] [PubMed] [Google Scholar]
- 37. Freixa X, Portillo K, Paré C, et al. Echocardiographic abnormalities in patients with COPD at their first hospital admission. Eur Respir J 2013;41:784–91. 10.1183/09031936.00222511 [DOI] [PubMed] [Google Scholar]
- 38. Houben-Wilke S, Spruit MA, Uszko-Lencer NHMK, et al. Echocardiographic abnormalities and their impact on health status in patients with COPD referred for pulmonary rehabilitation. Respirology 2017;22:928–34. 10.1111/resp.12968 [DOI] [PubMed] [Google Scholar]
- 39. MacDonald MI, Shafuddin E, King PT, et al. Cardiac dysfunction during exacerbations of chronic obstructive pulmonary disease. Lancet Respir Med 2016;4:138–48. 10.1016/S2213-2600(15)00509-3 [DOI] [PubMed] [Google Scholar]
- 40. Sapey E, Bafadhel M, Bolton CE, et al. Building toolkits for COPD exacerbations: lessons from the past and present. Thorax 2019;74:898–905. 10.1136/thoraxjnl-2018-213035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brunnée T, Graf K, Kastens B, et al. Bronchial hyperreactivity in patients with moderate pulmonary circulation overload. Chest 1993;103:1477–81. 10.1378/chest.103.5.1477 [DOI] [PubMed] [Google Scholar]
- 42. Dalsgaard M, Plesner LL, Schou M, et al. Prevalence of airflow obstruction in patients with stable systolic heart failure. BMC Pulm Med 2017;17:6. 10.1186/s12890-016-0351-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rabe KF, Hurst JR, Suissa S. Cardiovascular disease and COPD: dangerous liaisons? Eur Respir Rev 2018;27. 10.1183/16000617.0057-2018. [Epub ahead of print: 30 Sep 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Agostoni P, Bussotti M, Cattadori G, et al. Gas diffusion and alveolar-capillary unit in chronic heart failure. Eur Heart J 2006;27:2538–43. 10.1093/eurheartj/ehl302 [DOI] [PubMed] [Google Scholar]
- 45. Decramer M, Janssens W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med 2013;1:73–83. 10.1016/S2213-2600(12)70060-7 [DOI] [PubMed] [Google Scholar]
- 46. Metra M, Teerlink JR. Heart failure. Lancet 2017;390:1981–95. 10.1016/S0140-6736(17)31071-1 [DOI] [PubMed] [Google Scholar]
- 47. Canepa M, Franssen FME, Olschewski H, et al. Diagnostic and therapeutic gaps in patients with heart failure and chronic obstructive pulmonary disease. JACC Heart Fail 2019;7:823–33. 10.1016/j.jchf.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 48. Güder G, Rutten FH, Brenner S, et al. The impact of heart failure on the classification of COPD severity. J Card Fail 2012;18:637–44. 10.1016/j.cardfail.2012.05.008 [DOI] [PubMed] [Google Scholar]
- 49. Canepa M, Straburzynska-Migaj E, Drozdz J, et al. Characteristics, treatments and 1-year prognosis of hospitalized and ambulatory heart failure patients with chronic obstructive pulmonary disease in the European Society of cardiology heart failure long-term registry. Eur J Heart Fail 2018;20:100–10. 10.1002/ejhf.964 [DOI] [PubMed] [Google Scholar]
- 50. Hawkins NM, Virani S, Ceconi C. Heart failure and chronic obstructive pulmonary disease: the challenges facing physicians and health services. Eur Heart J 2013;34:2795–807. 10.1093/eurheartj/eht192 [DOI] [PubMed] [Google Scholar]
- 51. Du Q, Sun Y, Ding N, et al. Beta-blockers reduced the risk of mortality and exacerbation in patients with COPD: a meta-analysis of observational studies. PLoS One 2014;9:e113048. 10.1371/journal.pone.0113048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Salpeter S, Ormiston T, Salpeter E. Cardioselective beta-blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005:CD003566. 10.1002/14651858.CD003566.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. U.S. Department of Health and Human Services . Multiple chronic conditions: a strategic framework: optimum health and quality of life for individuals with multiple chronic conditions. Washington, DC: U.S. Department of Health and Human Services, 2010. [Google Scholar]
- 54. National Institute for Health and Care Excellence (NICE) . Multimorbidity: clinical assessment and management. National Institute for Health and Care Excellence, 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2020-216390supp001.pdf (3.2MB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. Linked pseudonymised mortality data from the Office for National Statistics (ONS), socioeconomic data from the Index of Multiple Deprivation (IMD) and secondary care data from Hospital Episode Statistics (HES) were provided for this study by CPRD for patients in England. Data are linked by NHS Digital, the statutory trusted third party for linking data, using identifiable data held only by NHS Digital. Select general practices consent to this process at a practice level, with individual patients having the right to opt-out. Use of HES and ONS data is Copyright © (2018), reused with the permission of The Health & Social Care Information Centre, all rights reserved. Data are available on request from the CPRD. Their provision requires the purchase of a license, and this license does not permit the authors to make them publicly available to all. This work used data from the version collected in January 2018 and have clearly specified the data selected in each Methods section. To allow identical data to be obtained by others, via the purchase of a license, the code lists will be provided upon request. Licenses are available from the CPRD (http://www.cprd.com): The Clinical Practice Research Datalink Group, The Medicines and Healthcare products Regulatory Agency, 10 South Colonnade, Canary Wharf, London E14 4PU.