Abstract
A 62-year-old woman with human T-lymphotropic virus type 1 cell lymphoma developed heart failure after mogamulizumab, an immunotherapy agent. Clinical presentation and cardiac magnetic resonance imaging were consistent with myocarditis, and a recurrence of heart failure occurred after rechallenge with the therapy. (Level of Difficulty: Advanced.)
Key Words: acute heart failure, cancer, cardiomyopathy, MR sequences
Abbreviations and Acronyms: CCR4, C-X-C chemokine receptor type 4; CHOPE, cyclophosphamide, doxorubicin, vincristine, prednisone, and etoposide; CMR, cardiac magnetic resonance; ECG, electrocardiogram; ICI, checkpoint inhibitor; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; PE, pulmonary embolus; Treg, T regulatory cells; TTE, transthoracic echocardiogram
Central Illustration
History of Presentation
A 62-year-old woman presented with weakness and was found to have hypercalcemia and a 4.7-cm anterior mediastinal mass with lymphadenopathy. She underwent lymph node and bone marrow biopsy that led to the diagnosis of T-cell lymphoma with staging showing bone and central nervous system involvement. She had a transthoracic echocardiogram (TTE) that showed a left ventricular ejection fraction (LVEF) of 61%, mild concentric left ventricular hypertrophy, and no significant abnormalities. She received multiple rounds of chemotherapy, including 6 cycles of cyclophosphamide, doxorubicin, vincristine, prednisone, and etoposide (CHOPE), but was refractory to treatment with subsequent development of bone and soft tissue lesions. Repeat TTE post-CHOPE showed a normal LVEF of 73% (Videos 1 and 2). She next underwent chemotherapy with 3 cycles of etoposide phosphate, prednisone, vincristine, cyclophosphamide, and doxorubicin; however, positron emission tomography/computed tomography showed progression of skeletal metastases. She was subsequently enrolled in a mogamulizumab trial, a humanized immunoglobulin G1 monoclonal antibody that targets C-X-C chemokine receptor type 4 (CCR4), which was recently approved for treatment of T-cell lymphoma (1).
Learning Objectives
-
•
To recognize a cardiac side effect of a novel immunotherapy agent in a cancer patient to decrease cardiovascular complications from the therapy.
-
•
To use CMR for evaluation of cardiomyopathy in a cancer patient to diagnose the underlying process and make informed recommendations for treatment.
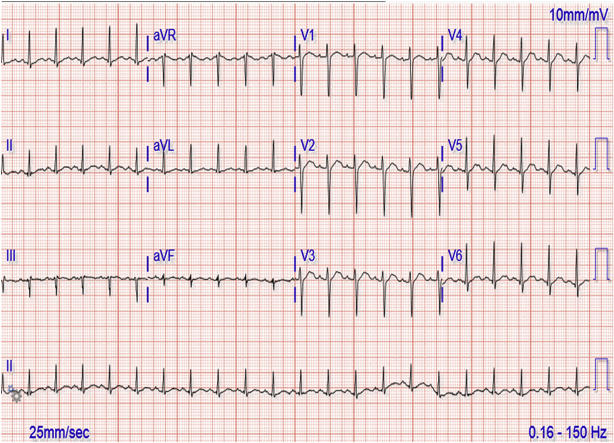
After the first infusion of mogamulizumab, she became febrile to 101°F, tachypneic, hypotensive, tachycardic at 130 beats/min, had saturation of 100% on room air, and experienced rigors, chills, and chest pain that prompted a visit to the emergency department. In the emergency department, her electrocardiogram (ECG) showed sinus tachycardia with new nonspecific ST-segment and T-wave changes (Figure 1). D-dimer was elevated to 14.3 mg/l, troponin I was elevated at 0.29 ng/ml, with subsequent troponin T at 0.10 ng/ml. N-terminal pro-hormone brain natriuretic peptide was elevated at 14,548 pg/ml. TTE on this admission demonstrated a newly depressed LVEF of 32% with global hypokinesis, prompting a cardiology consultation.
Figure 1.
Electrocardiogram Showing Sinus Tachycardia
Past Medical History
The patient has a history of hypertension and remote ovarian cancer, for which she underwent total abdominal hysterectomy and salpingo-oophorectomy.
Differential Diagnosis
Because of the temporal timing of the new immunotherapy agent, global hypokinesis, and clinical presentation, the mostly likely diagnosis was believed to be a side effect of the new cancer therapy or potentially a stress cardiomyopathy.
Investigations
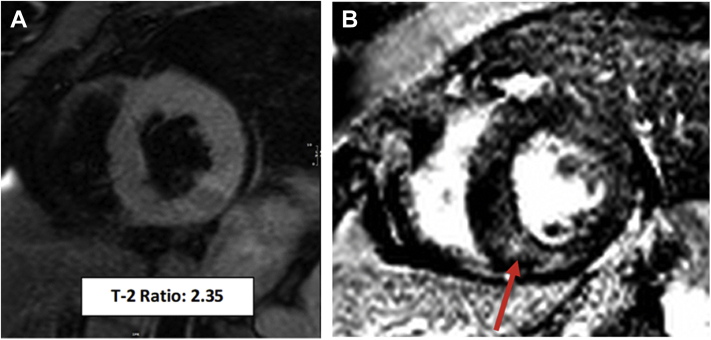
At her cardiology evaluation, her fever, tachypnea, and hypotension had resolved. Because of her malignancy and elevated D-dimer, she underwent chest computed tomography, which showed a possible pulmonary embolus (PE). Her examination by cardiology demonstrated a regular rhythm, absence of murmurs, rubs or gallops, decreased breath sounds at the bases, normal jugular venous pressure, and 1+ pitting edema bilaterally. Because of the elevated troponin and ECG and TTE changes, the patient underwent cardiac magnetic resonance (CMR) imaging. The CMR demonstrated decreased systolic function with a LVEF of 37% and a mildly reduced right ventricular ejection fraction of 47% (Video 3). There was diffusely increased signal intensity on T2-weighted images, with a T2 myocardial to skeletal muscle ratio of 2.4 (normal ≤1.9) (Figure 2A), which suggested diffuse myocardial inflammation. Late gadolinium enhancement (LGE) images demonstrated patchy mid-myocardial delayed enhancement throughout multiple areas (Figure 2B) that were consistent with a nonischemic cardiomyopathic process and with a quantitative LGE burden of 13%. These CMR findings were most consistent with myocarditis. Considering the temporal relationship of symptoms, laboratory, and imaging findings to mogamulizumab infusion, myocarditis was believed to have been caused by this immunotherapy agent.
Figure 2.
Cardiac Magnetic Resonance Obtained After Acute Heart Failure Following First Dose of Mogamulizumab
(A) Short axis T-2 weighted image showing diffuse high-signal intensity consistent with myocardial edema. (B) Single-shot, short-axis late-gadolinium enhancement image showing hazy mid-myocardial delayed enhancement in the basal inferoseptal wall (arrow).
Management
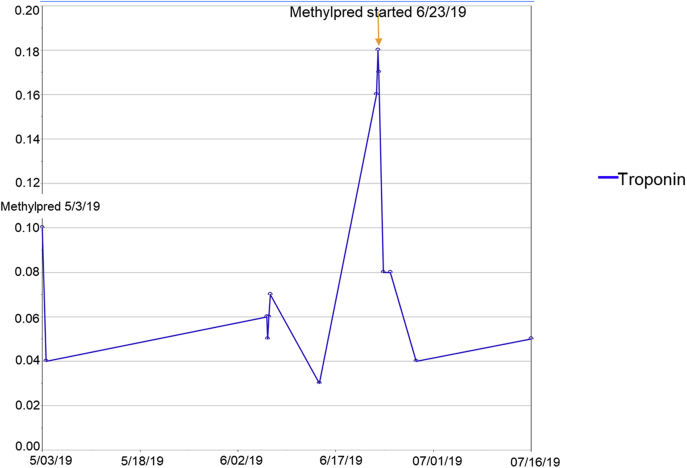
For her possible PE, because of known brain metastases, the decision was to not anticoagulate due to concern for intracranial bleeding. For possible immune therapy−mediated myocarditis, checkpoint inhibitor (ICI)−mediated myocarditis management was applied, and the patient was started on high-dose steroids. The patient was treated with 3 days of pulse dose steroids (1 g of methylprednisolone daily for 3 days) (2, 3, 4). With steroid therapy, the troponins down trended (Figure 3).The patient was discharged on a steroid taper and beta-blocker with follow-up in the cardio-oncology clinic. A repeat CMR 3 weeks after steroids showed interval improvement of LVEF from 37% to 45% and a similar LGE extent and pattern (Video 4). In addition, monitoring chest computed tomography found a new PE, for which the patient was started on therapeutic enoxaparin.
Figure 3.
Troponin Trend and Response to Steroids
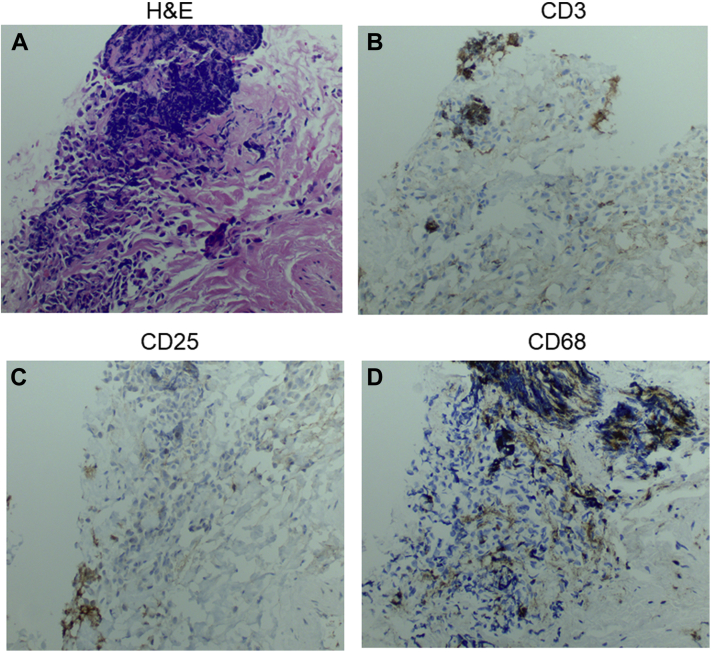
In light of the patient’s advanced cancer and lack of other treatment options, the patient received another dose of mogamulizumab, and 3 weeks later re-presented with tachycardia, hypotension, and undifferentiated shock. Infectious workup revealed a urinary source, for which she was treated with antibiotics. However, repeat TTE showed worsening of the LVEF to 24%. The main diagnostic differential for the decline in LVEF at this time included further immune-mediated cardiomyopathy due to mogamulizumab or potentially stress-induced cardiomyopathy. For concern of recurrent and/or progressive suspected immune-mediated myocarditis, the patient was once again started on pulse dose steroids with methylprednisolone 1 g/day for 3 days and continued on a steroid taper. She then underwent right heart catheterization, with an endomyocardial biopsy showing an elevated pulmonary capillary wedge pressure of 22 mm Hg and a cardiac output of 4.6 l with a cardiac index of 2.79. Pathology specimens showed fibrous tissue without myocardial tissue identified. Hematoxylin and eosin stain showed cell aggregates (Figure 4A). The focal lymphoid aggregates of clusters had positive staining for CD3+, CD25+, and CD68+ cells in non-overlapping areas (Figures 4B to 4D), which was deemed to have uncertain significance. Overall findings were believed to be nondiagnostic, although could be considered suggestive of myocarditis.
Figure 4.
EMB Pathology
EMB pathology with (A) hematoxylin and eosin stain 5A, (B) CD3 stain 5B, (C) CD25 stain 5C, and (D) CD68 staining 5D at 20× magnification. EMB = endomyocardial biopsy.
Discussion
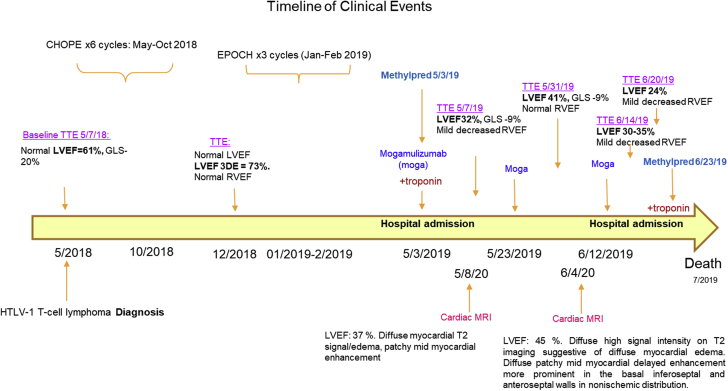
This is the first published case of likely CCR4 inhibitor−mediated myocarditis, based on the timing of the patient’s clinical presentation and course (Figure 5). CCR4 is a chemokine receptor expressed on circulating and tissue-resident T cells, as well as on other T-helper cells, including T-regulatory cells (Tregs). CCR4 is also highly expressed in T-cell lymphoma and has been shown to mediate cellular migration and proliferation (5,6). Thus, immune-mediated adverse effects on the heart may be due to immune cell activation and attack of the myocardium secondary to suppression of immune-suppressing cells such as Tregs. One other possibility could be increased tropism for T cells to the myocardium in the setting of CCR4 inhibition causing increased inflammation, cytokine release, and damage to the myocardium (6). Although extremely rare, human T-lymphotropic virus type 1 itself has been shown contribute to nonischemic cardiomyopathy (7).
Figure 5.
Clinical Events Timeline
CHOPE = cyclophosphamide, doxorubicin, vincristine, prednisone, and etoposide; EPOCH = etoposide phosphate, prednisone, vincristine, cyclophosphamide, and doxorubicin; HTLV-1 = human T-lymphotropic virus type 1; LVEF = left ventricular ejection fraction; RVEF = right ventricular ejection fraction.
Diagnosis of myocarditis normally requires clinical suspicion, positive biomarkers, imaging and/or biopsy. Sensitivity can be low for biopsy diagnosis of myocarditis (8) and may be low due to the patchy nature of myocardial involvement (9). Imaging such as CMR can help confirm the diagnosis, and our patient met the CMR Lake Louise criteria for myocarditis, with positive edema and LGE. Diagnostic criteria has been proposed for ICI-related myocarditis, which includes the use of CMR (10,11). Although there are no studies to guide treatment of CCR4 inhibitor−mediated myocarditis, support for steroid use was drawn from recommendations for ICI-mediated myocarditis (12). Additional therapies such as mycophenolate and monoclonal antibody therapies such as alemtuzumab could have been considered (13). Although the patient received previous chemotherapy agents, they were several months before heart failure presentation. However, previous agents might have contributed to subclinical cardiotoxicity, which was not readily identified.
Follow-up
The patient’s cancer continued to progress and given no further therapeutic options, the patient and family pursued hospice care and deferred additional CMR imaging to follow-up on myocarditis progression. The patient subsequently passed away, and the family declined autopsy.
Conclusions
This is the first published case of likely CCR4 inhibitor−mediated myocarditis, and ICI- mediated myocarditis management was applied. Evidence-based treatment guidelines are needed for CCR4 inhibition−mediated myocarditis. This case also demonstrated the usefulness of CMR in diagnosis of myocarditis, as well monitoring and response to therapy. In addition to mogamulizumab, other immunotherapy agents may be associated with myocarditis. Further surveillance and research are needed to understand the mechanisms behind cardiotoxicity and these immunotherapies.
Funding Support and Author Disclosures
This work was supported by American Heart Association (grant #18CDA34110361; LAB, 2018). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
TTE 4chamber view post CHOPE
TTE parasternal long axis view post CHOPE
Short axis stack cine during first heart failure presentation
Short axis stack cine after steroids 1 month after
References
- 1.Ollila T.A., Sahin I., Olszewski A.J. Mogamulizumab: a new tool for management of cutaneous T-cell lymphoma. Onco Targets Ther. 2019;12:1085–1094. doi: 10.2147/OTT.S165615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahmood S.S., Fradley M.G., Cohen J.V. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganatra S., Neilan T.G. Immune checkpoint inhibitor-associated myocarditis. Oncologist. 2018;23:879–886. doi: 10.1634/theoncologist.2018-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berlato C., Khan M.N., Schioppa T. A CCR4 antagonist reverses the tumor-promoting microenvironment of renal cancer. J Clin Invest. 2017;127:801–813. doi: 10.1172/JCI82976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugata K., Yasunaga J., Kinosada H. HTLV-1 Viral factor HBZ induces CCR4 to promote T-cell migration and proliferation. Cancer Res. 2016;76:5068–5079. doi: 10.1158/0008-5472.CAN-16-0361. [DOI] [PubMed] [Google Scholar]
- 6.Komarowska I., Coe D., Wang G. Hepatocyte growth factor receptor c-Met instructs T cell cardiotropism and promotes T cell migration to the heart via autocrine chemokine release. Immunity. 2015;42:1087–1099. doi: 10.1016/j.immuni.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abolbashari S., Ghayour-Mobarhan M., Ebrahimi M., Meshkat Z. The role of human T-lymphotropic virus (HTLV) in cardiovascular diseases: a review of literature. ARYA Atheroscler. 2018;14:183–187. doi: 10.22122/arya.v14i4.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott P., Arbustini E. The role of endomyocardial biopsy in the management of cardiovascular disease: a commentary on joint AHA/ACC/ESC guidelines. Heart. 2009;95:759–760. doi: 10.1136/hrt.2008.161166. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y.W., Zhu Y.J., Wang M.N. Immune checkpoint inhibitor-associated cardiotoxicity: current understanding on its mechanism, diagnosis and management. Front Pharmacol. 2019;10:1350. doi: 10.3389/fphar.2019.01350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonaca Marc P., Olenchock Benjamin A., Salem J.-E. Myocarditis in the setting of cancer therapeutics. Circulation. 2019;140:80–91. doi: 10.1161/CIRCULATIONAHA.118.034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyon A.R., Dent S., Stanway S. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020;22:1945–1960. doi: 10.1002/ejhf.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brahmer J.R., Lacchetti C., Schneider B.J. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esfahani K., Buhlaiga N., Thébault P., Lapointe R., Johnson N.A., Miller W.H. Alemtuzumab for immune-related myocarditis due to PD-1 therapy. N Engl J Med. 2019;380:2375–2376. doi: 10.1056/NEJMc1903064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TTE 4chamber view post CHOPE
TTE parasternal long axis view post CHOPE
Short axis stack cine during first heart failure presentation
Short axis stack cine after steroids 1 month after