Abstract
A patient underwent left atrial appendage occlusion due to recurrent stroke despite new oral anticoagulant therapy. The patient later presented with severe acute mitral regurgitation secondary to occluder device migration, which was retrieved percutaneously from the descending aorta via the femoral artery. Mitral surgical repair was required and successfully performed. (Level of Difficulty: Intermediate.)
Key Words: acute heart failure, atrial fibrillation, mitral valve, stroke
Abbreviations and Acronyms: AF, atrial fibrillation; LAAO, left atrial appendage occlusion; OAC, oral anticoagulant; TOE, transesophageal echocardiography
Central Illustration
History of Presentation
Two weeks after atrial fibrillation (AF) ablation and left atrial appendage occlusion (LAAO), a 50-year-old woman presented to the emergency department with sudden dyspnea and palpitations. Physical examination revealed new systolic heart murmur, grade 5 intensity, at the lower left sternal border and signs of acute heart and respiratory failure: arterial blood pressure 110/50 mm Hg, heart rate 150 beats/min, and arterial oxygen saturation 85%. An electrocardiogram showed left bundle branch block and atrial flutter with rapid ventricular response (150 beats/min), and chest radiography revealed bilateral perihilar haziness. A poor acoustic window did not allow transthoracic echocardiography evaluation. Pharmacological cardioversion was achieved under amiodarone and digoxin treatment, and heart failure stabilization under initial diuretic and antihypertensive treatment.
Learning Objectives
-
•
To review a rare complication: clinical presentation of acute mitral regurgitation with mitral chordae rupture secondary to LAAO device migration.
-
•
To discuss the importance of focused and timely diagnostic work-up for this rare complication.
-
•
To summarize current evidence, effectiveness, and safety of the LAAO procedure.
-
•
Percutaneous LAAO could be useful in cases of resistant stroke despite OAC therapy.
Past Medical History
The patient had breast neoplasm in 1995, treated with radical mastectomy, adjuvant radiotherapy, and later breast reconstruction. Paroxysmal AF was diagnosed in 2017 after a transient ischemic attack, and a new oral anticoagulant (OAC) therapy was started.
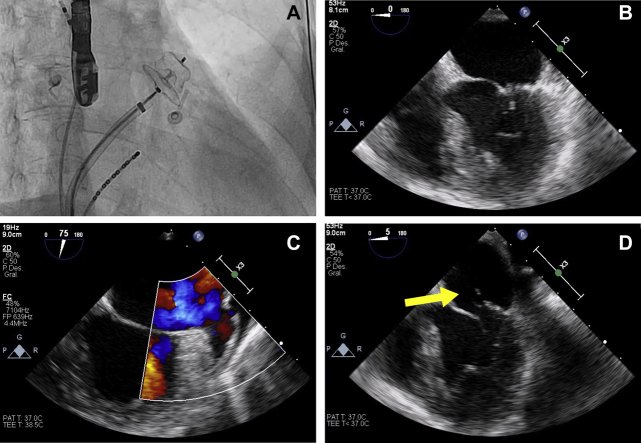
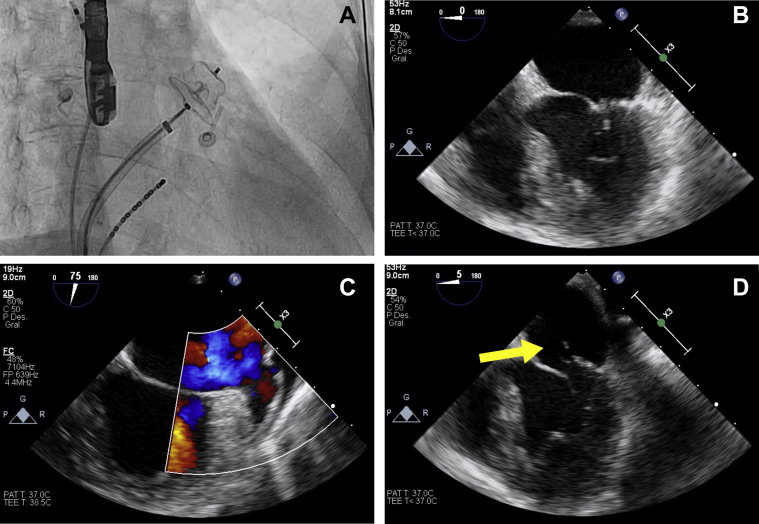
In 2018, the patient developed left middle cerebral artery stroke despite proper compliance with treatment and negative coagulopathy studies. In 2020, left atrial spontaneous echocardiographic contrast was evidenced in a control transesophageal echocardiography (TOE), treated with low-molecular-weight heparin for 3 months. Later, the patient underwent effective pulmonary vein cryoablation and LAAO (Amplatzer Amulet, St. Jude Medical, Plymouth, Minnesota) for secondary prevention of resistant stroke. Peri-procedural TOE showed a correct placement of the LAAO implant and the absence of peri-device residual flow (Figure 1).
Figure 1.
Pre- and Post-Procedural Imaging
(A) Amplatzer Amulet implanted in the left atrial appendage under fluoroscopic control. (B) Peri-procedural transesophageal echocardiography showing mitral valve with normal leaflet coaptation. 2-dimensional transesophageal echocardiography 2 weeks after the procedure showing occluder device absence (C) and a new-onset mitral prolapse of the posterior leaflet (yellow arrow) due to primary tendinous chordae rupture (D).
Differential Diagnosis
The differential diagnosis included acute ischemic mitral regurgitation, infective endocarditis, aortic stenosis, hypertrophic myocardiopathy, and interventricular communication.
Investigations
Nonsignificant coronary stenosis, negative troponin, and the absence of ventricle wall motion abnormalities rule out acute ischemic mitral regurgitation (type 1 myocardial infarction). TOE revealed normal aortic valve function, absence of hypertrophy, and normal left ventricular outflow tract pressure gradient, ruling out aortic stenosis and hypertrophic myocardiopathy. Negative blood culture findings and the absence of intracardiac vegetations rule out infective endocarditis.
Management
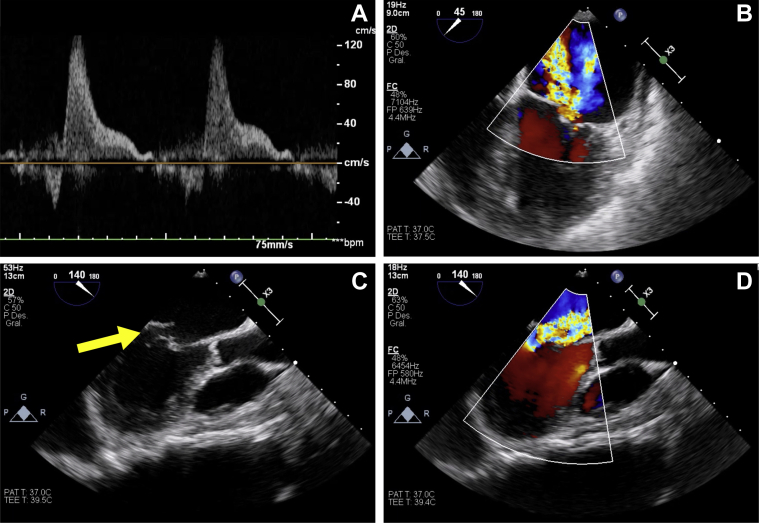
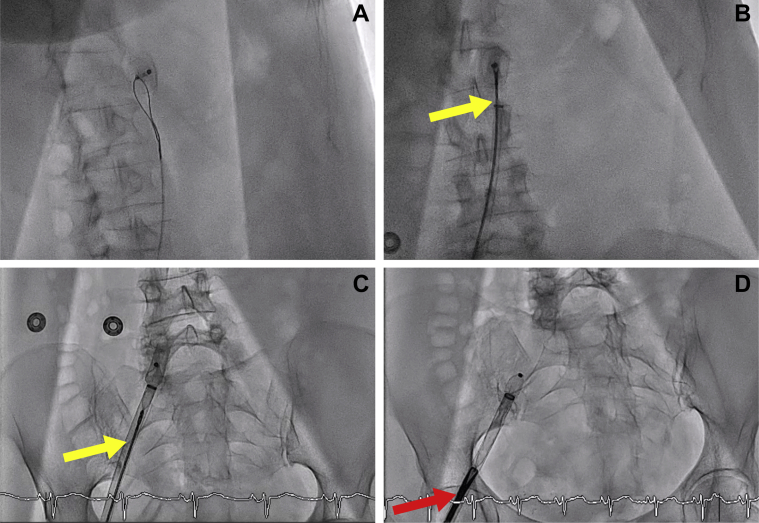
The patient was admitted to the cardiology area under the suspicion of mechanical peri-device complication and presented a favorable response within the first 48 h. On day 3, the patient was transferred to the coronary care unit due to acute pulmonary edema despite medical treatment. Intensive continuous furosemide infusion and sodium nitroprusside were administered. In the following 24 h, the patient exhibited progressive clinical improvement with hemodynamic stability and resolution of respiratory failure. TOE revealed severe eccentric mitral prolapse with posterior flail leaflet (P2 and P3 scallops), primary chordae tendineae rupture, and the absence of the LAAO device (Figures 1 and 2, Videos 1, 2, 3, 4, and 5). A device migration to the abdominal aorta was evidenced on the admission chest radiograph. Use of myotomy forceps (15-F diameter) was required for its removal, via right the femoral artery, after failed attempts with a goose-neck snare and endomyocardial biopsy forceps (Figure 3, Videos 6, 7, 8, and 9).
Figure 2.
Severe Acute Mitral Regurgitation
(A) Pulsed wave Doppler pulmonary veins showing systolic pulmonary flow reversal. (B and D) Color Doppler transesophageal echocardiography revealed severe eccentric mitral regurgitation. (C) 2-dimensional transesophageal echocardiography showing posterior flail leaflet (yellow arrow) with an eccentric jet directed to the interatrial septum.
Figure 3.
Device Retrieval Under Fluoroscopic Control
Goose-neck snare (A), endomyocardial biopsy forceps (yellow arrows) (B and C), and myotomy forceps (red arrow) (D) used for device retrieval through right femoral artery.
Discussion
AF is the most common cause of cardioembolic stroke, which is the subtype of ischemic stroke with greater size of the lesion, highest in-hospital mortality, and highest rate of functional limitation at discharge (1). Its prevalence is increasing exponentially in developed countries due to an increase in life expectancy and a higher detection of silent AF.
According to current guidelines, OAC therapy is the first-line treatment used to prevent stroke, based on the risk factors for stroke evaluated by using the CHA2DS2-VASc score (2).
LAAO has emerged as an alternative treatment to OAC therapy in patients with nonvalvular AF, as LAA is the primary substrate for thrombus formation (3).
LAAO has been shown to be noninferior to medical management for stroke prevention in patients with contraindication or intolerance to OAC therapy (4). LAAO should also be considered in patients who present with stroke on OAC despite therapy (resistant stroke), as in our case. Estimated frequency of resistant stroke is 11%, and there is insufficient evidence, to date, to decide the management of these patients. Nevertheless, new emerging cohort studies have shown similar effectiveness and safety outcomes for LAAO in this group of patients, suggesting that LAAO could be a viable alternative to medical treatment (5,6).
LAAO has been exponentially performed over the last decade due to the increasing prevalence of AF, as well as improvements in technique and operator expertise. Frequency of in-hospital adverse outcomes associated with the procedure is higher in the real-world population than in clinical trials (7).
The latest evidence, mainly based on multicenter registries, indicates a high rate of percutaneous LAAO success (97% to 99%) and a low rate of overall composite major adverse events (4% to 8%). The most common major adverse events are major bleeding and pericardial effusion or tamponade, which occur at a rate of 1.5% for both and usually appear within the first days after the procedure. Cardiac surgery is required in <3% of overall cases (5,6,8).
Device embolization is an infrequent (<1%) but severe complication. In most cases, it occurs in the peri-procedural period, but late embolization within the first months is possible, as in the current case. Its removal is most likely to be done percutaneously, as the device usually migrates to the aorta; otherwise, when the device is trapped in the mitral apparatus or the left ventricular outflow tract, cardiac surgery is needed (9). Implant dislocation and migration to mitral valve apparatus is very rare, and it is usually diagnosed as an incidental finding in a control echocardiography in asymptomatic patients (10).
This is the first case report described in which Amplatzer Amulet device aortic migration caused mitral chordae rupture and secondary symptomatic acute severe mitral regurgitation. Dislocation of the implant, defined as peri-device flow with a jet ≥5 mm width, may be due to incomplete LAAO, generally secondary to device oversizing, although effective LAA sealing was confirmed by TOE post-procedure in this case (2). Spontaneous atrial contraction in paroxysmal AF could also increase dislocation risk, although there is no current evidence.
Follow-Up
The patient underwent successful mitral valvuloplasty, mitral chordae replacement, and LAA surgical closure. The patient is in New York Heart Association functional class I in the first month after discharge from hospital.
Conclusions
Acute mitral regurgitation secondary to occluder device migration is a rare complication, and it should be considered in patients with recent percutaneous LAAO and acute heart failure. Flail mitral leaflet, chordae tendineae rupture, and LAAO device absence are specific echocardiographic features.
Emerging data support LAAO in the secondary prevention of resistant stroke as an effective and safe procedure, although randomized clinical trials are needed to provide solid evidence.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this article.
Appendix
Posterior Flail Leaflet: Mid-Esophageal 4-Chamber View
Color Doppler Mitral Regurgitation: Mid-Esophageal 4-Chamber View
Posterior Flail Leaflet: Mid-Esophageal Long-Axis View
Color Doppler Mitral Regurgitation: Mid-Esophageal Long-Axis View
LAAO Device Absence
Failed Retrieval With a Goose-Neck Snare
Failed Retrieval With an Endomyocardial Biopsy Forceps
Failed Retrieval With an Endomyocardial Biopsy Forceps
Successful Device Retrieval With a Myotomy Forceps
References
- 1.Arboix A., Alió J. Cardioembolic stroke: clinical features, specific cardiac disorders and prognosis. Curr Cardiol Rev. 2010;6:150–161. doi: 10.2174/157340310791658730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pison L., Potpara T.S., Chen J. Left atrial appendage closure-indications, techniques, and outcomes: results of the European Heart Rhythm Association Survey. Europace. 2015;17:642–666. doi: 10.1093/europace/euv069. [DOI] [PubMed] [Google Scholar]
- 3.Thambidorai S.K., Murray R.D., Parakh K. Utility of transesophageal echocardiography in identification of thrombogenic milieu in patients with atrial fibrillation (an ACUTE ancillary study) Am J Cardiol. 2005;96:935–941. doi: 10.1016/j.amjcard.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 4.Schellinger P.D., Tsivgoulis G., Steiner T., Köhrmann M. Percutaneous left atrial appendage occlusion for the prevention of stroke in patients with atrial fibrillation: review and critical appraisal. J Stroke. 2018;20:281–291. doi: 10.5853/jos.2018.02537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz-González I., González-Ferreiro R., Freixa X. Left atrial appendage occlusion for stroke despite oral anticoagulation (resistant stroke). Results from the Amplatzer Cardiac Plug registry. Rev Esp Cardiol (Engl Ed) 2020;73:28–34. doi: 10.1016/j.rec.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Hildick-Smith D., Landmesser U., Camm A.J. Left atrial appendage occlusion with the Amplatzer™ Amulet™ device: full results of the prospective global observational study. Eur Heart J. 2020;41:2894–2901. doi: 10.1093/eurheartj/ehaa169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badheka A.O., Chothani A., Mehta K. Utilization and adverse outcomes of percutaneous left atrial appendage closure for stroke prevention in atrial fibrillation in the United States: influence of hospital volume. Circ Arrhythm Electrophysiol. 2015;8:42–48. doi: 10.1161/CIRCEP.114.001413. [DOI] [PubMed] [Google Scholar]
- 8.Kleinecke C., Park J.W., Gödde M., Zintl K., Schnupp S., Brachmann J. Twelve-month follow-up of left atrial appendage occlusion with Amplatzer Amulet. Cardiol J. 2017;24:131–138. doi: 10.5603/CJ.a2017.0017. [DOI] [PubMed] [Google Scholar]
- 9.El-Gabry M., Shehada S.E., Wendt D., Mourad F. Emergent surgical removal of a migrated left atrial appendage occluder. Eur J Cardiothorac Surg. 2018;54:191–192. doi: 10.1093/ejcts/ezy005. [DOI] [PubMed] [Google Scholar]
- 10.Mester P., Dompnier A., Belle L. Asymptomatic migration of an AMPLATZER™ Amulet™ left atrial appendage occluder through the mitral valve. Catheter Cardiovasc Interv. 2017;90:346–349. doi: 10.1002/ccd.26557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Posterior Flail Leaflet: Mid-Esophageal 4-Chamber View
Color Doppler Mitral Regurgitation: Mid-Esophageal 4-Chamber View
Posterior Flail Leaflet: Mid-Esophageal Long-Axis View
Color Doppler Mitral Regurgitation: Mid-Esophageal Long-Axis View
LAAO Device Absence
Failed Retrieval With a Goose-Neck Snare
Failed Retrieval With an Endomyocardial Biopsy Forceps
Failed Retrieval With an Endomyocardial Biopsy Forceps
Successful Device Retrieval With a Myotomy Forceps