Abstract
Transcatheter mitral valve implantation is an emerging technology for the treatment of inoperable or high-risk patients with symptomatic severe mitral regurgitation. Known technical issues are obstruction of the left ventricular outflow tract, paravalvular leakage, and hemolysis. We report a case of valve retensioning successfully resolving paravalvular leakage and hemolysis. (Level of Difficulty: Intermediate.)
Key Words: hemodynamic status, mitral valve, valve replacement
Abbreviations and Acronyms: LVOT, left ventricular outflow tract; LVOTO, left ventricular outflow tract obstruction; MV, mitral valve; PVL, paravalvular leak; TMVI, transcatheter mitral valve implantation
Central Illustration
History of Presentation
A 75-year-old male patient with severe primary mitral regurgitation due to annular and focal calcifications of both leaflets (Figures 1A and 1B, Videos 1 and 2) presented with dyspnea on exertion (New York Heart Association functional class II) and secondary pulmonary hypertension (mean pulmonary artery pressure 28 mm Hg).
Learning Objectives
-
•
To become familiar with some of the key anatomic selection criteria for TMVI.
-
•
To learn about the specific technical issues associated with TMVI and their management, in particular the possibility to retension the Tendyne valve.
-
•
To understand the importance of medical management during and after TMVI.
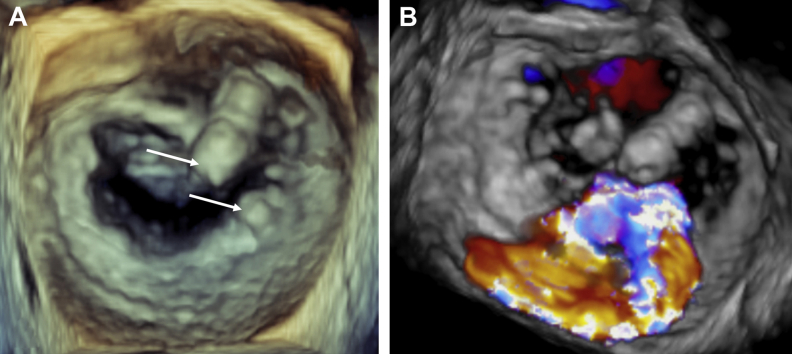
Figure 1.
Baseline Mitral Valve Anatomy
(A) 3-dimensional view of the mitral valve from the left atrium showing focal calcifications of the anterior and posterior valve leaflets (arrows) precluding transcatheter edge-to-edge repair. (B) 3-dimensional Doppler view showing an eccentric severe mitral regurgitation jet originating from the calcified zone of both leaflets.
Medical History
The patient underwent coronary artery bypass grafting in 1993, followed by surgical aortic valve replacement with a bioprosthesis 17 years later for treatment of symptomatic, severe aortic stenosis. Additional comorbidities comprised peripheral arterial disease and rheumatoid arthritis requiring chronic immunosuppressive therapy.
Differential Diagnosis
The differential diagnosis for dyspnea in this clinical context includes progression of coronary artery disease, decrease of left ventricular function, pulmonary hypertension, degeneration of the aortic bioprosthetic valve, and atrial arrhythmias (atrial flutter or fibrillation).
Investigations
Progressive coronary artery disease was excluded by coronary angiography revealing patent grafts, and the mean invasive aortic transprosthetic gradient was stable (14 mm Hg, planimetric aortic valve area 1.2 cm2). The Society of Thoracic Surgeons risk score was 7.8%, and the European System for Cardiac Operative Risk Evaluation II score was 8.1%.
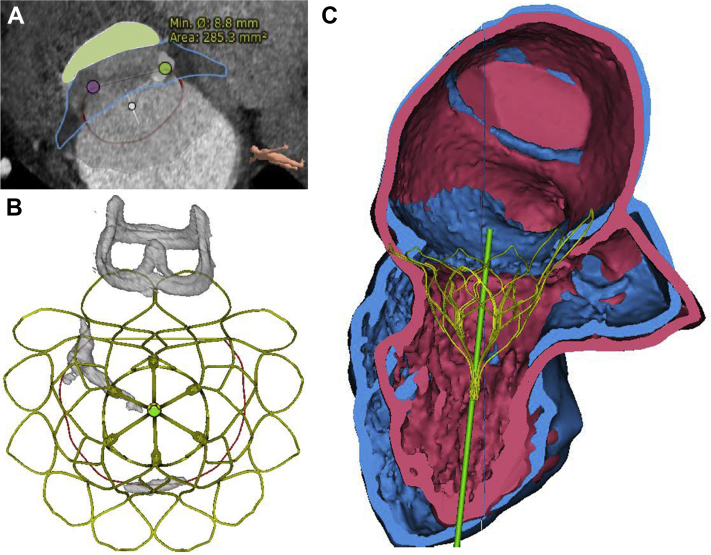
A third open-heart surgical procedure was deemed high risk by the Heart Team, and the patient was not eligible for transcatheter edge-to-edge repair, because of leaflet calcification in the grasping area (Figures 1A and 1B, Videos 1 and 2). Therefore, transcatheter mitral valve implantation (TMVI) with the Tendyne system (Abbott Vascular, Santa Clara, California) was evaluated. The system consists of a porcine bioprosthesis mounted in a self-expanding D-shaped Nitinol stent fixed at the apex of the left ventricle by a tether secured by a pad. Because of apical fixation, the system can be retensioned using the same surgical access. Analysis of pre-procedural cardiac computed tomography yielded a sufficient systolic simulated neo–left ventricular outflow tract (LVOT) area of 285 mm2 (Figure 2A) using a 35-mm low-profile valve placed with 6° of posterior bias (Figures 2B and 2C).
Figure 2.
Pre-Procedural Transcatheter Mitral Valve Implantation Planning
(A) Systolic measurement of neo–left ventricular outflow tract area (green) after delineation of the mitral valve (MV) annulus and simulation of the 35-mm (M) low-profile Tendyne valve (blue). (B) Interaction between Tendyne valve stent frame, MV calcifications, and aortic bioprosthesis. (C) Simulation of the Tendyne valve and tether during diastole (blue) and systole (pink).
Management
TMVI was performed under general anesthesia using both fluoroscopic and transesophageal echocardiographic guidance. After identification of the appropriate puncture site using transthoracic echocardiography, a left mini-thoracotomy was undertaken in the fifth intercostal space.
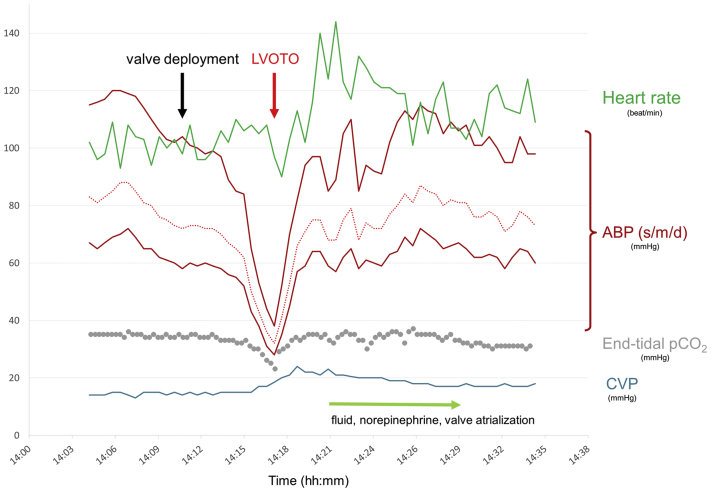
Immediately after deployment of the valve (Figures 3A and 3B), the patient experienced acute hemodynamic deterioration with a rapid blood pressure decrease attributable to severe LVOT obstruction (LVOTO) mediated by anterior displacement of the thickened and calcified native anterior mitral valve (MV) leaflet (Figure 3C, Video 3). Following administration of fluids and vasopressors (norepinephrine) and atrialization of the valve, the patient’s hemodynamic status quickly stabilized, and LVOTO resolved (Figure 4).
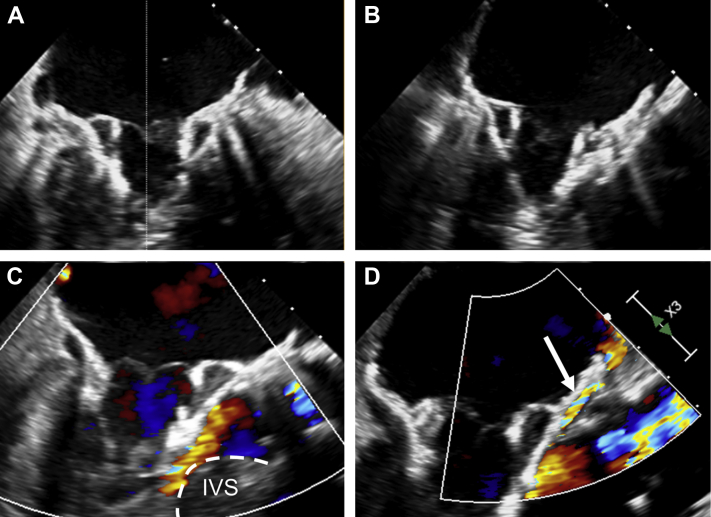
Figure 3.
Valve Implantation Procedure
(A) 2-chamber view of the Tendyne valve during implantation showing annular apposition of the D-shaped atrial stent frame. (B) Left ventricular outflow tract (LVOT) view of the Tendyne valve obtained using the x-plane function. (C) Acute LVOT obstruction during valve implantation with imminent circulatory arrest. (D) Anterior paravalvular leak (arrow) at the end of the procedure with resolved LVOT obstruction. IVS = interventricular septum.
Figure 4.
Evolution of Hemodynamic Parameters During Tendyne Valve Implantation
ABP (s/m/d) = arterial blood pressure (systolic/mean/diastolic); CVP = central venous pressure; LVOTO = left ventricular outflow tract obstruction; pCO2 = carbon dioxide pressure.
The self-expanding mitral bioprosthesis was then deployed (Video 4) and the tether secured at the left ventricular apex. A slightly atrial position of the valve with mild anterior paravalvular leak (PVL) was accepted to ensure a sufficient LVOT area (Figure 3D, Video 5).
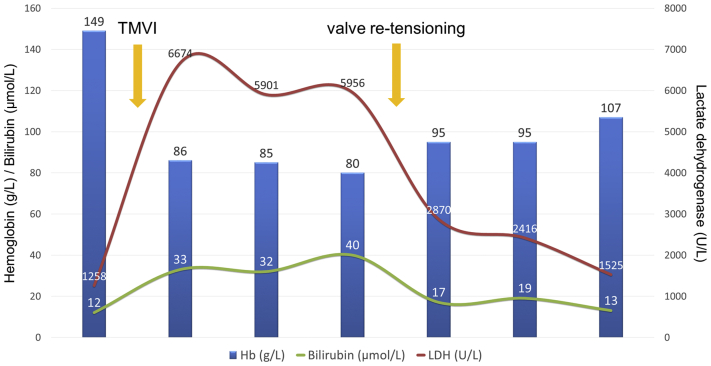
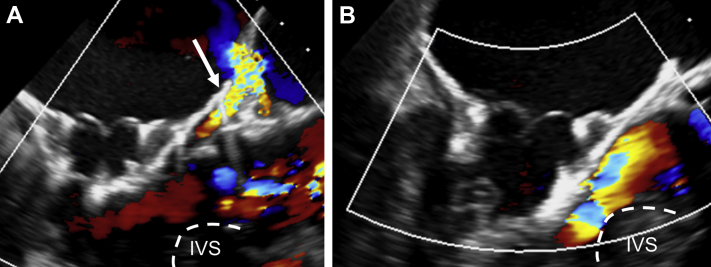
A few weeks later, the patient was rehospitalized with permanent atrial fibrillation and clinical signs of decompensated heart failure. Hemolytic parameters were elevated and haptoglobin was not detectable, with a minimum hemoglobin level of 80 g/l (Figure 5). Progressive hemolytic anemia was considered the main reason for persistent dyspnea and cardiac recurrent decompensation. Transesophageal echocardiography showed an increase of the PVL (Figure 6A, Video 6) related to the atrialization of the MV bioprosthesis.
Figure 5.
Evolution of Hemoglobin and Hemolytic Parameters During Therapy
Hb = hemoglobin; LDH = lactate dehydrogenase; TMVI = transcatheter mitral valve implantation.
Figure 6.
Valve Retensioning Procedure
(A) Left ventricular outflow tract (LVOT) view of the Tendyne valve showing worsening paravalvular leak (PVL) (arrow) due to valve loosening. (B) Improved apposition of the valve after successful retensioning with PVL abolishment and open LVOT. IVS = interventricular septum.
After interdisciplinary discussion, the decision was made to perform a valve retensioning procedure using the previous thoracotomy 11 weeks after TMVI, with the aims of reducing PVL, treating hemolysis, and resolving symptoms. The redo procedure was performed with a primed heart-lung machine in the hybrid room in case of valve dislocation. Transapical valve retrieval using a dedicated tool was trained before the intervention. The apical pad was unlocked and the remaining length of the tether secured with a clamp. Under transesophageal echocardiographic guidance, the tether was manually pulled out of the left ventricle by 1.4 cm, which resulted in improved apposition of the valve frame and almost abolished the anterior PVL (Figure 6B, Video 7). No significant increase of the LVOT gradient was observed. The patient recovered uneventfully with rapid alleviation of dyspnea.
Discussion
Patients with significant MV disease remain largely undertreated (1). TMVI is an emerging technology addressing the needs of inoperable or high-risk candidates and represents an alternative or complementary technique to transcatheter MV edge-to-edge repair. The efficacy and feasibility of TMVI have been demonstrated for the treatment of severe primary and secondary mitral regurgitation (2), as well as in patients with severe mitral annular calcification (3). The device obtained Conformité Européenne mark approval in early 2020. The 2 technical issues encountered during the reported TMVI case are separately discussed as follows.
Management of LVOTO
LVOTO is a potentially severe complication of TMVI, and pre-operative planning is essential to identify patients at risk (4). Known risk factors include left ventricular hypertrophy, pronounced septal thickness, an aortomitral angle of <90°, a small ventricular cavity, and a long anterior mitral leaflet (5). The complex interactions between these anatomic structures result in the exclusion of a considerable number of patients (60% to 70%) at the time of screening (6). However, pre-operative planning is not able to reliably predict sudden changes in pre- or afterload conditions that may occur during the procedure, as well as the dynamic behavior of the anterior leaflet that may result in systolic anterior motion.
Even if infrequent, LVOTO may therefore occur despite careful anatomic review. Medical management during the procedure is of paramount importance and, whenever possible, is preferred over valve repositioning. To avoid hypercontractile state and increased heart rate, vasopressors and fluid administration should be prioritized over inotropic agents at any time. Removal of the 34/36-F implant catheter from the left ventricle further facilitates diastolic filling and thereby opening of the LVOT. If these measures are not successful, bailout maneuvers include valve atrialization, as well as posterior angulation to minimize the projection of the mitral bioprosthesis into the LVOT. In our case, atrialization of the valve after onset of hemodynamic compromise resolved LVOTO but resulted in suboptimal annular apposition with PVL and subsequent hemolysis. Insufficient tether tension may also have contributed to the mild valve loosening observed during follow-up. Interestingly, LVOTO did not recur after valve retensioning, which emphasizes the central role of hemodynamic management.
Management of hemolysis
Hemolysis has been previously described as a consequence of PVL and is more frequent in the mitral than in the aortic position, particularly in the presence of annular calcifications (7). However, clinically relevant hemolysis leading to symptomatic anemia is rare and was observed in 3 of 100 patients during the Tendyne Global Feasibility Study (8,9). In another patient, valve retensioning was attempted to treat valve dislodgement with associated hemolysis (10). In our case, hemolysis was explained by the appearance of a PVL at the location of a focal calcification of the anterior leaflet, despite a still intra-annular prosthesis. If retensioning remains unsuccessful, transcatheter PVL closure has been described and may represent an alternative for selected patients (2,11).
Follow-Up
Subsequent follow-up showed stable valve position without recurrence of PVL and with resolution of heart failure symptoms and relevant hemolysis (Figure 5).
Conclusions
LVOTO and hemolysis are adverse events specific to TMVI and require multidisciplinary management. Medical management during implantation is of paramount importance for treating unexpected LVOTO and is preferred over valve repositioning whenever possible. Valve retensioning is feasible and can resolve PVL as well as clinically relevant hemolysis.
Funding Support and Author Disclosures
Dr. Reineke has a consultancy agreement with and has received proctoring fees and travel expenses from Abbott Vascular. Dr. Windecker has received research and educational grants to the institution from Abbott, Amgen, Bristol Myers Squibb, Bayer, Boston Scientific, Biotronik, Cardinal Health, Cardiovalve, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Johnson & Johnson, Medtronic, Querbet, Polares, Sanofi, Terumo, and Sinomed; has served as an unpaid member of the steering and executive groups of trials funded by Abbott, Abiomed, Amgen, Bristol Myers Squibb, Boston Scientific, Biotronik, Cardiovalve, Edwards Lifesciences, MedAlliance, Medtronic, Polares, Sinomed, V-Wave, and Xeltis, for which he received no personal payments from any pharmaceutical company or device manufacturer; and has been a member of the steering and executive committee groups of several investigated-initiated trials that receive funding from industry without impact on his personal remuneration. Dr. Praz has received travel expenses from Abbott Vascular, Edwards Lifesciences, and Polares Medical. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Baseline Mitral Valve Anatomy. Three-dimensional view of the mitral valve from the left atrium at baseline showing focal calcifications of the anterior and posterior valve leaflets
Baseline Mitral Regurgitation. Three-dimensional Doppler view showing an eccentric severe mitral regurgitation jet originating from the calcified zone of both leaflets
Left Ventricular Outflow Tract Obstruction. Acute left ventricular outflow tract obstruction during valve implantation with imminent circulatory arrest; the interventricular septum bulge is in contact with the valve during systole
Three-Dimensional Result of the Implantation Procedure. Three-dimensional atrial view of the final valve position
Two-Dimensional Result of the Implantation Procedure. X-plane view of the final valve position at the end of the implantation procedure with small residual anterior paravalvular leak and open left ventricular outflow tract
Follow-Up Transesophageal Echocardiography. Worsening paravalvular leak during follow-up due to valve loosening leading to clinically relevant hemolysis
Final Result After Retensioning. Final position of the valve after retensioning with almost complete resolution of paravalvular leak
References
- 1.Iung B., Delgado V., Rosenhek R. Contemporary presentation and management of valvular heart disease: the EURObservational Research Programme Valvular Heart Disease II Survey. Circulation. 2019;140:1156–1169. doi: 10.1161/CIRCULATIONAHA.119.041080. [DOI] [PubMed] [Google Scholar]
- 2.Sorajja P., Moat N., Badhwar V. Initial feasibility study of a new transcatheter mitral prosthesis: the first 100 patients. J Am Coll Cardiol. 2019;73:1250–1260. doi: 10.1016/j.jacc.2018.12.066. [DOI] [PubMed] [Google Scholar]
- 3.Sorajja P., Gossl M., Babaliaros V. Novel transcatheter mitral valve prosthesis for patients with severe mitral annular calcification. J Am Coll Cardiol. 2019;74:1431–1440. doi: 10.1016/j.jacc.2019.07.069. [DOI] [PubMed] [Google Scholar]
- 4.Blanke P., Naoum C., Webb J. Multimodality imaging in the context of transcatheter mitral valve replacement: establishing consensus among modalities and disciplines. J Am Coll Cardiol Img. 2015;8:1191–1208. doi: 10.1016/j.jcmg.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Bapat V., Pirone F., Kapetanakis S., Rajani R., Niederer S. Factors influencing left ventricular outflow tract obstruction following a mitral valve-in-valve or valve-in-ring procedure, part 1. Catheter Cardiovasc Interv. 2015;86:747–760. doi: 10.1002/ccd.25928. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig S., Ruebsamen N., Deuschl F. Screening for transcatheter mitral valve replacement: a decision tree algorithm. EuroIntervention. 2020;16:251–258. doi: 10.4244/EIJ-D-19-01051. [DOI] [PubMed] [Google Scholar]
- 7.Smolka G., Pysz P., Ochala A. Transcatheter paravalvular leak closure and hemolysis—a prospective registry. Arch Med Sci. 2017;13:575–584. doi: 10.5114/aoms.2016.60435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller D.W.M., Farivar R.S., Jansz P. Transcatheter mitral valve replacement for patients with symptomatic mitral regurgitation: a global feasibility trial. J Am Coll Cardiol. 2017;69:381–391. doi: 10.1016/j.jacc.2016.10.068. [DOI] [PubMed] [Google Scholar]
- 9.Duncan A., Quarto C., Ernst S. Transcatheter aortic valve replacement to treat left ventricular outflow tract obstruction and significant paravalvular leak following transcatheter mitral valve replacement. CASE (Phila) 2019;3:90–99. doi: 10.1016/j.case.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grinberg D., Pozzi M., Bernard C., Obadia J.F. A tensed Tendyne. Eur J Cardiothorac Surg. 2020;58:651–653. doi: 10.1093/ejcts/ezaa109. [DOI] [PubMed] [Google Scholar]
- 11.Perl L., Cohen A., Dadashev A. Long-term outcomes of catheter-based intervention for clinically significant paravalvular leak. EuroIntervention. 2021 Mar 16 doi: 10.4244/EIJ-D-20-01206. [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline Mitral Valve Anatomy. Three-dimensional view of the mitral valve from the left atrium at baseline showing focal calcifications of the anterior and posterior valve leaflets
Baseline Mitral Regurgitation. Three-dimensional Doppler view showing an eccentric severe mitral regurgitation jet originating from the calcified zone of both leaflets
Left Ventricular Outflow Tract Obstruction. Acute left ventricular outflow tract obstruction during valve implantation with imminent circulatory arrest; the interventricular septum bulge is in contact with the valve during systole
Three-Dimensional Result of the Implantation Procedure. Three-dimensional atrial view of the final valve position
Two-Dimensional Result of the Implantation Procedure. X-plane view of the final valve position at the end of the implantation procedure with small residual anterior paravalvular leak and open left ventricular outflow tract
Follow-Up Transesophageal Echocardiography. Worsening paravalvular leak during follow-up due to valve loosening leading to clinically relevant hemolysis
Final Result After Retensioning. Final position of the valve after retensioning with almost complete resolution of paravalvular leak