Abstract
There is still much we do not know about the impact of COVID-19 on the health of pregnant and postpartum women and pregnancy outcomes. Current evidence suggests that there is biological plausibility for worse outcomes among this population. This case report details the clinical care given to a postpartum Hispanic and obese woman diagnosed with COVID-19 in April 2020. We report the care she and her newborn received and her progression through the virus. We discuss the current knowledge surrounding COVID-19 among pregnant and postpartum women. While research supports COVID-19 outcomes being comparable to the general population, there is limited research in this area. Clinical trials, acting on the side of caution, have tended to exclude pregnant women from participation. Therefore, there is a need for further research that can inform evidence-based policy decisions related to COVID-19 in pregnant and postpartum women.
Keywords: COVID-19, emergency medicine, respiratory medicine, pregnancy
Background
Despite COVID-19 being a global pandemic, there is still much we do not know about the impact of the virus on the health of pregnant and postpartum women and pregnancy outcomes. Race and ethnicity, as well as the presence of comorbidities, have an impact on the risk of infection and COVID-19 outcomes. Our research forms part of the evidence base required to inform the provision of healthcare related to COVID-19 to this patient population.
Case presentation
Initial hospitalisation of our patient (26-year-old Hispanic woman—gravida 1, para 1) was for labour (at 37 weeks and 5 days) and delivery, via caesarean section, in Chicago, Illinois, USA. The patient was advised by her primary care physician (PCP) to visit labour and delivery to rule out the breech position. She had early and consistent prenatal care, and her body mass index during her first prenatal visit was 40.1 kg/m2. Her prenatal laboratory testing except for positive Group B Streptococcus (GBS) status was unremarkable. Anomaly ultrasound at 18 weeks and 6 days revealed a single live fetus in breech presentation, with a normal amniotic fluid index of 16.9 cm, normal placenta and a normal cervical length. The estimated fetal weight at the time was at the 53.2 percentile. The structural survey was unremarkable. She reported continued fetal movements, moderate myalgia but a lack of contractions, gross leakage of fluid, vaginal bleeding or other symptoms (eg, headache, sore throat, chills or ageusia). She denied recent travel or contact with individuals exhibiting symptoms of COVID-19. On initial assessment, the patient’s temperature was 39.5°C, pulse 130 beats per minute with blood pressure 117/66 mm Hg. Further to this, respiratory rate was 20 breaths per minute, oxygen saturation 95% on room air.
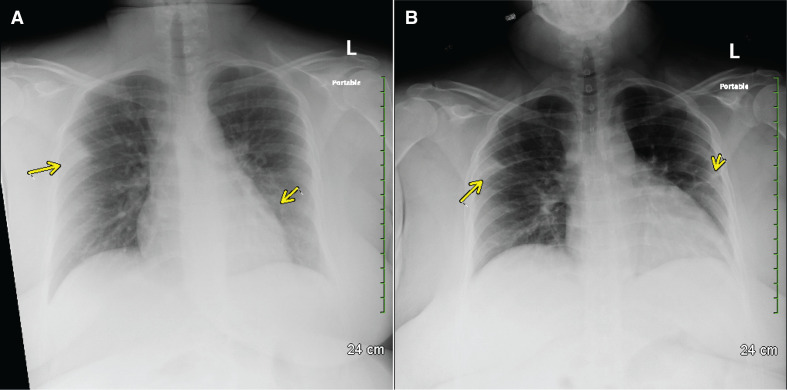
Given her fever and presence of symptoms in the context of pregnancy, the patient was admitted as a COVID-19 person under investigation (PUI) with a workup consisting of chest radiograph, PCR testing for COVID-19, PCR testing for influenza, legionella antigen, blood cultures, urine analysis and urine culture. Droplet and contact precautions were implemented as per COVID-19 recommendations. Chest radiographs revealed peripheral right upper lobe and left perihilar airspace opacity (figure 1A). Laboratory results are shown in table 1.
Figure 1.
Chest radiographs of patient, a postpartum woman with COVID-19 pneumonia. (A) Chest radiograph on admission (day of delivery), showing peripheral right upper lobe and left perihilar airspace opacity. (B) Chest radiograph on postpartum day 1, showing persistent peripheral airspace opacity in the right upper lobe, a slightly increased interstitial opacity in the left perihilar region, and a new peripheral airspace opacity in the left upper lobe.
Table 1.
Pertinent laboratory results by clinical course progression, including prenatal visit and first and second hospital admissions for COVID-19 positive primigravida who died on PPD 22, Chicago, Illinois, USA
| Variable | Reference range |
Laboratory results* | ||||
| First admission† | Second admission‡ | |||||
| Prenatal visit | PPD 0–2 | PPD 11–14 | PPD 15–18 | PPD 19–22 | ||
| Prenatal laboratory tests | ||||||
| RPR test for syphilis | – | Negative | – | – | – | – |
| Rubella IgG | – | Immune | – | – | – | – |
| Blood group and Rh factor | – | A+ | A+ | – | – | – |
| Ab screen | – | Negative | Negative | – | – | – |
| HIV 1/2 Ab and Ag | – | Non-reactive | – | Non-reactive | – | – |
| Microbiology/immunology laboratory tests | ||||||
| SARS-CoV-2 by RT-PCR | – | – | Positive | – | – | – |
| Influenza A | – | – | Negative | – | – | – |
| Influenza B | – | – | Negative | – | – | – |
| QuantiFERON-TB Gold | – | – | – | Negative | – | – |
| HBS Antigen | – | – | – | Negative | – | – |
| Antihepatitis C virus | – | – | – | Negative | – | – |
| Legionella antigen | – | – | Negative | – | – | – |
| Inflammatory markers | ||||||
| CRP (mg/L) | <8.1 | – | 45.0 | 218.8–239.8 | 159.9 | – |
| ESR (mm) | 0–20 | – | – | 32 | – | – |
| Procalcitonin (ng/mL) | <0.06 | – | – | 0.55 | – | – |
| Ferritin (ng/mL) | 11–241 | – | – | 565–1256 | 504 | – |
| Serum IL-6 (pg/mL) | <5.0 | – | – | 80.1 | – | – |
| D-dimer (ng/mL) | <500 | – | – | 7341–62 236 | 6545 | 6311–18 416 |
| Serum electrolytes | ||||||
| Na (mmol/L) | 133–144 | – | 127–132 | 137–142 | 139–147 | 138–143 |
| K (mmol/L) | 3.5–5.1 | – | 3.0–3.9 | 3.4–4.6 | 2.3–5.3 | 3.7–5.3 |
| Cl (mmol/L) | 98–108 | – | 98–101 | 105–112 | 108–113 | 104–120 |
| CO (mmol/L) | 20–32 | – | 20–22 | 11–19 | 22–26 | 11–21 |
| Ca (mg/dL) | 8.6–10.3 | – | 7.3–8.3 | 7.4–7.9 | 7.4–8.3 | 7.7–8.4 |
| Glucose (mg/dL) | 70–100 | – | 74–93 | 109–162 | 84–122 | 39–126 |
| Total protein (mg/dL) | 6.4–8.3 | – | 5.1–5.8 | 5.0–6.1 | 5.3–5.8 | 5.6–6.1 |
| Mg (mg/dL) | 1.6–2.6 | – | 1.3 | 1.9–2.1 | 1.9–2.3 | 1.6–2.1 |
| P (mg/dL) | 2.5–4.7 | – | – | 6.6 | 1.3–6 | 4.6–6.5 |
| Blood cell counts | ||||||
| WCC (x109/L) | 4–11 | – | 5–8.9 | 20.9–38.3 | 14.6–20.7 | 13.3–39.2 |
| Neutrophil (%) | – | 72 | 76.3–83.5 | 72–90 | 71–81 | 35–82 |
| Lymphocyte (%) | – | 20 | 9.1–14.7 | 2–7 | 1–8 | 6–24 |
| Monocyte (%) | – | – | 7.1–12.8 | 1–7 | 3–13 | 6–14 |
| Meta myelocytes (%) | – | – | – | 1–5 | 1–6 | 2–10 |
| Myelocyte (%) | – | – | – | 1–4 | 1–5 | 2–7 |
| Eosinophil (%) | – | – | 0 | – | 1 | 1 |
| Basophil (%) | – | – | 0.1–0.5 | – | – | 1 |
| RCC (x1012/L) | 3.8–5.4 | – | 2.96–4.61 | 3.46–4.43 | 3.36–3.57 | 3.28–3.54 |
| Hb (g/L) | 115–155 | – | 76–120 | 91–114 | 89–97 | 86–93 |
| Hct (%) | 34–46.5 | – | 23.2–36.4 | 28.9–38 | 27.7–29.9 | 27.7–32.0 |
| Platelet count (k/mm cu) | 150–450 | – | 126–160 | 81–188 | 151–254 | 255–329 |
| Band (%) | – | – | – | 3–15 | 3–10 | 2–18 |
| Nucleated RCC (%) | <1 | – | – | 4–23 | 1–9 | 1–13 |
| Coagulation Parameters | ||||||
| PT (seconds) | 9.7–13.1 | – | – | 12.4 | – | 20.8–20.9 |
| INR | 0.8–1.2 | – | – | 1.2 | – | 1.8 |
| aPTT (seconds) | 26.8–36.0 | – | – | 29.2–81.9 | 52.8–110.3 | 46.9–177.5 |
| Liver function tests | ||||||
| AST (U/L) | 10–40 | – | 26–27 | 33–1856 | 173–409 | 94–1084 |
| ALT (U/L) | 7–35 | – | 17–21 | 23–1103 | 269–806 | 107–284 |
| Albumin (mg/dL) | 3.5–5.7 | – | 2.7–3.2 | 1.7–2.5 | 1.8–2.1 | 1.5–1.8 |
| Total bilirubin (mg/dL) | 0.0–1.4 | – | 0.8 | 4.3–6.5 | 6.2–7.7 | 3.6–5.7 |
| Direct bilirubin (mg/dL) | 0.0–0.3 | – | – | 3.5–4.3 | 4.2–4.2 | – |
| ALP (U/L) | 30–110 | – | 67–95 | 66–88 | 75–80 | 89–137 |
| TG (mg/dL) | <150 | – | – | 526 | 426 | 320–482 |
| Renal Function Tests | ||||||
| BUN (mg/dL) | 7–25 | – | 4–8 | 15–58 | 42–61 | 41–64 |
| Creatinine (mg/dL) | 0.5–1.4 | – | 0.61–0.65 | 1.01–2.75 | 1.42–2.20 | 1.53–4.25 |
| Estimated GFR (mL/min/1.73m2)t | >89 | >60 | 23–77 | 30–51 | 14–46 | |
| BUN/creatinine | 6.0–20.0 | – | 6.2–13.1 | 14.9 | 44.0–61.0 | – |
| Anion gap (mmol/L) | 6.2–14.7 | – | 8.0–10.0 | 10.0–20.0 | 5.0–13.0 | 10.0–16.0 |
| Cardiac function markers | ||||||
| BNP (pg/mL) | 1–100 | – | – | 898–1035 | – | 1391 |
| Troponin (ng/mL) | 0.00–0.02 | – | – | 0.07–0.24 | 0.03–0.06 | 0.06 |
| CK (u/L) | 35–200 | – | – | 70–101 | – | 150 |
| LDH (u/L) | 108–212 | – | – | 1751–2737 | – | – |
| Lactic acid (mm/L) | – | – | – | – | ||
| Arterial | 0.4–1.3 | – | – | 2.3–3.1 | – | – |
| Venous | 0.0–2.5 | – | 1.2 | 7.6 | – | – |
| Blood gas parameters | ||||||
| pH | 7.36–7.46 | – | – | 7.17–7.30 | 7.38–7.49 | 6.86–7.44 |
| pCO2 (mm Hg) | 32–46 | – | – | 32–48 | 38–42 | 31–72 |
| pO2 (mm Hg) | 83–108 | – | – | 36–203 | 60–320 | 37–124 |
| hCO3 (mm/L) | 21–29 | – | – | 14–22 | 22–29 | 12–23 |
| BE, calculated (mmol/L) | – | – | (−8) – (−12) | (-2)–5 | (−1) – (−21) | |
| Carboxyhaemoglobin (%) | 0.0–2.0 | – | – | 1.3–1.7 | – | – |
| Methaemoglobin (%) | 0.0–1.5 | – | – | 1.3–1.5 | – | – |
| Ca, ionised (mm/L) | 1.15–1.30 | – | – | 1.06 | – | – |
| Ca, ionised pH corrected (mm/L) | 1.15–1.30 | – | – | 0.98 | – | – |
*Results provided as a 3-day range for simplicity of presentation.
†First admission: prenatal visit, 6 September 2019; PPD 0–2, 1–3 April 2020.
‡Second admission: PPD 11–14, 12–15 April 2020; PPD 15–18, 16–19 April 2020; PPD 19–22, 20–23 April 2020; PPD 11–22, 12–23 April 2020.
Ab, antibody; ALP, alkaline phosphatase; ALT, alanine aminotransferase; aPTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BE, base excess; BHCG, beta-human chorionic gonadotropin; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; Ca, calcium; CK, creatine kinase; Cl, chloride; CO, carbon monoxide; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; GFR, glomerular filtration rate; Hb, haemoglobin; HBS, hepatitis B surface antigen; hCO3, bicarbonate; hct, haematocrit; IL-6, interleukin 6; INR, international normalised ratio; K, potassium; LDH, lactate dehydrogenase; Mg, magnesium; Na, sodium; NP, nasopharyngeal; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; PPD, postpartum day; PT, prothrombin time; RCC, red cell count; Rh, Rhesus; RPR, rapid plasma reagin; TB, tuberculosis; TG, triglyceride; WCC, white cell count.
Due to persistent non-reassuring fetal heart tracing, a primary caesarean section with low transverse incision was performed—severe oligohydramnios was suspected. On postpartum day (PPD) 1, the patient became tachypneic and tachycardic with a temperature of 37.8°C, as well as a heart rate of 103 beats per minute, blood pressure 96/54 mm Hg and a respiratory rate of 24 breaths per minute. Maternal COVIDc1-19 PCR resulted came back positive. Chest radiographs revealed a persistent peripheral airspace opacity in the right upper lobe, a slightly increased interstitial opacity in the left perihilar region, and a new peripheral airspace opacity in the left upper lobe (figure 1B). Baseline ECG and BMP were ordered. At the time of diagnosis, we followed the treatment regimen recommended by health organisations (Centers for Disease Control and Prevention (CDC) and Food and Drug Administration (FDA)) within the U.SA.1 This consisted of initial treatment with plaquenil 400 mg two times per day for 1 day, followed by 400 mg daily and azithromycin 500 mg once followed by 250 mg daily.
Overnight, the patient reported shortness of breath; her temperature peaked at 37.8°C. Blood pressure dropped to 83/47 but improved for a brief period with IV fluid bolus. Her pulse remained stable at 96 beats per minute, respirations remained stable at 24 breaths per minute, and oxygen saturation was at 98% on room air. Zosyn 3.375 g every 8 hours was added to her antibiotic regimen but discontinued after four doses as cultures were negative and she improved clinically. She continued to tolerate a general diet with mild nausea and vomiting.
On PPD 2, her vitals remained stable—CBC with differential was significant for haemoglobin of 7.8, which was likely due to the C-section, compounded with administration of intravenous fluids. Blood cultures produced negative results after 24 hours. Due to her stable condition, the patient was discharged on azithromycin, plaquenil, colace 100 mg by mouth once a day and ferrous sulfate 324 mg three times a day. Appropriate postpartum education was given, and she was asked to follow up in the clinic in 2 weeks to remove her C-section staples.
On PPD 11, she presented to the emergency department. She had shortness of breath and increased leg swelling. She was in hypoxic respiratory failure, with 60% oxygen saturation on the non-rebreather mask utilised by emergency medical services. Her temperature was 38.6°C, blood pressure 96/73 mm Hg, pulse 153 beats per minute and respiration 40 breaths per minute. The patient was kept prone, which improved saturation to 80%, but tachypnea persisted between 40 and 50 breaths per minute. She was intubated for acute hypoxic and hypercapnic respiratory failure (arterial blood gas (ABG) revealed pH 7.13, PO2 97.0, PCO2 54, HCO3 17.9). She needed mechanical ventilation on high ventilatory settings, with fraction of inspired oxygen (FiO2) of 100% and PEEP of 10–15 cm H2O, due to severe COVID-19-induced acute respiratory distress syndrome (ARDS). Eventually, she was transferred to a tertiary-level care centre.
Treatment
At the tertiary care centre, an infectious disease workup (including repeat blood cultures, urinalysis and a chest radiograph) was ordered. Her blood work revealed significant leukocytosis and raised C reactive protein (CRP) (table 1, online supplemental table S1). She was given vancomycin, zosyn and plaquenil 400 mg × 2 doses, 200 mg of two times per day for 5–10 days was also administered. Lactate dehydrogenase (LDH), ferritin, CRP, D-Dimer, troponin, creatine phosphokinase (CPK), erythrocyte sedimentation rate (ESR) and procalcitonin were ordered every 48 hours. A course of vasopressors was also administered. Vancomycin, cefepime and metronidazole were administered, and a course of hydroxychloroquine restarted. Lactose dehydrogenase, ferritin, CRP, D-dimer, troponin, creatine phosphokinase, erythrocyte sedimentation rate and procalcitonin were ordered every 24–48 hours. Because the patient was too unstable to undergo a CT pulmonary angiography, cardiology was consulted for a bedside echocardiogram. The echocardiogram revealed normal mitral, aortic and pulmonary valves. However, the tricuspid valve demonstrated mild tricuspid regurgitation. The right ventricle (RV) showed enlargement with an RV to right atrial gradient of 35 mm Hg and a pulmonary velocity (PV) acceleration with a time of 67 ms. There was also increased RV/left ventricle (LV) ratio with akinesis of the right ventricular free wall, and preserved motion of the RV apex (McConnell sign), suggestive of pulmonary embolism. This prompted initiation of therapeutic doses of heparin with close monitoring of coagulation.
bcr-2021-242819supp001.pdf (226.2KB, pdf)
Chest radiograph findings were further suggestive of pneumonia secondary to COVID-19. Liver and renal function, coagulation profile and respiratory parameters were significantly abnormal on PPD 11 and PPD 12 but showed mild improvement from PPD 13 to PPD 20.
Outcome and follow-up
On PPD 19, the patient developed a fever and circulatory failure. Micafungin was added to her antibiotic regimen for suspected fungal sepsis, along with an increased requirement of vasopressors over the next 2 days. Full ventilatory support was continued with low tidal volume. On PPD 21, her renal and liver function, coagulation profile and respiratory parameters began to deteriorate quickly, needing high ventilator support, and by PPD 22 there was evidence of multiorgan failure. During the last 6 hours of her admission, she had further deterioration of her clinical status. This included an elevated temperature of 42.2°C and a need for maximum vasopressor and ventilatory support. Her physical examination revealed bilateral fixed and dilated pupils and her laboratory profile revealed sudden deterioration of blood gases with low PO2 on 100% FiO2, and the vasopressor requirements increased to maximum. Because intracranial haemorrhage was suspected, protamine sulfate and mannitol were initiated. The patient was not stable enough to undergo a CT of her head. At this point, endotracheal tube was exchanged for cuff leak or to rule out tube blocks. Respiratory rate was increased to 40 for hyperventilation and, subsequently, she went into cardiac arrest. She developed pulseless electrical activity before undergoing three rounds of cardiopulmonary resuscitation (CPR) and achieving a return of spontaneous circulation. Pulseless electrical activity ensued again 20 min later, followed by four rounds of CPR with no return of spontaneous circulation. Time of death was called shortly thereafter.
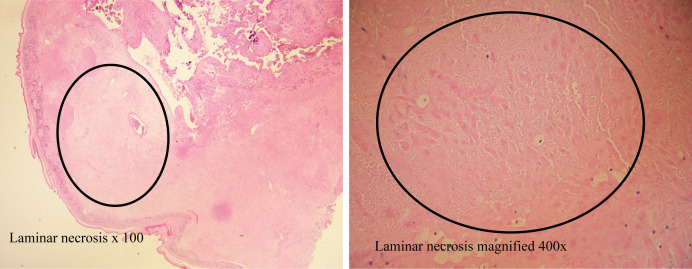
The clinical course of her newborn boy was unremarkable. The placental pathology report revealed a placenta weighing 345 grams with 5% laminar necrosis of fetal membranes (figure 2). Despite the presence of placental vasculopathy, the infant did not exhibit any symptoms associated with COVID-19 and two PCR nasopharyngeal swab tests came back negative. At this juncture, the baby was sent home to the care of family members. No healthcare workers or close relatives were infected with COVID-19 as a result of her infection.
Figure 2.
Placental pathology showing laminar necrosis.
Discussion
Across the USA, the number of postpartum deaths due to the complications of COVID-19 is increasing. At the time (April 2020), this was one of the first cases of death attributed to COVID-19 in a postpartum mother in the USA. Rates of infection with COVID-19, asymptomatic versus symptomatic cases, and morbidity and mortality appear to be similar among pregnant or postpartum and the general population, although additional research is much needed.2 Pregnancy leaves individuals more vulnerable to respiratory tract infections due to hormonal changes, reduces tolerance to hypoxia and creates a state of hypoimmunity.3 The increased susceptibility of infection in pregnant women was evident in previous large-scale infectious disease pandemics, including increased need for the use of ventilators to assist with breathing.4 Infection with SARS-Cov-2, and coronaviruses similar to it in nature such as Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS), have also been found to increase the risk of preterm births, miscarriage and complications with pregnancy.5–7
Our patient was both from a Hispanic background and had an underlying comorbid condition in obesity. At the time of her diagnosis to death, we did not have a clear picture of the impact of race and ethnicity, or overweight and obesity, on COVID-19 infection and outcomes. As data have continued to be collected, and the pandemic has progressed, both have been implicated as independent strong risk factors for severe illness with increased risk of mortality.8–11 One of the reason for obesity as a maker for disease severity could be increased pro-inflammatory response observed in overweight individuals. In addition, obese individuals are often difficult to ventilate and rehabilitate. The individuals from Hispanic background are specifically at increased risk of due to higher prevalence of obesity in Hispanics compared with other ethnic groups in USA.12 Another possible reason for higher mortality rate in Hispanics could be attributed to and poorer access to healthcare among racial and ethnic minority communities in USA.13 14 As physicians, we continue to monitor how race and ethnicity, weight and the presence of comorbidities alter the impact of COVID-19 on our patients.
In terms of pregnancy outcomes for those with COVID-19, there are now a substantial number of systematic reviews and meta-analysis producing heterogeneous results.15 16 A study of 141 pregnant patients with COVID-19 compared their pregnancy outcomes with those of women who did not have the infection.17 They found no significant impact of COVID-19 on either the health of the mother or child. In contrast, a second study found that approximately 30% of women with COVID-19 had a preterm delivery—which is a significantly higher rate compared with that found in the general population.18 There is also a certain difficulty in ascribing a miscarriage as a consequence of COVID-19 infection that needs to be considered.19 The gathering of evidence related to vertical transmission is ongoing, with results appearing to support a low risk.20 A systematic review and meta-analysis that included 86 studies noted that vertical transmission was biologically plausible but appeared to be rare,21 while a second reported a low risk of neonatal infection.22 Preterm babies may also be considered to have increased risk of infection due to the presence of an immature immune system.20At the start of the pandemic, those seeking to understand morbidity and mortality from COVID-19 among pregnant and postpartum populations relied on a limited number of case reports and case series. The first to be published in the United States detailed a 36-year-old postpartum woman who presented with symptoms of COVID-19, required a caesarean section and immediate admission to the intensive care unit, and rapidly declined to multiorgan failure and death.23 This clinical course differs from ours in that the patient experienced an accelerated decline in health that was untreatable, while our patient was able to go home following the birth but then subsequently required readmission to intensive care due to worsening COVID-19 and a deteriorating clinical course, possibly due to postpartum state. Existing comorbidities could be attributed to worsening of the clinical status. Moreover, the severity of COVID-19 illness in our patient was clinically high enough to require high ventilatory support. Her clinical status was also labile and critical needing continuous vasopressors for the most part of her second admission. Additionally, her hypercoagulable status complicated her clinical profile causing pulmonary embolism needing anticoagulation, which all together resulted in rapid multiorgan failure leading to her sudden deterioration. Since these two cases, it has become clear that the path of COVID-19 in this population is as diverse as that for the general population, and that there is a need for ongoing research focused on the virus among those who are pregnant or post partum.
Learning points.
The impact of COVID-19 on the health of those who are pregnant or postpartum appears similar to the general population.
Our case report highlights the rapidity with which the virus can cause the deterioration of health within a young patient.
Although we cannot know the impact of obesity on the outcomes of our patient, research has consistently demonstrated the associations between obesity and mortality due to severe COVID-19.
Acknowledgments
The authors thank the doctors and staff at McNeal Hospital, Oak Berwyn, Illinois, USA, Loyola University Medical Center, Maywood, Illinois, USA and AMITA Health Saints Mary and Elizabeth Medical for Center, Chicago, Illinois, USA, for caring for the patient during the course of her illness. We also thank all three hospitals for sharing the patient’s clinical information with Dr Younes, the patient’s primary doctor. The patient’s parents and sibling graciously consented to the publication of this case report.
Footnotes
Contributors: JM planned the study, conceptualised the idea, helped in data acquisition, writing the first draft of the manuscript and supervised the case report. LY, MS and TT collected the data and contributed to writing the first draft of the manuscript. All authors read and approved the final manuscript for submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.CDC . Considerations for inpatient obstetric healthcare settings. secondary considerations for inpatient obstetric healthcare settings, 2020. Available: https://www.cdc.gov/coronavirus/2019-ncov/hcp/inpatient-obstetric-healthcare-guidance.html
- 2.Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020;370:m3320. 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qi M, Li X, Liu S, et al. Impact of the COVID-19 epidemic on patterns of pregnant women's perception of threat and its relationship to mental state: a latent class analysis. PLoS One 2020;15:e0239697. 10.1371/journal.pone.0239697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. The Lancet 2009;374:451–8. 10.1016/S0140-6736(09)61304-0 [DOI] [PubMed] [Google Scholar]
- 5.Wong SF, Chow KM, Leung TN, et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol 2004;191:292–7. 10.1016/j.ajog.2003.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Souza Silva GA, da Silva SP, da Costa MAS, et al. Sars-Cov, MERS-CoV and SARS-CoV-2 infections in pregnancy and fetal development. J Gynecol Obstet Hum Reprod 2020;49:101846. 10.1016/j.jogoh.2020.101846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Toro F, Gjoka M, Di Lorenzo G, et al. Impact of COVID-19 on maternal and neonatal outcomes: a systematic review and meta-analysis. Clin Microbiol Infect 2021;27:36–46. 10.1016/j.cmi.2020.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sze S, Pan D, Nevill CR, et al. Ethnicity and clinical outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine 2020;29-30:100630. 10.1016/j.eclinm.2020.100630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreira A, Chorath K, Rajasekaran K, et al. Demographic predictors of hospitalization and mortality in US children with COVID-19. Eur J Pediatr 2021;180:1659–63. 10.1007/s00431-021-03955-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu T, Mack JA, Salvatore M, et al. Characteristics associated with racial/ethnic disparities in COVID-19 outcomes in an academic health care system. JAMA Netw Open 2020;3:e2025197. 10.1001/jamanetworkopen.2020.25197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajifathalian K, Kumar S, Newberry C, et al. Obesity is associated with worse outcomes in COVID-19: analysis of early data from New York City. Obesity 2020;28:1606–12. 10.1002/oby.22923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Chaar M, King K, Galvez Lima A. Are black and Hispanic persons disproportionately affected by COVID-19 because of higher obesity rates? Surg Obes Relat Dis 2020;16:1096–9. 10.1016/j.soard.2020.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackey K, Ayers CK, Kondo KK, et al. Racial and Ethnic Disparities in COVID-19-Related Infections, Hospitalizations, and Deaths : A Systematic Review. Ann Intern Med 2021;174:362–73. 10.7326/M20-6306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panagiotakopoulos L, Myers TR, Gee J, et al. SARS-CoV-2 infection among hospitalized pregnant women: reasons for admission and pregnancy characteristics — eight U.S. health care centers, March 1–May 30, 2020. MMWR Morb Mortal Wkly Rep;69:1355–9. 10.15585/mmwr.mm6938e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martini AE. COVID-19 in pregnancy. Glob Reprod Health 2020;5:e47. 10.1097/GRH.0000000000000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akhtar H, Patel C, Abuelgasim E, et al. COVID-19 (SARS-CoV-2) infection in pregnancy: a systematic review. Gynecol Obstet Invest 2020;85:295–306. 10.1159/000509290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nayak AH, Kapote DS, Fonseca M, et al. Impact of the coronavirus infection in pregnancy: a preliminary study of 141 patients. J Obstet Gynaecol India 2020;70:256–61. 10.1007/s13224-020-01335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yee J, Kim W, Han JM, et al. Clinical manifestations and perinatal outcomes of pregnant women with COVID-19: a systematic review and meta-analysis. Sci Rep 2020;10:18126. 10.1038/s41598-020-75096-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong TC, Lee ZY, Sia TLL, et al. Miscarriage risk in COVID-19 infection. SN Compr Clin Med 2020;2:1449–52. 10.1007/s42399-020-00443-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandrasekharan P, Vento M, Trevisanuto D, et al. Neonatal resuscitation and Postresuscitation care of infants born to mothers with suspected or confirmed SARS-CoV-2 infection. Am J Perinatol 2020;37:813–24. 10.1055/s-0040-1709688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalil A, Kalafat E, Benlioglu C, et al. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine 2020;25:100446. 10.1016/j.eclinm.2020.100446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capobianco G, Saderi L, Aliberti S, et al. COVID-19 in pregnant women: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 2020;252:543–58. 10.1016/j.ejogrb.2020.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vallejo V, Ilagan JG. A postpartum death due to coronavirus disease 2019 (COVID-19) in the United States. Obstet Gynecol 2020;136:52–5. 10.1097/AOG.0000000000003950 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bcr-2021-242819supp001.pdf (226.2KB, pdf)