Abstract
Left ventricular assist devices (LVADs) are surgically implanted mechanical devices indicated for patients with advanced heart failure and are known to come with several complications. Here we present a case series, and review 1 documented report, of LVAD vasculitis, a presumed new LVAD immune/humoral related phenomenon. (Level of Difficulty: Advanced.)
Key Words: autoimmune, cardiac assist devices, cardiomyopathy, cardiovascular disease, chronic heart failure, systolic heart failure, vascular disease
Abbreviations and Acronyms: AKI, acute kidney infection; C. jeikeium, Corynebacterium jeikeium; HLA, human leukocyte antigen; LCV, leukocytoclastic vasculitis; LVAD, left ventricular assist device; PIGN, postinfectious glomerulonephritis; S.aureus, Staphylococcus aureus
Central Illustration
Case 1
A 59-year-old man with nonischemic cardiomyopathy and a HeartMate II left ventricular assist device (LVAD) (Abbott Laboratories) (implanted 5 years before) presented with several days of weakness, fatigue, hemoptysis, and dyspnea.
Learning Objectives
-
•
To understand the importance of immediate skin biopsy in LVAD recipients who present with maculopapular/petechial rash, especially if underlying vasculitis is suspected.
-
•
To understand the need for thorough investigations in LVAD recipients who are diagnosed with any form of a vasculitis.
-
•
To be able to treat LVAD vasculitis (biopsy-confirmed, systemic vasculitis in LVAD recipients) through appropriate steroid/immune therapy, in combination with other interventions/therapies that target the etiology of the vasculitis.
Three months before, he was seen by a dermatologist for an unspecified maculopapular rash of the lower extremities, and later developed microscopic hematuria and acute kidney injury (AKI). On admission, he was without rash, but had AKI, a supratherapeutic international normalized ratio, and developed acute hypoxic respiratory failure requiring immediate mechanical ventilation. Imaging demonstrated pulmonary infiltration, bronchoscopy confirmed alveolar hemorrhage, and sampling revealed elevated p-antineutrophil cytoplasmic antibody levels (1:5,120) and positive reflexive myeloperoxidase testing. The patient received a diagnosis of secondary microscopic polyangiitis, and respiratory cultures grew methicillin-resistant Staphylococcus aureus (S.aureus) (appropriately treated with vancomycin). He later experienced spontaneous bleeding requiring transfusion, persistent respiratory failure requiring tracheostomy, and worsening renal failure (peak creatinine 3.3 mg/dL). Renal and pulmonary biopsies were not performed because of the patient’s unstable condition. He was initiated on steroids, intravenous immunoglobulin, and subsequent rituximab, and renal function normalized in 1 week. Unfortunately, the patient later developed Klebsiella bacteremia, septic shock, and multiorgan failure, and died (hospitalization 8 weeks).
Case 2
A 62-year-old man with ischemic cardiomyopathy and a HeartMate II LVAD (implanted 4 years before) and recurrent driveline infections (methicillin-sensitive S.aureus and Enterococcus faecium) on amoxicillin-clavulanate for long-term suppression presented to the hospital with a 1-week history of fevers and a 3-day rash. Petechial, maculopapular lesions were present on the lower extremities and buttocks. AKI was present, and erythrocyte sedimentation rate and C-reactive protein levels were elevated. In-hospital class I human leukocyte antigen (HLA) testing was positive for B7, B13, B27, B42, B48, B60, and B81, and was weakly positive for B8, B41, B55, B67, B76, and B82. Class II HLA testing was negative (no prior HLA testing). Skin biopsy revealed leukocytoclastic vasculitis (LCV) and blood cultures grew Corynebacterium jeikeium (C. jeikeium), which was appropriately treated with doxycycline. Positron emission tomography imaging showed new, increased moderate metabolic activity surrounding the LVAD inflow cannula. One week later, creatinine peaked at 4.1 mg/dL but steadily decreased after initiation of prednisone 60 mg daily. Renal biopsy was consistent with postinfectious glomerulonephritis (PIGN). The patient was discharged (hospitalization 5 weeks) on a course of prednisone and doxycycline. After 2 months, all symptoms and renal function normalized, and his health remained at baseline several months thereafter.
Case 3
A 70-year-old man with ischemic cardiomyopathy and a HeartMate III LVAD (implanted 1 year before), and stage IIIA chronic kidney disease with baseline creatinine of 2.2 mg/dL presented to the hospital with several days of chills, fatigue, lethargy, and gross hematuria. Three months before presentation he developed an unspecified maculopapular rash of the upper extremities, 2 months before presentation he was admitted with interstitial lung findings and was treated for presumed pneumonia, and 3 weeks before presentation he had hematochezia. The rash, dyspnea, and hematochezia resolved before his presentation. Admission diagnostics revealed anemia, AKI, and methicillin-sensitive S.aureus bacteremia, and he was appropriately treated with blood transfusions and cefazolin. After 10 days, he developed a petechial rash again, but was over the lower extremities. Skin biopsy revealed LCV. His renal function continued to gradually worsen (peak creatinine 6.98 mg/dL) requiring hemodialysis and renal biopsy demonstrated immunoglobulin A glomerulonephritis as a PIGN. Steroids were never started as AKI was believed to be solely infectious. He remained on dialysis and later developed an additional Klebsiella oxytoca infection and diffuse intravascular coagulation, and he died (hospitalization 14 weeks).
Discussion
LVADs are durable, surgically implanted mechanical devices that support the hearts of patients with advanced heart failure. However, these devices come with their own complications, such as hemodynamic alterations, thromboembolic phenomena, coagulopathy, and humoral sensitization (antibody development against specific human antigens) (1).
We present 3 LVAD recipients affected with vasculitis within the past 3 years at University Hospital Cleveland Medical Center (proper ethical oversight obtained). We defined LVAD vasculitis as the biopsy-confirmed presence of any vasculitis, with systemic (multiorgan) dysfunction, in an LVAD recipient. All 3 cases fit this description. All other predisposing factors or causes of vasculitis (hematologic, dermatologic, infectious, medication-induced, or connective tissue diseases and malignancy) were ruled out through thorough history, examination, and investigative laboratory data/cultures. Transesophageal echocardiography ruled out endocarditis. All positive tests are reported in the previous text. All patients were on the same medications for several months to years, with no new medications before initial symptoms.
All patients developed rash before hospitalization and vasculitis (all infectious-related) with systemic end-organ dysfunction (all with renal failure). All clinical courses, however, were not uniform. The initial rashes of patients 1 and 3 were not biopsied on outpatient evaluations. Additionally, they were without in-hospital HLA testing, although prior testing was negative. In our third case, the patient’s rash reoccurred (days after cefazolin) and was biopsied. Although medication-induced vasculitis was considered, there was no improvement in end-organ dysfunction even weeks after discontinuing cefazolin, and skin biopsy demonstrated predominant neutrophilic infiltration (without predominant mononuclear infiltrates or prominent eosinophilia) (2) consistent with LCV.
Despite variations in cases 1 and 3, both presented months after the initial rash, with multiorgan failure and subsequent mortality. If skin biopsies were performed and steroid/immunotherapy was implemented sooner, less ambiguity would prevail, with potentially less fatalities. Thus, we share this first case series on LVAD vasculitis to highlight this under-recognized and rare phenomenon, because there are no current guidelines on its evaluation or management.
Vasculitis
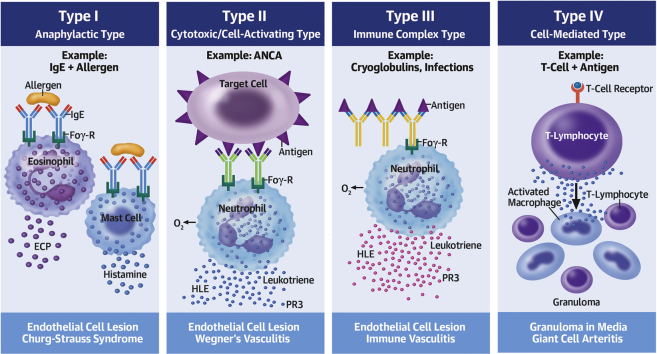
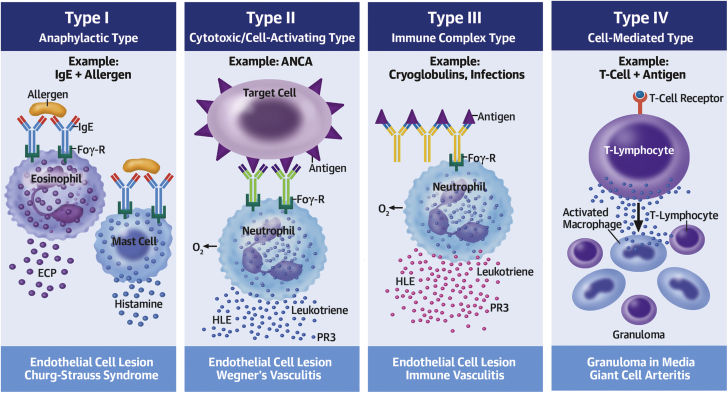
Vasculitis is any inflammatory vessel disease that is definitively diagnosed on biopsy. A maculopapular rash is a heralding sign, and rash was noted first in all of our cases, and in one other documented report of LVAD vasculitis (3). LCV is a small-vessel vasculitis defined as neutrophilic inflammation, which results from immune complex deposition (type III reaction, Figure 1) and can be idiopathic or caused by other systemic vasculitides, connective tissue diseases, malignancy, paraprotein disorders, infections, or medications. LCV can be localized to the skin, or can be systemic involving the skin and other organ(s), with mainstay treatment including steroids (4).
Figure 1.
Vasculitis and the Related Types of Immune-Mediated Interactions
© 2013 Choucair J. Published in Updates in the Diagnosis and Treatment of Vasculitis under CC BY 3.0 license. Available from: http://dx.doi.org/10.5772/55189. ANCA = antineutrophilic cytoplasmic antibody; ECP = eosinophilic cation protein; Eos = eosinophil; FcƐR = immunoglobulin E receptor; FcyR = immunoglobulin G receptor; HLE = human leukocyte elastase; IgE = immunoglobulin E; Ly = lymphocyte; M0 = macrophage; O2- = superoxide/oxygen free radical; PR3 = proteinase 3; T-Ly = T-lymphocyte.
Microscopic polyangiitis is a type II systemic, myeloperoxidase-antineutrophilic cytoplasmic antibody–associated vasculitis. It has several causes, but infections are a major differential (5), and therapy includes steroids and immunomodulators (eg, rituximab).
To further evaluate the relationship between vasculitis and LVAD recipients, we queried a large, multi-institutional electronic health care database (Explorys). The Explorys platform provides records from 26 major US health care systems with over 50 million patients. For our retrospective cohort analysis of baseline characteristics (Table 1), of the 4,130 LVAD recipients analyzed, 340 had vasculitis. Higher proportions of cardiovascular disease, smoking, and sepsis were noted in the vasculitis group. As this trend of risk in these LVAD recipients is similar to that in the general population, we found further credence toward infection as the primary etiology of LVAD vasculitis.
Table 1.
Characteristics of 4,130 LVAD Recipients From 26 Hospital Systems in the IBM Explorys Database
| LVAD Recipient and No Vasculitis (n = 3,790) | LVAD Recipient and Vasculitis (n = 340) | P Value | |
|---|---|---|---|
| Age >65 y | 1,860 (49) | 180 (53) | 0.17 |
| Male | 2,740 (72) | 270 (79) | 0.0047 |
| White | 2,810 (74) | 280 (82) | 0.00001 |
| Coronary artery disease | 2,860 (75) | 290 (85) | 0.000045 |
| Myocardial infarction | 3,470 (59) | 220 (65) | 0.00001 |
| Stroke | 720 (19) | 100 (29) | 0.00001 |
| Diabetes | 1,910 (50) | 230 (68) | 0.00001 |
| Hypertension | 3,100 (82) | 320 (94) | 0.00001 |
| Smoker | 1,670 (44) | 170 (50) | 0.035 |
| Sepsis | 1,050 (28) | 160 (47) | 0.00001 |
Values are n (%).
LVAD = left ventricular assist device.
Infections and potential immune factors
The impact of LVAD implantation on the cell-mediated and humoral immune systems is speculated to be one factor that predisposes recipients to infectious complications (Figure 1). Infections are associated with immune-mediated interactions (commonly type III) in certain vasculitides through the stimulation of antigen presentation, B cells, and prime neutrophils (6,7). Thus, one may deduce that the cumulative presence of an LVAD and an infectious state augment humoral stimulation/sensitization, creating the ideal milieu for LVAD vasculitis. However, this presumption requires further investigation, and to date, there is no known/established relationship between class I HLA and LCV.
In all noted LVAD cases with LCV (3), acute gram-positive infection was the determined cause. C.jeikeium is known to be a multidrug resistant organism that may cause various infections (8). However, no cases have reported C.jeikeium causing LCV, let alone in an LVAD recipient. Furthermore, as LCV results from immune complex-mediated inflammation and PIGN from immune complex glomerular injury, we presume type III interactions in all LCV cases (3), as all involved an infectious etiology.
Renal failure
In our series, when AKI was present, daily increases in creatinine averaged from 0.2-0.5 mg/dL. Full renal recovery occurred in 2 of 3 patients, both of whom received steroids/immunological therapy in addition to antibiotics. With steroid/immune therapy, there was immediate, consistent daily improvement until normalization, while the lack thereof led to persistent renal failure (3).
Mortality
Including the case by Bunker et al. (3), all but 1 patient died of LVAD vasculitis. The characteristics associated with survival among these patients include early presentation, early biopsy, and combined antibiotic and steroid/immune therapy. Considering the little known of LVAD vasculitis and its current high mortality rates, awareness is imperative.
Conclusions
To date, LVAD vasculitis remains a poorly described and under-recognized phenomenon. Of the aforementioned reported and documented cases of LVAD vasculitis (3) (most common type: LCV), all had rash as a heralding sign, all had acute infection (most common type gram positive), all had renal failure (most common type PIGN), and all but 1 died (mean hospitalization 9 weeks).
Given the observed clinical courses and mortality, we recommend that all LVAD recipients with a new petechial/maculopapular rash undergo immediate biopsy with no delay. We also recommend a full course of steroid/immunotherapy in addition to antibiotics for infection-induced LVAD vasculitis.
Funding Support and Author Disclosures
Dr Garcia is supported by the National Heart, Lung, and Blood Institute, Institutes of Health, under Aware Number 5T32HL110837. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
In the highest forms of honor and regard to all of our patients and their families, the authors acknowledge their very meaningful lives and what they have added to our lives, and what they will yet add to life itself every time we remember and reflect on them.
Footnotes
This work was accepted as an abstract to ISHLT 2020 with the ePoster title of “Vasculitis With Acute Renal Failure In Left Ventricular Assist Device (LVAD) Patients And Associated Mortality.” All 3 patients are unable to provide letters of permission as they are now deceased. Attempts were made to determine and access active contact information to family members of all patients; however, we were unsuccessful and thus unable to attain any other means of permission. As a result, proper ethical oversight was obtained.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Drakos S.G., Kfoury A.G., Kotter J.R. Prior human leukocyte antigen-allosensitization and left ventricular assist device type affect degree of post-implantation human leukocyte antigen-allosensitization. J Heart Lung Transplant. 2009;28(8):838–842. doi: 10.1016/j.healun.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahrami S., Malone J.C., Webb K.G. Tissue eosinophilia as an indicator of drug-induced cutaneous small-vessel vasculitis. Arch Dermatol. 2006;142(2):155–161. doi: 10.1001/archderm.142.2.155. [DOI] [PubMed] [Google Scholar]
- 3.Bunker D.R., Sullivan T. A case of leukocytoclastic vasculitis caused by Listeria monocytogenes bacteremia. Case Rep Infect Dis. 2016;2016:1093453. doi: 10.1155/2016/1093453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouiller K., Audia S., Devilliers H. Etiologies and prognostic factors of leukocytoclastic vasculitis with skin involvement: a retrospective study in 112 patients. Medicine (Baltimore) 2016;95(28):e4238. doi: 10.1097/MD.0000000000004238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choucair J. Infectious causes of vasculitis. In: Sakkas L., editor. Updates in the Diagnosis and Treatment of Vasculitis. IntechOpen; 2013. [Google Scholar]
- 6.Ooi J.D., Jiang J.-H., Eggenhuizen P.J. A plasmid-encoded peptide from Staphylococcus aureus induces anti-myeloperoxidase nephritogenic autoimmunity. Nat Commun. 2019;10(1):3392. doi: 10.1038/s41467-019-11255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García-Porrúa C., González-Gay M.A. Bacterial infection presenting as cutaneous vasculitis in adults. Clin Exp Rheumatol. 1999;17(4):471–473. [PubMed] [Google Scholar]
- 8.Bechara C., Gousseff M., Passeron A. Corynebacterium jeikeium pacemaker infection associated with antineutrophil cytoplasmic antibodies: a single positive blood culture could be sufficient for diagnosis. J Med Microbiol. 2011;60(Pt 2):249–251. doi: 10.1099/jmm.0.023283-0. [DOI] [PubMed] [Google Scholar]