Abstract
Background
Iatrogenic pneumothorax is a common and clinically important transbronchial cryobiopsy (TBCB) complication. A study was conducted to assess the diagnostic accuracy and clinical impact of immediate post-procedure lung ultrasound for diagnosing iatrogenic pneumothorax in patients suspected of interstitial lung disease (ILD) undergoing TBCB.
Study design and methods
In patients undergoing TBCB due to suspected ILD, lung ultrasound of the anterior surface of the chest was performed immediately after the TBCB procedure prior to extubation. Presence of lung point was used as a definite sign of pneumothorax. Chest radiography was routinely performed 2 h after TBCB and was used as the reference standard.
Results
A total of 141 consecutive patients were included. Post-procedure lung ultrasound identified definite pneumothorax in five patients (3.6%, 95% confidence interval (CI) 1.5–8.3%). Chest radiography at 2 h identified 19 patients (13.5%, 95% CI 8.7–20.2%) with pneumothorax following TBCB. The diagnostic accuracy of lung ultrasound for diagnosing pneumothorax was as follows: sensitivity: 21.1% (95% CI 6.1–45.6%), specificity: 99.2% (95% CI 95.5–100.0%), positive predictive value (PPV): 80.0% (95% CI 28.4–99.5%) and negative predictive value (NPV): 89.0% (95% CI 82.5–93.7%). Post-procedure lung ultrasound had a clinical impact in five patients (3.6%, 95% CI 1.5–8.3), of which four had a pleural drain inserted prior to extubation and one underwent prolonged observation prior to extubation.
Interpretation
Lung ultrasound performed immediately following TBCB has a clinical impact by identifying patients with pneumothorax in need of immediate treatment prior to extubation and by monitoring pneumothorax size in the operating room. Supplementary imaging prior to patient discharge is still needed however, as the majority of pneumothoraxes develop later in the post-procedure period.
Short abstract
Lung ultrasound immediately following transbronchial lung cryobiopsy can identify early pneumothorax development. Supplementary imaging is, however, still needed since most pneumothoraxes develop later in the post-procedure period. https://bit.ly/3ubcDLh
Introduction
The novel use of lung transbronchial cryobiopsy (TBCB) as an invasive diagnostic tool in patients suspected of interstitial lung disease (ILD) has been increasingly studied over recent years [1–3]. However, concerns have been raised regarding safety issues due to procedure-related complications, the major risks being bleeding and pneumothorax [1, 2].
Despite several studies assessing the diagnostic role of TBCB, studies assessing optimal post-procedure diagnosis and management of pneumothorax are limited [4, 5]. A TBCB expert statement recommends post-procedure chest radiography or lung ultrasound examination, either immediately if signs or symptoms of pneumothorax are present or 2 h after the procedure if the patient is asymptomatic [1].
Several studies have found the diagnostic accuracy of lung ultrasound for pneumothorax comparable or superior to conventional chest radiography [6–10]. Two studies have specifically assessed the diagnostic accuracy of lung ultrasound for diagnosing pneumothorax following TBCB, but the studies used different time points at which lung ultrasound was performed and the prevalence of pneumothorax varied significantly [4, 5]. In other lung biopsy procedures, the time from procedure to pneumothorax development varies and should be considered when deciding the time point for control imaging [11]. Another important factor concerning the timing of control imaging is that pleural drain insertion is experienced as a very painful procedure by many patients [12]. From a patient perspective, early diagnosis of pneumothorax in the operating room while the patient is still sedated is preferable. Additional advantages of early diagnosis include the physician performing TBCB being able to readily initiate treatment in an optimal setting and help to identify patients with small pneumothoraces needing extended observation in the operating room. A prospective study in patients suspected of ILD undergoing TBCB was conducted to assess the following research question: what is the diagnostic accuracy and clinical impact of immediate post-procedure lung ultrasound for diagnosing iatrogenic pneumothorax in ILD patients undergoing TBCB?
Methods
The study was conducted as a prospective, diagnostic accuracy study at the South Danish Center for Interstitial Lung Diseases (SCILS), Odense University Hospital, Odense, Denmark. The hospital is a tertiary hospital serving the Region of Southern Denmark (approx. 1.2 million inhabitants). A more detailed description of the organisation and technical aspects of the TBCB setup, diagnostic yield, overall complications and learning curves at SCILS has previously been published [13].
Patients
Patients were selected for TBCB when the following criteria were fulfilled: 1. unclassified ILD; 2. high-resolution computed tomography (HRCT) performed within 3 months; 3. forced vital capacity (FVC) ≥50% pred; 5. diffusing capacity of the lung for carbon monoxide (DLCO) ≥40%; 6. normal transthoracic echocardiography with a tricuspid valve gradient ≤40 mmHg; 7. body mass index (BMI) <35 kg·m−2. The exclusion criteria were: 1. patient age <18 years; 2. informed consent not obtained; 3. permanent mental disability; 4. TBCB procedure not performed in the operating room.
TBCB procedure
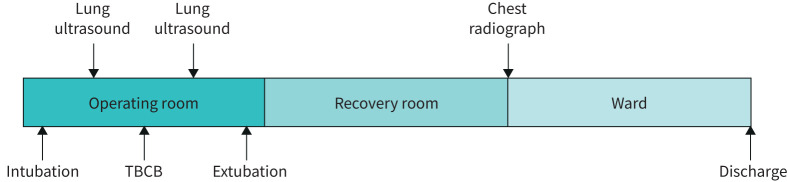
TBCB was performed in an operating room, with the patients under general anaesthesia and mechanically ventilated using an endobronchial tube. A flexible bronchoscope and flexible cryoprobe (Erbe, Thübingen, Germany) were used. Bronchoalveolar lavage (BAL) was initially performed, followed by four TBCBs from the same lung. Following TBCB, the patient was transferred to a recovery room. If the patient was clinically stable after 2 h, chest radiography was performed. The patient was discharged the same day if clinically stable and if the chest radiograph showed no signs of pneumothorax (figure 1).
FIGURE 1.
Timing of the transbronchial cryobiopsy (TBCB) procedure and imaging
Lung ultrasound procedure
Lung ultrasound was performed immediately following intubation, prior to TBCB and post-procedure following the last cryobiopsy and removal of the Fogarty balloon (figure 1). The lung ultrasound technique used was based on protocols validated for diagnosing pneumothorax in trauma patients and by post-operative assessment [14, 15]. The transducer was placed anteriorly and transversally above the intercostal spaces, at the highest point of each hemithorax and just lateral of the sternum. If lung sliding, lung pulse and B-lines were absent, the transducer was rotated counter-clockwise along the intercostal space. The transducer was then gradually moved laterally along the intercostal space to determine whether a lung point could be identified or not. Lung ultrasound was performed using a uSmart3200T ultrasound machine (Terason, Burlington, MA, USA) with a linear transducer (15–4 MHz). 2D-mode and the standard lung pre-set were used for the examinations. The respiratory physicians performing TBCB also performed the lung ultrasound and all had a competency level corresponding to a European Federation of Societies for Ultrasound in Medicine and Biology level III qualification [16].
The diagnostic criteria for pneumothorax were divided into two categories [17–22]: 1. definite pneumothorax (change from the presence of lung sliding prior to TBCB to the absence of lung sliding, lung pulse and B-lines, but with the presence of a lung point following TBCB); 2. possible pneumothorax (change from the presence of lung sliding prior to TBCB to the absence of lung sliding, lung pulse and B-lines, but with the absence of a lung point following TBCB; or subcutaneous emphysema). If a definite pneumothorax was present the size was estimated using the following criteria [23]: 1. small pneumothorax (lung point anterior to the midaxillary line); 2. large pneumothorax (lung point posterior to the midaxillary line). Immediate treatment with placement of a pleural drain was performed in case of: 1. a large pneumothorax; 2. a small pneumothorax with clinical deterioration (when other obvious causes of clinical deterioration have been excluded).
If a pneumothorax was diagnosed but immediate drain treatment was not indicated, the patient was kept under general anaesthesia and assessment of pneumothorax size was repeated every fifth minute or in case of clinical deterioration. If the patient was determined to be stable and two repeated lung ultrasound assessments did not show any signs of progression in pneumothorax size, the general anaesthesia would be stopped and the patient prepared for extubation.
Reference standard and blinding
The chest radiograph 2 h after the TBCB procedure was used as a reference standard. Pneumothorax was considered present if diagnosed by the radiologist assessing the images. To establish the prevalence of pneumothorax following discharge, electronic patient charts were reviewed to determine whether any of the patients had been readmitted with pneumothorax within the first week following TBCB.
Lung ultrasound results were recorded prior to any reference standard tests. The physicians performing the scans were thus blinded to the reference standard results. The radiologist assessing the chest radiograph did not have access to the lung ultrasound results.
Clinical impact
Clinical impact of lung ultrasound was defined as: 1. insertion of a pleural drain immediately following TBCB; 2. prolonged observation in the operating room following TBCB.
Statistics
Descriptive statistics of baseline characteristics, lung ultrasound findings and clinical impact were performed using numbers, percentages, means, medians and interquartile ranges (IQRs). IQRs were expressed as the 25th and 75th percentiles. The “rule of three” was used for calculating 95% confidence intervals (CIs) of events not observed. Diagnostic accuracy calculations used post-TBCB lung ultrasound as an index test when compared to the reference standard. The calculations included sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), positive likelihood ratio (LR+), negative likelihood ratio (LR−) and corresponding 95% CI. Data analysis was conducted using Stata version 15 (StataCorp LLC, College Station, TX, USA).
Ethics and approvals
The Regional Ethics Board waived approval of the project. All patients provided written informed consent for study participation. The project was approved by the local branch of the Data Protection Agency (18/613). This study was conducted in accordance with the amended Declaration of Helsinki. Data are reported according to STARD guidelines [24].
Results
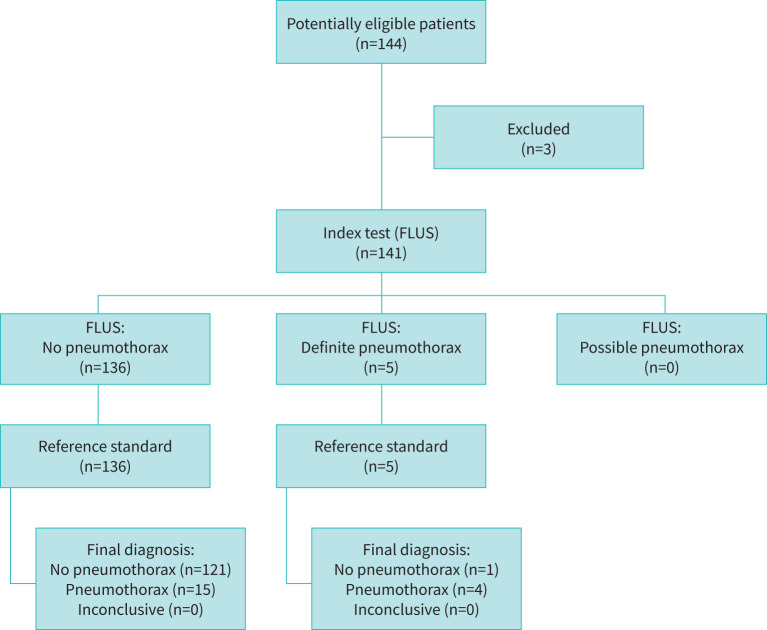
In a 3-year period from February 2017 to March 2020, a total of 144 consecutive patients booked in for TBCB due to suspected ILD were screened for eligibility. One declined informed consent and two patients were excluded since another endobronchial intervention than TBCB was performed in the operating room. This left 141 patients for inclusion. The study flow diagram can be seen in figure 2. Patient baseline characteristics are given in table 1.
FIGURE 2.
Study flow diagram. FLUS: focused lung ultrasound
TABLE 1.
Baseline characteristics of the patients (n=141)
| Characteristic | Result |
| Age years | |
| Median | 69 (60–74) |
| Range | 26–85 |
| Sex | |
| Female | 55 (39.0) |
| Male | 86 (61.0) |
| Smoking status | |
| Never smoker | 55 (39.0) |
| Current smoker | 23 (16.3) |
| Previous smoker | 63 (44.7) |
| Pulmonary function tests | |
| FEV1 L | 2.5 (2.0–2.9) |
| FEV1 % pred | 89.0 (76.0–98.0) |
| FVC L | 3.1 (2.6–3.7) |
| FVC % pred | 87.1 (77.8–102.0) |
| TLC L | 4.8 (4.1–5.6) |
| TLC % pred | 77.0 (68.2–86.0) |
| DLCO % pred | 57.0 (40.0–64.0) |
| 6MWD m | 435 (99–520) |
| Primary presumptive diagnosis prior to TBCB | |
| UIP/IPF | 49 (34.5) |
| NSIP | 23 (16.2) |
| HP | 17 (12.0) |
| CTD-ILD | 16 (11.3) |
| Unclassified ILD | 14 (9.9) |
| Unclassifiable ILD | 7 (4.9) |
| Other | 15 (10.6) |
| TBCB biopsy site | |
| Right upper lobe | 0 (0) |
| Right middle lobe | 2 (1.4) |
| Right lower lobe | 124 (87.9) |
| Left upper lobe | 0 (0) |
| Left lower lobe | 15 (10.6) |
Date are presented as n–n, n (%) or median (IQR) unless otherwise stated. FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; TLC: total lung capacity; DLCO: diffusing capacity of the lung for carbon monoxide; 6MWD: 6-min walk distance; TBCB: transbronchial cryobiopsy; UIP: usual interstitial pneumonia; IPF: idiopathic pulmonary fibrosis; NSIP: nonspecific interstitial pneumonia; HP: hypersensitivity pneumonitis; CTD-ILD: connective tissue disease-associated ILD.
Lung ultrasound was feasible in all patients prior to and following TBCB. Prior to TBCB, lung sliding was present bilaterally in all patients, but only 14 patients (9.9%) had a normal appearance of the visceral pleura and no B-lines. The lung ultrasound findings prior to TBCB are summarised in table 2.
TABLE 2.
Lung ultrasound findings prior to transbronchial cryobiopsy (TBCB)
| Lung ultrasound findings | n (%) | 95% CI |
| Normal findings | ||
| Normal visceral pleura, no B-lines, lung sliding present | 14 (9.9) | 5.9–16.1 |
| Pleural line movement | ||
| Bilateral lung sliding | 141 (100) | 97.9–100 |
| B-lines | ||
| Few B-lines in intercostal space (3>B-lines>0) | 8 (5.7) | 2.9–11.1 |
| Multiple B-lines in intercostal space (≥3 B-lines) | 113 (80.1) | 72.7–86.0 |
| Abnormal visceral pleura | ||
| Unilaterally fragmented and thickened visceral pleura | 1 (0.71) | 0.1–4.9 |
| Bilaterally fragmented and thickened pleura | 118 (83.7) | 76.6–89.0 |
Post-procedure lung ultrasound prior to extubation identified 136 patients (96.5%, 95% CI 91.7–98.5%) with no signs of pneumothorax. In five patients (3.6%, 95% CI 1.5–8.3%) lung ultrasound findings were consistent with definite pneumothorax, whereas no patients (0%, 95% CI 0.0–2.1%) fulfilled the criteria for possible pneumothorax. Based on the lung point placement, four patients (2.84%, 95% CI 1.1–7.4%) had a small pneumothorax and one (0.71%, 95% CI 0.1–4.9%) had a large pneumothorax.
The reference standard identified 19 patients (13.5%, 95% CI 8.7–20.2%) with pneumothorax following TBCB. No patients were diagnosed with pneumothorax in the recovery room due to development of clinical symptoms or signs. Of the 19 patients with pneumothorax, 13 (9.2%, 95% CI 5.4–15.3%) received treatment with a pleural drain while the remainder were treated conservatively. Chart review following patient discharge identified three patients (2.1%, 95% CI 0.7–6.4%) who developed pneumothorax within the first week following discharge. One of these patients had been diagnosed and treated for pneumothorax initially. The remaining two patients had no signs indicating pneumothorax following TBCB.
When compared to the reference standard, the diagnostic accuracy of lung ultrasound for diagnosing pneumothorax was as follows: sensitivity: 21.1% (95% CI 6.1–45.6%), specificity: 99.2% (95% CI 95.5–100.0%), PPV: 80.0% (95% CI 28.4–99.5%), NPV: 89.0% (95% CI 82.5–93.7%), LR+ 25.7 (95% CI 3.03–218) and LR− 0.796 (95% CI 0.631–1). The corresponding 2×2 table for post-procedure lung ultrasound compared to the reference standard is presented as table 3.
TABLE 3.
Post-procedure lung ultrasound compared with a reference standard: 2×2 table
| Reference standard | Post-procedure lung ultrasound | Total | |
| Definite pneumothorax | No pneumothorax | ||
| Pneumothorax present | 4 | 15 | 19 |
| Pneumothorax absent | 1 | 121 | 122 |
| Total | 5 | 136 | 141 |
In one patient, the lung ultrasound finding of pneumothorax was a false positive when compared to the chest radiograph. In this patient, lung ultrasound prior to the TBCB showed bilateral lung sliding, multiple B-lines and a fragmented, thickened visceral pleura. Following TBC on the anterior right hemithorax, lung sliding, lung pulse and B-lines were no longer present, while the visible pleura line was without movement, thin and well defined. A lung point could be identified just medially to the midclavicular line, corresponding to a small pneumothorax. The patient was clinically stable and no immediate intervention was performed. Prior to extubation, lung ultrasound was repeated at 5 mins and 10 mins following the initial post-procedure lung ultrasound. There was no change in the placement of the lung point and, following extubation, the patient was still clinically stable and the placement of the lung point on repeated lung ultrasound prior to transfer to the recovery room did not change. Chest radiography 2 h following TBCB had no signs of pneumothorax and the patient was discharged in the afternoon and did not subsequently develop pneumothorax. There were no apparent differences in lung ultrasound findings among the 15 patients having a false negative lung ultrasound, as all exhibited the presence of lung sliding prior to and following the TBCB procedure.
Post-procedure lung ultrasound had a clinical impact in five patients (3.6%, 95% CI 1.5–8.3%) as follows: 1) a pleural drain was placed in one patient with a large pneumothorax; 2) one patient with a small pneumothorax was clinically unstable and immediate pleural drain insertion subsequently stabilised them; 3) two patients with a small pneumothorax were clinically stable, but lung ultrasound monitoring in the operating room showed progression of pneumothorax size from small to large requiring pleural drain insertion prior to extubation; 4) the fifth patient was the lung ultrasound false positive patient described above. In this patient the observation period in the operating room prior to extubation was prolonged but no drain was inserted. There were no observed adverse events related to lung ultrasound.
Discussion
Lung ultrasound conducted immediately following TBCB had a low sensitivity for procedure-related pneumothorax and was only able to identify approximately every fifth patient developing pneumothorax. Lung ultrasound did, however, have a clinical impact, since four patients benefitted from early identification and treatment of pneumothorax prior to being extubated.
The observed diagnostic accuracy is substantially lower than reported in two previous studies assessing lung ultrasound's ability to diagnose pneumothorax following TBCB [4, 5]. In a prospective study of 43 patients by Viglietta et al. [4], the reported sensitivity and specificity of lung ultrasound were 90% (95% CI 55.5–99.7%) and 94% (95% CI 79.8–99.3%), respectively. The lung ultrasound criteria for pneumothorax were comparable to the ones used in this study and chest radiography was also used as a reference standard. In a small retrospective study, Matus et al. [5] described findings in 24 patients who had lung ultrasound performed prior to and following TBCB, and who also used chest radiography as a reference standard. In this study only one patient developed pneumothorax and this was identified by lung ultrasound. However, with only one event, the reported prevalence of pneumothorax is low when compared to other TBCB studies [2, 3, 25].
Compared to these two studies, the most likely explanation for the marked difference in results is the time point after TBCB at which lung ultrasound was performed. In the studies by Matus et al. and Viglietta et al., both lung ultrasound and chest radiography were performed at 1 h and 3 h following TBCB, respectively. As demonstrated in other studies, pneumothorax is not always apparent immediately after a lung biopsy procedure, but may gradually develop within hours or even days following the procedure [26, 27]. In transthoracic lung biopsies, control imaging 1 h following the procedure has been recommended [11]; however, no studies have established a certain time frame in which most patients develop pneumothorax following TBCB. That said, based on the results of this study and those presented by Viglietta et al., control imaging at 2 h–3 h following TBCB would probably allow identification of most pneumothoraces with a low prevalence of patients being readmitted with pneumothorax (2.1% in the present study).
Another reason for the low sensitivity could be the use of a lung ultrasound protocol limited to the anterior surface of the thorax. However, similar protocols have been validated with high diagnostic accuracy in settings resembling patient positioning and practical limitations in intubated patients in an operating room [14, 28, 29]. A more comprehensive protocol might have had a higher sensitivity, but since the pre-procedural lung ultrasound findings did not indicate the presence of extensive pleural adhesions, larger pneumothoraces would most likely also have been identified using a limited protocol [30, 31].
Even though immediate post-procedure lung ultrasound missed many pneumothoraces, the study highlights the potential clinical impact in a small proportion of the patients. The main advantages being identification and bedside monitoring of early evolving pneumothoraces and the possibility of pleural drain placement being performed while the patient is still under general anaesthesia, thus eliminating pain related to pleural drain insertion [12]. One could argue that the value of lung ultrasound in patients clinically unstable due to rapidly evolving tension pneumothoraces is limited, since this is a clinical diagnosis and should be treated promptly when suspected. However, most physicians would have to balance clinical confidence in a suspected diagnosis with the threshold for performing an invasive procedure. Using a setup with lung ultrasound readily available in the operation room, the time used for performing focused lung ultrasound in these patients is minimal. As such, the time used for lung ultrasound could be considered as being balanced by increased diagnostic confidence and subsequently a lowered threshold for performing an invasive procedure. Additionally, pneumothorax is not the sole reason for patients becoming clinically unstable following TBCB (e.g. bleeding into central airways). Rapidly ruling out pneumothorax in these patients alerts the physician to the necessity of searching for alternative causes (e.g. bronchoscopic reassessment of the central airways), rather than using the time to insert a pleural drain. Interestingly, no patients on our study developed a pneumothorax needing immediate treatment in the recovery room. Whether this was a direct result of early lung ultrasound guided intervention in the operating room is speculative, but, from a patient safety viewpoint, this is an important incidental finding. The study clearly demonstrates that a focused lung ultrasound approach, despite being simple in theory, might not be as simple when integrated into all clinical settings. Lung ultrasound findings should always be integrated with information regarding the clinical context, other findings and dynamic changes in the underlying disease, as well as the operator being fully aware of the technique's limitations and pitfalls.
Strengths and limitations
The presented study is the largest, prospective diagnostic accuracy study on pneumothorax following TBCB. All patients were included consecutively, and index tests and reference standards were standardised and feasible in every patient. A significant limitation is the time difference between the index test and the reference standard, as this should ideally be as short as possible. However, the aim of the study was to assess the use of immediate post-procedure lung ultrasound and any chest radiography in the operating room would have been as single plane, supine chest radiography, or alternatively as fluoroscopy, and these alternatives also possess significant limitations [32, 33]. A more ideal study design would have included a third lung ultrasound examination either prior to or immediately after the chest radiograph. This could possibly have helped to differentiate whether the reported low sensitivity of lung ultrasound in the operating room might be due to timing or whether in fact it reflects a significantly lower diagnostic accuracy of lung ultrasound for pneumothorax in this setting. Unfortunately, a third lung ultrasound being performed was logistically not feasible in our study setting, as the physician performing TBCB and lung ultrasound could not leave the operating room to go to another part of the hospital to repeat the lung ultrasound prior to chest radiography.
Conclusion
Despite the low sensitivity observed, lung ultrasound prior to extubation still had a clinical impact by helping to identify the following: 1) patients with pneumothorax needing immediate treatment; and 2) patients in which an expanded observation period in the operating room prior to extubation may be needed. However, lung ultrasound immediately following TBCB should still be supplemented with chest radiography or with lung ultrasound (either immediately if signs or symptoms of pneumothorax are present or 2 h after the end of the procedure if the patient is asymptomatic).
Acknowledgements
The authors thank Lars C. Lund (Dept of Clinical Biochemistry and Pharmacology, Odense University Hospital, Odense, Denmark) for valuable help and support for the data analysis.
Footnotes
Conflict of interest: C.B. Laursen has nothing to disclose.
Conflict of interest: P.I. Pietersen has nothing to disclose.
Conflict of interest: N. Jacobsen has nothing to disclose.
Conflict of interest: C. Falster has nothing to disclose.
Conflict of interest: A.D. Juul has nothing to disclose.
Conflict of interest: J.R. Davidsen reports financial support to attend the ERS International Congress and personal fees for teaching from Roche and Boehringer Ingelheim, and personal fees for teaching from Chiesi, outside the submitted work.
References
- 1.Hetzel J, Maldonado F, Ravaglia C, et al. . Transbronchial cryobiopsies for the diagnosis of diffuse parenchymal lung diseases: expert statement from the cryobiopsy working group on safety and utility and a call for standardisation of the procedure. Respiration 2018; 95: 188–200. doi: 10.1159/000484055 [DOI] [PubMed] [Google Scholar]
- 2.Sethi J, Ali MS, Mohananey D, et al. . Are transbronchial cryobiopsies ready for prime time?: a systematic review and meta-analysis. J Bronchology Interv Pulmonol 2019; 26: 22–32. doi: 10.1097/LBR.0000000000000519 [DOI] [PubMed] [Google Scholar]
- 3.Troy LK, Grainge C, Corte TJ, et al. . Diagnostic accuracy of transbronchial lung cryobiopsy for interstitial lung disease diagnosis (COLDICE): a prospective, comparative study. Lancet Respir Med 2020; 8: 171–181. doi: 10.1016/S2213-2600(19)30342-X [DOI] [PubMed] [Google Scholar]
- 4.Viglietta L, Inchingolo R, Pavano C, et al. . Ultrasonography for the diagnosis of pneumothorax after transbronchial lung cryobiopsy in diffuse parenchymal lung diseases. Respiration 2017; 94: 232–236. doi: 10.1159/000477818 [DOI] [PubMed] [Google Scholar]
- 5.Matus I, Raja H. Protocolized thoracic ultrasonography in transbronchial lung cryobiopsies: a potential role as an exclusion study for pneumothorax. J Bronchology Interv Pulmonol 2019; 26: 172–178. doi: 10.1097/LBR.0000000000000541 [DOI] [PubMed] [Google Scholar]
- 6.Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest 2012; 141: 703–708. doi: 10.1378/chest.11-0131 [DOI] [PubMed] [Google Scholar]
- 7.Alrajab S, Youssef A, Akkus N, et al. . Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Crit Care 2013; 17: R208. doi: 10.1186/cc13016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahmarde H, Parooie F, Salarzaei M. Accuracy of ultrasound in diagnosis of pneumothorax: a comparison between neonates and adults-a systematic review and meta-analysis. Can Respir J 2019; 2019: 5271982. doi: 10.1155/2019/5271982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reissig A, Kroegel C. Accuracy of transthoracic sonography in excluding post-interventional pneumothorax and hydropneumothorax. Comparison to chest radiography. Eur J Radiol 2005; 53: 463–470. doi: 10.1016/j.ejrad.2004.04.014 [DOI] [PubMed] [Google Scholar]
- 10.Kreuter M, Eberhardt R, Wenz H, et al. . Diagnostic value of transthoracic ultrasound compared to chest radiography in the detection of a post-interventional pneumothorax. Ultraschall in der Medizin 2011; 32 Suppl. 2, E20–E23. doi: 10.1055/s-0031-1273316 [DOI] [PubMed] [Google Scholar]
- 11.Manhire A, Charig M, Clelland C, et al. . Guidelines for radiologically guided lung biopsy. Thorax 2003; 58: 920–936. doi: 10.1136/thorax.58.11.920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luketich JD, Kiss M, Hershey J, et al. . Chest tube insertion: a prospective evaluation of pain management. Clin J Pain 1998; 14: 152–154. doi: 10.1097/00002508-199806000-00011 [DOI] [PubMed] [Google Scholar]
- 13.Davidsen JR, Skov IR, Louw IG, et al. . Implementation of transbronchial lung cryobiopsy in a tertiary referral center for interstitial lung diseases: a cohort study on diagnostic yield, complications, and learning curves. BMC Pulm Med 2021; 21: 67. doi: 10.1186/s12890-021-01438-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkpatrick AW, Sirois M, Laupland KB, et al. . Hand-held thoracic sonography for detecting post-traumatic pneumothoraces: the extended focused assessment with sonography for trauma (EFAST). J Trauma 2004; 57: 288–295. doi: 10.1097/01.ta.0000133565.88871.e4 [DOI] [PubMed] [Google Scholar]
- 15.Kwan RO, Miraflor E, Yeung L, et al. . Bedside thoracic ultrasonography of the fourth intercostal space reliably determines safe removal of tube thoracostomy after traumatic injury. J Trauma Acute Care Surg 2012; 73: 1568–1573. doi: 10.1097/TA.0b013e318265fc22 [DOI] [PubMed] [Google Scholar]
- 16.European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) . Appendix 11: thoracic ultrasound. In: Minimal training requirements for the practice of medical ultrasound in Europe. 2008. https://efsumb.org/wp-content/uploads/2020/12/2009-04-14apx11.pdf Date last accessed: March 01, 2016.
- 17.Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest 1995; 108: 1345–1348. doi: 10.1378/chest.108.5.1345 [DOI] [PubMed] [Google Scholar]
- 18.Lichtenstein D, Meziere G, Biderman P, et al. . The comet-tail artifact: an ultrasound sign ruling out pneumothorax. Intensive Care Med 1999; 25: 383–388. doi: 10.1007/s001340050862 [DOI] [PubMed] [Google Scholar]
- 19.Lichtenstein D, Meziere G, Biderman P, et al. . The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med 2000; 26: 1434–1440. doi: 10.1007/s001340000627 [DOI] [PubMed] [Google Scholar]
- 20.Lichtenstein DA, Lascols N, Prin S, et al. . The “lung pulse”: an early ultrasound sign of complete atelectasis. Intensive Care Med 2003; 29: 2187–2192. doi: 10.1007/s00134-003-1930-9 [DOI] [PubMed] [Google Scholar]
- 21.Blaivas M, Tsung JW. Point-of-care sonographic detection of left endobronchial main stem intubation and obstruction versus endotracheal intubation. J Ultrasound Med 2008; 27: 785–789. doi: 10.7863/jum.2008.27.5.785 [DOI] [PubMed] [Google Scholar]
- 22.Volpicelli G, Elbarbary M, Blaivas M, et al. . International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012; 38: 577–591. doi: 10.1007/s00134-012-2513-4 [DOI] [PubMed] [Google Scholar]
- 23.Volpicelli G, Boero E, Sverzellati N, et al. . Semi-quantification of pneumothorax volume by lung ultrasound. Intensive Care Med 2014; 40: 1460–1467. doi: 10.1007/s00134-014-3402-9 [DOI] [PubMed] [Google Scholar]
- 24.Bossuyt PM, Reitsma JB, Bruns DE, et al. . Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Standards for reporting of diagnostic accuracy. Clin Chem 2003; 49: 1–6. doi: 10.1373/49.1.1 [DOI] [PubMed] [Google Scholar]
- 25.Ravaglia C, Bonifazi M, Wells AU, et al. . Safety and diagnostic yield of transbronchial lung cryobiopsy in diffuse parenchymal lung diseases: a comparative study versus video-assisted thoracoscopic lung biopsy and a systematic review of the literature. Respiration 2016; 91: 215–227. doi: 10.1159/000444089 [DOI] [PubMed] [Google Scholar]
- 26.Brown KT, Brody LA, Getrajdman GI, et al. . Outpatient treatment of iatrogenic pneumothorax after needle biopsy. Radiology 1997; 205: 249–252. doi: 10.1148/radiology.205.1.9314993 [DOI] [PubMed] [Google Scholar]
- 27.Charig MJ, Phillips AJ. CT-guided cutting needle biopsy of lung lesions – safety and efficacy of an out-patient service. Clin Radiol 2000; 55: 964–969. doi: 10.1053/crad.2000.0964 [DOI] [PubMed] [Google Scholar]
- 28.Blaivas M, Lyon M, Duggal S. A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad Emerg Med 2005; 12: 844–849. doi: 10.1197/j.aem.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 29.Soldati G, Testa A, Sher S, et al. . Occult traumatic pneumothorax: diagnostic accuracy of lung ultrasonography in the emergency department. Chest 2008; 133: 204–211. doi: 10.1378/chest.07-1595 [DOI] [PubMed] [Google Scholar]
- 30.Cassanelli N, Caroli G, Dolci G, et al. . Accuracy of transthoracic ultrasound for the detection of pleural adhesions. Eur J Cardiothorac Surg 2012; 42: 813–818. doi: 10.1093/ejcts/ezs144 [DOI] [PubMed] [Google Scholar]
- 31.Corcoran JP, Psallidas I, Hallifax RJ, et al. . Ultrasound-guided pneumothorax induction prior to local anaesthetic thoracoscopy. Thorax 2015; 70: 906–908. doi: 10.1136/thoraxjnl-2014-206676 [DOI] [PubMed] [Google Scholar]
- 32.Wilkerson RG, Stone MB. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med 2010; 17: 11–17. doi: 10.1111/j.1553-2712.2009.00628.x [DOI] [PubMed] [Google Scholar]
- 33.Rowan KR, Kirkpatrick AW, Liu D, et al. . Traumatic pneumothorax detection with thoracic US: correlation with chest radiography and CT – initial experience. Radiology 2002; 225: 210–214. doi: 10.1148/radiol.2251011102 [DOI] [PubMed] [Google Scholar]