Abstract
Gemella morbillorum is increasingly implicated in infectious endocarditis. Our patient presented with anaemia and renal failure with evidence of infarcts and embolic disease. He was found to have endocarditis with an organism that could not speciate with standard culture methods requiring matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF) for identification and susceptibilities. While involvement of mitral and aortic valves can be expected with Gemella, he had rare involvement of the pulmonic valve in a structurally normal heart. Although bacteriological cure was achieved, due to the locally destructive nature of Gemella, he ultimately required valve replacements for heart failure resolution. Workup for commonly implicated pathologies associated with G. morbillorum led to suspicion of gastrointestinal malignancy with findings of occult bleeding prompting an ongoing evaluation. With improved access to advanced diagnostics, G. morbillorum has been increasingly identified in infectious endocarditis. Given its destructive nature, it is important for clinicians to consider this organism is difficult to identify isolates.
Keywords: heart failure, valvar diseases, infectious diseases, medical management, drugs: infectious diseases
Background
At an incidence rate of 11–15 per 100 000 population, infective endocarditis is an important infectious disease for clinicians to recognise.1 Though the most common predisposing risk factors for developing infectious endocarditis include a history of intravenous drug use, structural, valvular, or congenital heart disease, poor dentition, or recent dental procedures, intracardiac devices and immunocompromised status with Staphylococci most frequently implicated (31%–42%), followed by Streptococci (17%–30%) and Enterococci (11%), not all cases follow the typical template. We present a case of endocarditis lacking traditional risk factors for Gemella morbillorum—a rare organism, but one with increasing frequency and importance for clinicians.2 3
Grouped as a genus of nine species,4 Gemella are commensal organisms of the mucous membranes often found in the respiratory system, the genitourinary system, gastrointestinal tract and oropharynx.5 They are considered opportunistic pathogens but have been implicated in central nervous system infections, embolic strokes, septic arthritis, abscesses and as mentioned here, endocarditis.6 Predisposing factors for infections with this species relate to their niche and include colonic pathologies/malignancies, inflammatory intestinal disorders, recent gastrointestinal/genitourinary procedures or respiratory tract manipulation.7 Thus, once a Gemella infection has been identified, the source of bacteraemia should be investigated to assess for predisposing factors.
Since 1992, there have been approximately 40 reported cases of endocarditis with G. morbillorum. We speculate that this rise is because the organism has been historically difficult to grow and identify using standard gram staining and culture methods. Gemella species are often misidentified as they can be decolourised during the gram staining process and often present as gram-negative or gram-variable organisms.8 More recently, as molecular identification methods advance, G. morbillorum has been increasingly identified in the literature. Additionally, from our review of the literature, 6/32 (18.75%) reported cases of G. Morbillorum endocarditis resulted in a mortality endpoint due to infection sequelae. Given the growing incidence and high mortality rate, we propose maintaining a higher index of suspicion for infection with Gemella in difficult to identify cases to facilitate early detection. To illustrate this, we report a case of infective endocarditis due to G. morbillorum presenting with renal compromise and multi-valvular disease that successfully resolved with antibiotic therapy and surgical correction. Additionally, our patient is the only known case of G. morbillorum with involvement of the pulmonary valve in the setting of a structurally normal heart.
Case presentation
A 72-year-old man with a history of hypertension, hyperlipidaemia and chronic degenerative disk disease presented to a community-based hospital at the behest of his primary care physician due to chronic anaemia that had acutely worsened, a concomitant decline in renal function and a 60-pound unintentional weight loss over the past 3 months.
He reported of worsening fatigue, poor appetite, dysphagia to food and pills as well as progressive muscle weakness. He denied chest pain, fevers, chills, night sweats or other signs of systemic infection at this time. He reported no history of intrathoracic or intracardiac procedures, congenital heart disease, gastrointestinal tract pathology or recent infections. He also denied any history of intravenous drug use, smoking, recent dental work or oral manipulation.
He remained afebrile and relatively normotensive, however, was tachycardic to the 130 s. His examination was notable for a previously undocumented and presumably new holosystolic heart murmur heard throughout the precordium, clear lung fields to auscultation and diffuse tenderness over the low back midline and paraspinal muscles. Rectal examination was negative for blood. There were no obvious stigmata of endocarditis
Previous anaemia workup by his primary care physician suggested anaemia of chronic disease. Guiac test was negative at that time and thus endoscopy and colonoscopy were deferred.
His history was notable for an emergency department visit at another hospital 1 month prior due to subacute muscle weakness, for which an MRI of the brain showed small bilateral punctate parietal and right frontal lobe infarcts but was negative for an acute intervenable cause. Additionally, he had concomitantly undergone MRI of the thoracic and lumbar spine due to his progressive back pain, which did not show any acute abnormalities, but rather consistent chronic degenerative changes, not warranting neurosurgical intervention. At that presentation, screening lab-work including complete blood count, basic metabolic panel, blood glucose, cardiac biomarkers and clotting factors were normal. CT angiography of the head and neck was not pursued at that time. Transthoracic echocardiogram with agitated bubble study was not indicative of a patent foramen ovale and did not comment on abnormal valvular pathology such as vegetations. ECG did not show any evidence of arrhythmias or recent ischemia. He was discharged with a 2-week external cardiac rhythm monitor, which demonstrated paroxysmal atrial fibrillation. On review, he was started on anticoagulation and beta blockade. Definitive cause of his multiple punctate infarcts however, was not determined.
Investigations
Laboratory workup was notable for creatinine of 3.35 mg/dL (baseline <1.0 mg/dL), haemoglobin of 73 g/L (baseline 13–15 g/dL), elevated C reactive protein to 66 mg/L, erythrocyte sedimentation rate of 30 mm/hr and no leucocytosis. ECG demonstrated atrial fibrillation. Renal, bladder and prostate imaging were negative for acute pathology. Creatine phosphokinase was negative and urinalysis was negative for infection. Urine studies were indicative of a pre-renal azotaemia.
Transthoracic echo obtained on admission was notable for mild left ventricular hypertrophy, normal systolic function, severe left atrial dilatation and dilated right ventricle with thickened mitral leaflets with moderate mitral regurgitation and moderate aortic regurgitation without evidence of vegetation.
Abdominal and pelvic imaging was later obtained, which showed splenic infarcts (figure 1), which, in the context of his previous brain imaging with multiple infarcts prompted a re-read of the echocardiogram which now raised suspicion of possible vegetations.
Figure 1.
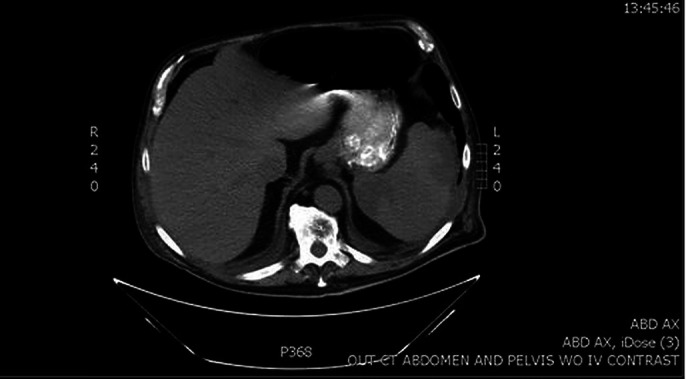
Cross-sectional CT of the abdomen demonstrating wedge-shaped hypodensities with the spleen measuring 5.3×8.3 cm anteriorly and 5.6×3.9 cm posteriorly, indicative of splenic infarcts.
Blood cultures from admission resulted with gram-positive cocci 3 days later. At this point, our patient met ‘possible’ endocarditis per Duke’s criteria. He met major criteria of possible evidence of endocardial involvement on echocardiogram, but was negative for persistently positive blood cultures, and did not speciate to a common pathogenic organism causative of endocarditis. Additionally, he only met two out of five minor criteria of: vascular phenomena (splenic infarcts and bilateral punctate brain lesions) and microbiological evidence.
A transoesophageal echo was then performed which showed vegetations on the mitral, pulmonary and aortic valves, which precluded the ability to cardiovert for atrial fibrillation. The mitral valve A2 scallop vegetation measured 1.2×0.6 cm; A3 scallop was described as a frond-like vegetation measuring 1.9×1.1 cm (figure 2). There was severe mitral regurgitation. The pulmonic valve leaflets were thin and pliable, but with normal valve motion. A small vegetation was noted, measuring 0.5×0.4 cm with mild pulmonic regurgitation (figure 3). The aortic valve was sclerotic, but without functional abnormality. A small vegetation was present in the left coronary cusp measuring 0.5×0.9 cm (figure 4). The tricuspid valve was normal in structure and function.
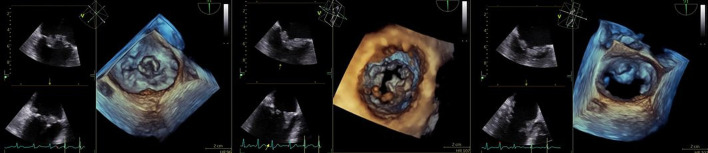
Figure 2.
Transoesophageal echocardiogram with two vegetations seen on the mitral valve. On the A2 scallop, the vegetation measures 1.2×0.6 cm. Second vegetation on the A3 scallop is frond-like and measures 1.9×1.1 cm. (Left: two-dimensional representation; right: three-dimensional reproduction.)
Figure 3.
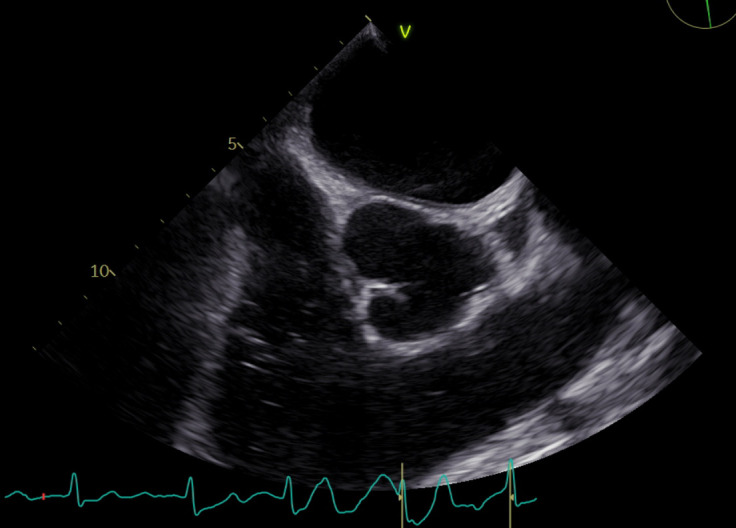
Short axis view of the aortic valve and pulmonary artery outflow demonstrating 0.5×0.4 cm vegetation on the pulmonic valve.
Figure 4.
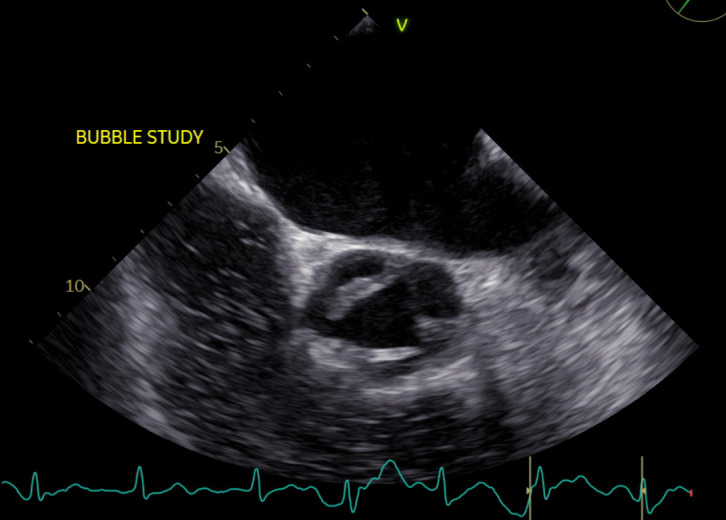
Short axis view of the aortic valve leaflets demonstrating sclerotic aortic valve with a small vegetation on the aortic valve measuring 0.5×0.9 cm in the left coronary cusp.
The organism was unable to be speciated via cultures drawn in hospital, thus isolates were sent for matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF) identification and susceptibilities to be determined via minimum inhibitory concentration. These returned identifying G. morbillorum, with susceptibility to ceftriaxone and penicillin (table 1).
Table 1.
Antibiotic susceptibilities of G. morbillorum
| Antimicrobial | MIC |
| Penicillin | 0.03 |
| Ceftriaxone | 0.16 |
| Meropenem | Susceptible |
| Vancomycin | Susceptible |
| Erythromycin | Resistant |
MIC, minimum inhibitory concentration.
Additionally, given acute renal injury due to glomerulonephritis of undetermined aetiology as well as progressive deterioration of renal function requiring dialysis during admission, an autoimmune workup was initiated, although infectious glomerulonephritis was suspected. Complement levels were depressed (C3:47, C4:15) and repeat inflammatory markers remained elevated, though lower since the initial evaluation. Cryoglobulins with a hepatitis panel, antinuclear antibody (ANA), antineutrophil cytoplasmic antibody (ANCA), IgG, streptozyme were negative, as was HIV. Given the high suspicion for infectious glomerulonephritis, a renal biopsy was deferred.
Differential diagnosis
Our patient presented with an anaemia as well as progressive renal disease that ultimately led to initiation of dialysis. On presentation, the differential was broad and included causes such as infectious endocarditis and septic thrombophlebitis, but also conditions such as malignancy, valvular, thrombotic disease, as well as emboli in the setting of relatively new-onset atrial fibrillation.
Given new onset of atrial fibrillation, initial suspicion was highest for intracardiac pathology, thus a transthoracic echo was obtained, which initially read as having no significant valvular disease. To characterise the renal failure and due to concern for intra-abdominal or thoracic malignancy, chest, abdomen and pelvis imaging was obtained. There was no malignancy noted on imaging, however it was notable for splenic infarcts. In the context of the recent imaging showing parietal and frontal lobe infarcts, this was suspicious for thromboembolic pathology, raising suspicion for endocarditis, thrombophlebitis and embolic phenomena from atrial fibrillation. Physical examination and imaging were not indicative of deep vein thrombosis in the extremities.
With his progressive decline, renal failure, anaemia of chronic disease and infarcts, infective endocarditis was again considered and a re-review of the echocardiogram was requested. Subsequent review concerning for suspected vegetations and diagnosis was later confirmed via transoesophageal echocardiogram.
Treatment
Once imaging findings raised suspicion for endocarditis, our patient was empirically started on vancomycin. This decision was supported when the blood cultures grew gram-positive cocci. However, because the rapid diagnostic testing via available BIOFIRE blood culture identification did not recognise the organism, the gram stain was compared with images of known gram-positive cocci pathogens while the isolate was sent for MALDI-TOF identification and susceptibility testing.
MALDI-TOF conducted on the isolate identified G. morbillorum and mean inhibitory concentration showed susceptibility to ceftriaxone (0.016), penicillin (0.03), meropenem and vancomycin with resistance to erythromycin (table 1). By the time susceptibilities had resulted, the patient had received 14 days of vancomycin. The patient was then transitioned to cefazolin with an additional 4 weeks of continued intravenous antibiotics, totalling 6 weeks of planned antibiotics due to the extensive burden of disease.
Given the valvular deformities from endocarditis and new-onset heart failure, cardiothoracic surgery was consulted for possible surgical options during the hospitalisation. Per 2016 American Association for Thoracic Surgery guidelines on surgical treatment of infective endocarditis, the patient met criteria for surgical intervention given development of heart failure and evidence of embolic disease.9 However, during his hospitalisation, valve replacement surgery was initially deferred to allow for antibiotics to decrease the bacterial load given isolation of infection to the leaflets and to allow for optimisation of patient for surgery considering high-risk features and poor surgical candidacy.
Outcome and follow-up
The patient’s renal function declined throughout the admission, which was suspected due to infection-related immune complex glomerulonephritis vs acute tubular necrosis in a background of longstanding non-steroidal anti-inflammatory drug use, hypertension and new sepsis. He continued to have oliguria with proteinuria and haematuria, for which the time course and hypocomplementemia suggested glomerulonephritis. Renal biopsy was deferred by nephrology as it was not expected to change management given the negative autoimmune workup. Haemodialysis was initiated for volume overload, uraemia and electrolyte disturbances which continued to the outpatient setting.
The patient presented to outpatient infectious diseases clinic approximately 4 weeks postdischarge. The antibiotics were discontinued after a completed 6 weeks course given his marked clinical improvement. A follow-up transthoracic echo was ordered to evaluate disease postantibiotic therapy and potential need for surgical correction or valve surgery.
Compared with the in-hospitalisation echo, follow-up imaging showed persistent vegetation (approximately 1.2 cm) on the mitral valve with ruptured chordal structure and a persistent aortic valve vegetation. Additionally, his postdischarge course was complicated by episodic volume overload with suspected contribution from severe mitral regurgitation. Given findings of mitral regurgitation, mitral vegetation size >1 cm and heart failure symptomology, cardiothoracic surgery was reconsulted. The patient was deemed a more appropriate surgical candidate at this juncture and mitral and aortic valve replacements were then performed without complication.
Future plans were made for dental evaluation, colonoscopy and endoscopy to evaluate sources of possible infection. However, delays in non-emergent medical follow-up occurred due to the coronavirus pandemic. Faecal immunochemical testing was performed and indicative of bleeding. At most recent contact, the patient has met with gastroenterology for consideration of esophagogastroduodenoscopy/colonoscopy (with no known prior evaluation). With regards to his renal injury, the patient has recovered some renal function no longer necessitating haemodialysis and has made strides towards renal recovery at sequential follow-ups. He remains with a stable, recovering anaemia, but is no longer requiring erythropoietin supplementation.
Discussion
The incidence of Gemella causing endocarditis in the literature is rare, with less than 40 documented cases due to G. morbillorum. However, the frequency of this organismal cause of infectious endocarditis has been increasingly documented. Rather than an increased prevalence, we suspect this rise is due to advancements in diagnostics and increased utilisation of molecular-based diagnostic techniques in the setting of indeterminate culture results. Microbiological identification of endocarditis based on blood cultures, tissue cultures or infected emboli are successful in 92%–95% of cases when an organism is present.10 However, conventional cultures are thus unsuccessful in 5%–8% of infective endocarditis cases, and can pose a challenge to diagnose. These cases can be associated with slow-growing or non-culturable organisms or settings where the patient has received antibiotics prior to specimen collection.11
Our patient grew gram-positive-cocci on conventional culture methodology but was unable to speciate further. This is thought to be due to the observation that G. morbillorum, which exists as a facultative anaerobic, catalase-negative, gram-positive-coccus is often easily decolorized during gram staining and thus found to be gram variable.12 This has been repeatedly seen with Gemella species, necessitating utilisation of advanced PCR or spectrometry. On review of literature, >20 case reports acknowledged the diagnostic difficulty in culturing this organism with standard methodology, thereby requiring more advanced culture technology. Our institution used BIOFIRE for blood culture identification, which would have been expected to pick up commonly implicated organisms such as Staphylococcus, Streptococcus and Enterococcus, among others. The VITEK ID did not identify the organism either, thus isolates were sent out for advanced identification.
Broad-range PCR has been commonly used in the literature for identification of Gemella species when traditional culture methods fail and allows for diagnosis of fastidious organisms.13 14 Following isolation, susceptibilities are often determined via disc-diffusion methods.8 14 However, it is worth noting no specific Clinical and Laboratory Standards Institute standard method exists for interpretation of Gemella susceptibility testing.15 In addition to 16s rRNA PCR, MALDI-TOF identification has been gaining traction in diagnosis of Gemella species, as was used in our patient. It rapidly identifies proteins and analyses specimens without requiring sequencing methodology and can predict antibiotic susceptibility of bacteria.8 16
Once identified, Gemella isolates are generally susceptible to beta lactams and vancomycin.17 Initially, a majority of G. morbillorum isolates were thought to be susceptible to penicillin G and ampicillin with most bacteriological cure achievable through penicillin G with accompanying aminoglycoside.18 However, documented resistance patterns with penicillin-resistant and macrolide-resistant strains that have increasingly been reported.13
Our patient’s presentation was atypical given that he did not display generally suspected symptoms of infective endocarditis nor did he possess common predisposing risk factors for developing endocarditis. Additionally, he was found to have vegetations on the mitral, aortic and pulmonary valves without a prior history of valvular heart disease. Typically, endocarditis due to G. morbillorum is thought to affect the mitral and aortic valve in approximately the same proportion occurring more commonly than involvement of tricuspid and pulmonary valves. Infective endocarditis with involvement of the pulmonary valve is rare, occurring only in 2% of hospital admissions for endocarditis.19 There are even fewer cases of Gemella infectious endocarditis with involvement of the pulmonary valves (table 2), each of which, in the literature, occurred in the setting of congenital heart defects.7 Thus, while our patient’s infection of the aortic and mitral valve fits the general paradigm of Gemella endocarditis, the involvement of the pulmonary valve is unique.
Table 2.
Reported cases of Gemella endocarditis
| Authors reported | Age/gender | Risk factors for endocarditis | Presentation | Comorbidities | Valves involved | Antimicrobial therapy | Valve replacement | Survival outcome |
| Bell and McCartney,199222 | 19/M | IVDU—heroin, buprenorphine; saliva inoculation of venipuncture sites | 2 weeks of malaise, nights sweats, intermittent fever | Hepatitis B infection | Tricuspid valve | Flucloxacillin, benzylpenicillin and gentamicin | No | Alive |
| Kerr et al, 199423 | 29/F | HOCM, recent wisdom teeth extraction | Persistent lethargy, flu-like symptoms, night sweats, dry cough | Not reported | Non-valvular | Benzylpenicillin and gentamicin; became sensitised to penicillin and switched to oral erythromycin and oral rifampicin | No | Alive |
| Terada et al, 199424 | 64/M | Dental caries | Low-grade fever, nocturnal dyspnoea | Not reported | Mitral and aortic valve | Penicillin | Yes, aortic and mitral valve replacement | Alive |
| Martin, Wright and Jones, 199525 | 75/M | Childhood rheumatic fever | Weight loss, lethargy | Not reported | Mitral valve | Benzylpenicillin and gentamicin; developed rash and switched to rifampin and erythromycin | No | Alive |
| Lopez-Dupla et al21 | 73/F | Colonic adenomatous polyps in rectum; adenocarcinoma in situ in transverse colon | 3 months of anorexia, asthenia, malaise, fever, weight loss | Not reported | Mitral and aortic valve | Benzylpenicillin and gentamicin | Yes, aortic and mitral valve replacement | Deceased during surgery |
| Vasishtha and Sood, 199626 | 2/F | Single ventricle, transposition of the great arteries, pulmonic stenosis who underwent Fontan procedure | Fever and respiratory failure after Fontan surgery; developing septic shock | Down syndrome | Non-valvular | Vancomycin, ceftazidime, amikacin | No | Deceased. multisystem organ failure |
| Nandakumar and Raju, 199727 | 71/M | Chronic gingivitis and extensive dental caries; villous adenoma with severe atypia | Pulmonary infiltrates, acute onset fever, left lower lobe infiltrate, pleural effusion, weight loss, anaemia, renal insufficiency | ETOH liver disease | Tricuspid valve | Penicillin | No | Alive |
| Farmaki et al, 200028 | 9/F | Dental caries and periodontal disease with multiple recent dental procedures | 6 weeks of intermittent fever | Not reported | Mitral valve | Penicillin and gentamicin | No | Alive |
| Purcell et al, 200129 | 12/F | Congenital heart disease (mitral stenosis, VSD, PDA) repaired at 6 months | Cough, fatigue, dec appetite; no fever | Not reported | Mitral valve | Vancomycin then transitioned to gentamicin and penicillin | No | Alive |
| Akiyama et al, 200130 | 55/M | Aortic regurgitation; prior endocarditis; previous aortic valve replacement; dental caries | Persistent fever, nocturnal dyspnoea | DM2, hepatitis B infection | Aortic valve | Cefotiam then transitioned to tobramycin, cefmetazole, fosfomycin | Yes, aortic valve replacement | Alive |
| Woo et al13 | 66/M | Not reported | 1 month of abdominal and low back pain | Abdominal aortic aneurysm | Mitral and aortic valve | Penicillin, netilmicin | No | Alive |
| Zakir et al, 200431 | 44/M | IVDU, prosthetic heart valve, recent mitral valve replacement | Pleuritic chest pain, dyspnoea, fever, chills | HIV | Bioprosthetic mitral valve and aortic valve | Ceftriaxone and gentamicin | Met criteria, but deferred due to active IVDU | Alive |
| Gimigliano et al, 200532 | 10/F | Transposition of the great arteries, an interventricular septal defect and aortic coarctation | Nocturnal fevers | Not reported | Mitral and aortic valve | Vancomycin and gentamicin | No | Alive |
| Kofteridis et al5 | 46/M | Recent dental procedure, poor ulcerative gingivitis | Fever, headache, vomiting, progressive confusion | Aneurysmal dilation of the ascending aorta with moderate aortic regurgitation | Aortic valve | Penicillin and gentamicin then transitioned to vancomycin | Yes, aortic valve replacement | Alive |
| Kofteridis et al5 | 53/M | Previous infective endocarditis | Persistent fever, sweats, myalgias | Not reported | Mitral valve | Penicillin and gentamicin then transitioned to vancomycin | No | Alive |
| Murai et al, 200633 | 53/M | Dental caries | 1 week of high fevers | Not reported | Aortic valve | Cefotiam then transitioned to ampicillin and gentamicin | Yes, aortic valve replacement | Alive |
| Al-Hujailan et al, 200734 | 37/M | Bicuspid aortic valve with prior prosthetic valve replacement | 10 days of sweats and chills | Not reported | Aortic valve | Vancomycin, gentamicin, rifampicin; vancomycin later switched to penicillin | No | Alive |
| Zheng et al, 200835 | 67/M | Aortic regurgitation, aortic syphilis; poor dentition | 3 days of chills and rigours | HTN, ESRD on haemodialysis | Mitral and aortic valve | Ampicillin and gentamicin | Deceased prior to surgery | Deceased, multisystem organ failure |
| Chekakie et al., 200936 | 44/M | Bicuspid aortic valve with prior prosthetic valve replacement | Dyspnoea and decreased urine output | Cardiomyopathy with EF 15% with defibrillator placement | Aortic valve | Vancomycin, gentamicin, rifampin and switched to ampicillin and gentamicin then later switched to imipenem and metronidazole to treat E. coli bacteraemia and clostridium difficile | Yes, aortic valve replacement | Deceased, multisystem organ failure and LVAD related bleeding |
| Taimur et al, 201037 | 31/F | Bicuspid aortic valve, aortic regurgitation, repair of large aortic arch aneurysm | 1 month of fever, exertional dyspnoea, myalgias, oedema, anorexia | Not reported | Mitral and aortic valve | Amikacin, ceftriaxone, vancomycin then transitioned to ceftriaxone and gentamicin | No | Alive |
| Massoure et al, 201038 | 22/M | Dental caries and periodontitis | Fever and dyspnoea | Tobacco use, khat chewer | Aortic valve | Amoxicillin and gentamicin | Deceased prior to surgery | Deceased, multisystem organ failure |
| Carano et al, 201039 | 18/F | Oral piercing on lower lip | Heart failure and recurrent fever | Not reported | Mitral valve | Penicillin | Yes, mitral valve replacement | Alive |
| Hull, 201040 | 87/M | Mitral regurgitation with ruptured chordae | 5 days of confusion, cough, nausea, diarrhoea; 20 lbs weigh loss over 2 months | Atrial fibrillation | Mitral valve | ‘Broad spectrum antibiotics’ | Considered, but deemed a poor candidate | Alive |
| Godinho et al, 201341 | 72/M | Aortic valve disease, recent endoscopies | Fever, abdominal pain, decompensated heart failure | Iron deficiency anaemia, hypertension | Aortic, mitral and tricuspid valve | Vancomycin and gentamicin | Yes, aortic and mitral valve replacement; tricuspid valve annuloplasty | Alive |
| Ural et al18 | 67/M | Recent EGD, colonoscopy | Anaemia, fever, fatigue, failure to thrive | Not reported | Aortic valve | Ampicillin-sulbactam and gentamicin then transitioned to vancomycin and meropenem | Yes, aortic valve replacement | Alive |
| Agrawal et al, 201442 | ‘Middle-aged man’ | Atrial septal defect | Exertional fatigue and dyspnoea | Not reported | Pulmonary valve | Not reported | Yes, pulmonary bioprosthetic valve and patch closure of ASD | Alive |
| Kolhari et al, 201443 | 34/M | Recent dental extraction | 2 weeks of low-grade fever, lower extremity oedema, dyspnoea on exertion | Hypertrophic cardiomyopathy | Mitral valve | Penicillin and levofloxacin | Yes, mitral valve replacement | Alive |
| Shahani, 201444 | 73/M | Aortic valve replacement, dental caries | 5 days of fever and lower extremity oedema | Coronary artery disease, CABG, diabetes, hyperlipidaemia | Aortic valve | Penicillin and gentamicin | Yes, aortic valve replacement | Alive |
| Constantinos and Marios, 201545 | 80/F | Prior aortic valve replacement | 2 weeks of fever and chest pain | Hypertension, diabetes | Tricuspid valve | Ceftriaxone and gentamicin | No | Alive |
| Rosa et al15 | 72/M | Prior CABG | Dyspnoea, dry cough, fever, anorexia, >10% weight loss over past 30 days |
Hypertension, diabetes, peripheral arterial disease | Mitral valve | Ceftriaxone, gentamicin then transitioned to penicillin and gentamicin | Yes, mitral valve replacement | Deceased, during surgery |
| Shinha, 201746 | 37/M | IVDU | 1 month of fever, chills, fatigue | Not reported | Aortic valve | Not reported | Not reported | Not reported |
| Li et al, 20177 | 28/M | Congenital VSD, ASD and double chambered right ventricle | 3 months of fever, chills, dyspnoea, decreasing exertional capacity, weight loss | Previous admission for bilateral pneumonia | Pulmonary valve | Ceftriaxone and then later added vancomycin | Yes, pulmonary and aortic valve replacement; closure of VSD and ASD, reconstruction of RVOT, excision of vegetations | Alive |
| Kumar et al, 201747 | 12/F | None | Fevers, chills, weight loss, palpitations, night sweats | None | Mitral valve | Vancomycin, gentamicin, penicillin | Offered mitral valve replacement, but family refused | Alive |
ASD, Atrial Septal Defect; CABG, Coronary Artery Bypass Graft; DM2, Type 2 Diabetes Mellitus; EF, Ejection Fraction; EGD, Esophagogastroduodenoscopy; ESRD, End-Stage Renal Disease; ETOH, Alcohol; HOCM, Hypertrophic Obstructive Cardiomyopathy; HTN, Hypertension; IVDU, Intravenous Drug Use; LVAD, Left Ventricular Assist Device; PDA, Patent Ductus Arteriosus; VSD, Ventricular Septal Defect.
As previously mentioned, the source of bacteraemia with G. morbillorum should be identified. The literature suggests a correlation between Gemella and gastrointestinal pathology and cancers. Gemella has been found to be prevalent in the stool of patients with colorectal cancer and also in the cancerous tissues of the gastrointestinal tract specifically. It is suspected that compromised mucosa, especially of the gastrointestinal tract, allows Gemella bacteria to translocate, causing bacteraemia and subsequently endocarditis.20 However, given presence of Gemella in the oral cavity, respiratory tract and genitourinary pathways, proper examination should be conducted to rule-out predisposing pathology in all these segments.21 Specifically in our patient, given the concern for gastrointestinal source and no prior history of colonoscopy nor endoscopy, he underwent repeat faecal occult blood test which was positive and subsequently was referred to gastroenterology.
As our diagnostic methods improve and we see increasing reports of Gemella endocarditis, we encourage clinicians to consider Gemella in the differential when evaluating patients for endocarditis in the setting of difficult to identify gram-positive-cocci and pursue advanced diagnostics not only to solidify the diagnosis but also considering proper identification can assist in the detection of underlying comorbidities such as malignancy.
Patient’s perspective.
My journey began in August 2019 with a back injury while playing golf. I became semi-inactive due to this injury, not feeling well due to nausea and constant pain. After a month of weight loss of 20 lbs due to the loss of appetite as well as trying the chiropractic route of dealing with the back pain which did not help it was decided around the 1st of November that I should contact my specialist who had done my surgeries on my hips and knee. Before deciding on setting up a new surgery procedure for my back they gave me cortisone shots, which really did not help with the excruciating pain every time I tried to walk.
Around December 1st my primary doctor became genuinely concerned why the continued weight loss and fatigue checking blood, etc. Everything seemed to always come back to the back issue but through the holidays I continued to become more lethargic, no real appetite and not wanting to move due to pain. This went on until the end of January 2020 after a series of blood tests showed that my kidneys were not functioning properly. I received the call to come into emergency where I was given two units of blood. I was immediately scheduled to begin kidney dialysis during which time the doctors were trying to figure out what was causing all my mounting problems with my heart as well as my kidneys.
From January to May when I was finally allowed due to coronavirus rules to have my heart surgery, I was not very coherent, bed ridden with no energy, loss of some of my bodily functions at times and really not aware of what I was experiencing other than taking it day by day to just taking it 1 day at a time trying make sure that I was healthy and strong enough to be able to have my open heart surgery.
After the surgery in May, I struggled to regain my strength and mobility, learning to walk again and get self-sufficient. With 3 days a week kidney dialysis, it was a slow process, but I continued to improve on so may levels including after 4 months to be taken off dialysis since miracle of miracles my kidneys begin to function on there own once again!
I just finished my 36 sessions cardiac rehab (my heart has gone back to sinus rhythm), have added 20 lbs of muscle weight with the waist size remaining the same. The back pain has gone away, I am back to three rounds of golf a week. I still do not have the strength back in my legs and arms but working on it every day. I am struggling with balance and equilibrium, but I am starting physical therapy next week to address these issues. I have come a long way with a lot of great help, most important being my wife.
Learning points.
Clinicians should maintain a high index of suspicion for organisms such as Gemella morbillorum that are becoming increasingly prevalent in difficult to identify isolates due to improvements in molecular diagnostics.
In patients who present with G. morbillorum, clinicians should pursue an expedited evaluation of the gastrointestinal tract for possible malignancy.
G. morbillorum typically affects the mitral and aortic valve in equal distributions, but can involve the pulmonary valve in structurally normal hearts.
Gemella can cause significant valvular destruction and embolic phenomena thereby requiring surgery, thus a vigorous attempt to culture and accurately identify such organisms as early as possible in their presentation is warranted.
Footnotes
Twitter: @AnishDesai15
Contributors: Substantial contributions by both AKD and EMMB. Literature search and review, manuscript planned and composed by AKD with significant edits contributed by EMMB, the senior author who was part of the patient’s original treatment team. Idea for the article was composed by EMMB.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015;65:2070–6. 10.1016/j.jacc.2015.03.518 [DOI] [PubMed] [Google Scholar]
- 2.Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International collaboration on Endocarditis-Prospective cohort study. Arch Intern Med 2009;169:463-73. 10.1001/archinternmed.2008.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tleyjeh IM, Abdel-Latif A, Rahbi H, et al. A systematic review of population-based studies of infective endocarditis. Chest 2007;132:1025–35. 10.1378/chest.06-2048 [DOI] [PubMed] [Google Scholar]
- 4.Ruoff KL. Miscellaneous catalase-negative, gram-positive cocci: emerging opportunists. J Clin Microbiol 2002;40:1129–33. 10.1128/JCM.40.4.1129-1133.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kofteridis DP, Anastasopoulos T, Panagiotakis S, et al. Endocarditis caused by Gemella morbillorum resistant to beta-lactams and aminoglycosides. Scand J Infect Dis 2006;38:1125–7. 10.1080/00365540600740538 [DOI] [PubMed] [Google Scholar]
- 6.Jayananda S, Gollol-Raju NS, Fadul N. Gemella Species Bacteremia and Stroke in an Elderly Patient with Respiratory Tract Infection. Case Rep Med 2017;2017:1–2. 10.1155/2017/1098527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li D, Zhu Z, Zheng X, et al. Gemella morbillorum endocarditis of pulmonary valve:a case report. J Cardiothorac Surg 2017;12:16. 10.1186/s13019-017-0579-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hikone M, Sakamoto N, Ota M, et al. The first case report of infective endocarditis caused by Gemella taiwanensis. J Infect Chemother 2017;23:567–71. 10.1016/j.jiac.2017.02.012 [DOI] [PubMed] [Google Scholar]
- 9.AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs, Pettersson GB, Coselli JS, et al. 2016 the American association for thoracic surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: Executive summary. J Thorac Cardiovasc Surg 2017;153:1241–58. 10.1016/j.jtcvs.2016.09.093 [DOI] [PubMed] [Google Scholar]
- 10.Gauduchon V, Chalabreysse L, Etienne J, et al. Molecular diagnosis of infective endocarditis by PCR amplification and direct sequencing of DNA from valve tissue. J Clin Microbiol 2003;41:763–6. 10.1128/JCM.41.2.763-766.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Scola B, Raoult D. Molecular identification of Gemella species from three patients with endocarditis. J Clin Microbiol 1998;36:866–71. 10.1128/JCM.36.4.866-871.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maraki S, Plevritaki A, Kofteridis D, et al. Bicuspid aortic valve endocarditis caused by Gemella sanguinis: case report and literature review. J Infect Public Health 2019;12:304–8. 10.1016/j.jiph.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 13.Woo PCY, Lau SKP, Fung AMY, et al. Gemella bacteraemia characterised by 16S ribosomal RNA gene sequencing. J Clin Pathol 2003;56:690–3. 10.1136/jcp.56.9.690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boujelben I, Gdoura R, Hammami A. A broad-range PCR technique for the diagnosis of infective endocarditis. Braz J Microbiol 2018;49:534–43. 10.1016/j.bjm.2017.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosa RG, Rosa MD, Ascoli AM, et al. Cardiogenic shock due to Gemella morbillorum native mitral valve endocarditis. Clin Case Rep 2015;3:342–4. 10.1002/ccr3.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dingle TC, Butler-Wu SM. Maldi-Tof mass spectrometry for microorganism identification. Clin Lab Med 2013;33:589–609. 10.1016/j.cll.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 17.Buu-Hoï A, Sapoetra A, Branger C, et al. Antimicrobial susceptibility of Gemella haemolysans isolated from patients with subacute endocarditis. Eur J Clin Microbiol 1982;1:102–6. 10.1007/BF02014200 [DOI] [PubMed] [Google Scholar]
- 18.Ural S, Gul Yurtsever S, Ormen B, et al. Gemella morbillorum endocarditis. Case Rep Infect Dis 2014;2014:456471. 10.1155/2014/456471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Revilla A, López J, Villacorta E, et al. Isolated right-sided valvular endocarditis in non-intravenous drug users. Rev Esp Cardiol 2008;61:1253–9. 10.1016/s1885-5857(09)60052-9 [DOI] [PubMed] [Google Scholar]
- 20.Youssef D, Youssef I, Marroush TS, et al. Gemella endocarditis: a case report and a review of the literature. Avicenna J Med 2019;9:164–8. 10.4103/AJM.AJM_3_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Dupla M, Creus C, Navarro O, et al. Association of Gemella morbillorum endocarditis with adenomatous polyps and carcinoma of the colon: case report and review. Clin Infect Dis 1996;22:379–80. 10.1093/clinids/22.2.379 [DOI] [PubMed] [Google Scholar]
- 22.Bell E, McCartney AC. Gemella morbillorum endocarditis in an intravenous drug abuser. J Infect 1992;25:110–2. 10.1016/0163-4453(92)93753-D [DOI] [PubMed] [Google Scholar]
- 23.Kerr JR, Webb CH, McGimpsey JG, et al. Infective endocarditis due to Gemella morbillorum complicating hypertrophic obstructive cardiomyopathy. Ulster Med J 1994;63:108–10. [PMC free article] [PubMed] [Google Scholar]
- 24.Terada H, Miyahara K, Sohara H, et al. Infective endocarditis caused by an Indigenous bacterium (Gemella morbillorum). Intern Med 1994;33:628–31. 10.2169/internalmedicine.33.628 [DOI] [PubMed] [Google Scholar]
- 25.Martin MJ, Wright DA, Jones AR. A case of Gemella morbillorum endocarditis. Postgrad Med J 1995;71:188. 10.1136/pgmj.71.833.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasishtha S, Isenberg HD, Sood SK. Gemella morbillorum as a cause of septic shock. Clin Infect Dis 1996;22:1084–6. 10.1093/clinids/22.6.1084 [DOI] [PubMed] [Google Scholar]
- 27.Nandakumar R, Raju G. Isolated tricuspid valve endocarditis in nonaddicted patients: a diagnostic challenge. Am J Med Sci 1997;314:207–12. 10.1097/00000441-199709000-00011 [DOI] [PubMed] [Google Scholar]
- 28.Farmaki E, Roilides E, Darilis E, et al. Gemella morbillorum endocarditis in a child. Pediatr Infect Dis J 2000;19:751–3. 10.1097/00006454-200008000-00015 [DOI] [PubMed] [Google Scholar]
- 29.Purcell LK, Finley JP, Chen R, et al. Gemella species endocarditis in a child. Can J Infect Dis 2001;12:317–20. 10.1155/2001/960734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akiyama K, Taniyasu N, Hirota J, et al. Recurrent aortic valve endocarditis caused by Gemella morbillorum--report of a case and review of the literature. Jpn Circ J 2001;65:997–1000. 10.1253/jcj.65.997 [DOI] [PubMed] [Google Scholar]
- 31.Zakir RM, Al-Dehneh A, Dabu L, et al. Mitral bioprosthetic valve endocarditis caused by an unusual microorganism, Gemella morbillorum, in an intravenous drug user. J Clin Microbiol 2004;42:4893–6. 10.1128/JCM.42.10.4893-4896.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gimigliano F, Carletti M, Carducci G, et al. Gemella morbillorum endocarditis in a child. Pediatr Infect Dis J 2005;24:190. 10.1097/01.inf.0000153173.47612.ab [DOI] [PubMed] [Google Scholar]
- 33.Murai M, Fukumoto H, Negoro N, et al. Evidence of active endocarditis, caused by Gemella morbillorum, related to acute embolic stroke. Int J Cardiol 2006;112:E17–18. 10.1016/j.ijcard.2006.01.071 [DOI] [PubMed] [Google Scholar]
- 34.Al-Hujailan G, Lagacé-Wiens P. Mechanical valve endocarditis caused by Gemella morbillorum. J Med Microbiol 2007;56:1689–91. 10.1099/jmm.0.47389-0 [DOI] [PubMed] [Google Scholar]
- 35.Zheng M, Ng OT, Teo BW. Aortic and mitral valve endocarditis caused by Gemella morbillorum in a haemodialysis patient. Singapore Med J 2008;49:e385–7. [PubMed] [Google Scholar]
- 36.Al Chekakie MO, Heroux A, Montpetit M, et al. Gemella morbillorum prosthetic valve endocarditis. Congest Heart Fail 2009;15:291–2. 10.1111/j.1751-7133.2009.00061.x [DOI] [PubMed] [Google Scholar]
- 37.Taimur S, Madiha R, Samar F, et al. Gemella morbillorum endocarditis in a patient with a bicuspid aortic valve. Hellenic J Cardiol 2010;51:183–6. [PubMed] [Google Scholar]
- 38.Massoure P-L, Lions C, Caumes J-L, et al. [Lethal aortic endocarditis due to Gemella morbillorum in a Djiboutian khat user]. Rev Med Interne 2010;31:e7–9. 10.1016/j.revmed.2009.07.021 [DOI] [PubMed] [Google Scholar]
- 39.Carano N, Agnetti A, Allegri V, et al. Infective endocarditis following body piercing: presentation of one case due to Gemella morbillorum and review of the literature. Med Sci Monit 2010;16:CS124–8. [PubMed] [Google Scholar]
- 40.Hull JE. Multisystem organ failure due to Gemella morbillorum native valve endocarditis. Mil Med 2010;175:923–5. 10.7205/MILMED-D-10-00121 [DOI] [PubMed] [Google Scholar]
- 41.Godinho AR, Tomé E, Vaz A, et al. [Gemella endocarditis: an aggressive entity]. Rev Port Cardiol 2013;32:1027–30. 10.1016/j.repc.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 42.Agrawal N, Kariyappa M, Kolhari VB, et al. Cauliflower-like deformation of pulmonary valve in a case of infective endocarditis by a rare organism: Gemella morbillorum. BMJ Case Rep. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolhari VB, Kumar VV, Agrawal N, et al. Gemella morbillorum endocarditis in hypertrophic cardiomyopathy: a rare organism causing a large vegetation and abscess in an uncommon setting. Case Rep Child Meml Hosp Chic 2014;2014:bcr2014203951. 10.1136/bcr-2014-203951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shahani L. Gemella morbillorum prosthetic aortic valve endocarditis. BMJ Case Rep 2014;2014. 10.1136/bcr-2014-207304. [Epub ahead of print: 18 Nov 2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Constantinos M, Marios S. Gemella morbillorum tricuspid valve endocarditis resulting in septic pulmonary emboli in a patient with intracranial hemorrhage. Int J Cardiol 2015;184:769–71. 10.1016/j.ijcard.2015.02.094 [DOI] [PubMed] [Google Scholar]
- 46.Shinha T. Endocarditis due to Gemella morbillorum. Intern Med. 2017;56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar G, Al Ali AS, Gulzar Bhatti N. Rare bacteria infecting the heart and affecting the kidney of a young child. Case Rep Nephrol Dial 2017;7:138–43. 10.1159/000484474 [DOI] [PMC free article] [PubMed] [Google Scholar]