Abstract
This report presents a rare case of acute decompensated heart failure with technetium-99m-pyrophosphate accumulation in extracardiac sites, such as chest and abdominal walls, in addition to intense myocardial uptake of the tracer. Subsequently, an abdominal fat pad fine-needle aspiration biopsy, which provided positive findings for transthyretin amyloidosis, was performed. (Level of Difficulty: Advanced.)
Key Words: abdominal fat, amyloidosis, bone-avid radiotracer, heart failure, transthyretin
Abbreviations and Acronyms: ADHF, acute decompensated heart failure; ATTR-CM, transthyretin cardiac amyloidosis; CT, computed tomography; DPD, 3,3-diphosphono-1,2-propanedicarboxylic acid; HMDP, hydroxymethylene diphosphonate; LV, left ventricle; MR, mitral regurgitation; PYP, pyrophosphate; SPECT, single photon emission computed tomography; 99mTc, technetium-99m
Central Illustration
History of Presentation
An 89-year-old Japanese woman was admitted to our hospital with a history of peripheral edema and dyspnea at rest; the oxygen saturation in room air was 80%, pulse rate 73 beats/min with an irregular rhythm, systemic blood pressure 116/73 mm Hg, and respiratory rate 14 breaths/min with oxygen saturation of 93% at a flow rate of 2 l/min over an oxygen mask. On auscultation, there was a third heart sound and a systolic regurgitant murmur; wet rales in the lung fields were audible. The liver was palpable by a 2-finger breadth below the costal margin. Marked pretibial edema and jugular venous distension were observed.
Learning Objectives
-
•
To identify electrocardiogram and echocardiographic changes over time that are “red flags” for cardiac amyloidosis, which may occur at the timing of the subsequent ADHF in patients with chronic heart failure.
-
•
To appropriately regulate the dosage of the antihypertensive and heart-failure drugs, such as calcium-channel blockers or renin-angiotensin-aldosterone system inhibitors, which are often poorly tolerated in cardiac amyloidosis.
Past Medical History
The patient’s medical history included long-standing hypertension, and Alzheimer’s disease resulting in a gradual cognitive decline; however, she had no history indicating a high index of suspicion for cardiac amyloidosis, such as carpal tunnel syndrome, lumbar spinal stenosis, biceps tendon rupture, or autonomic dysfunction.
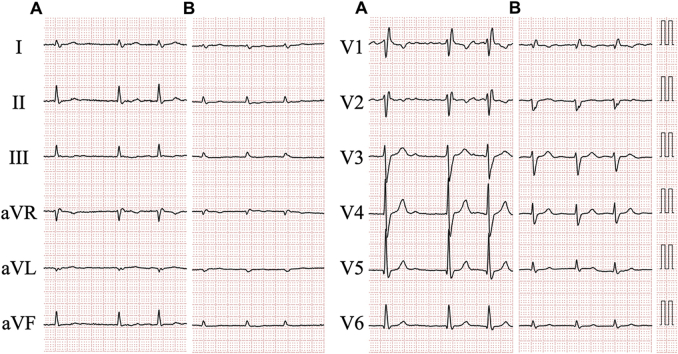
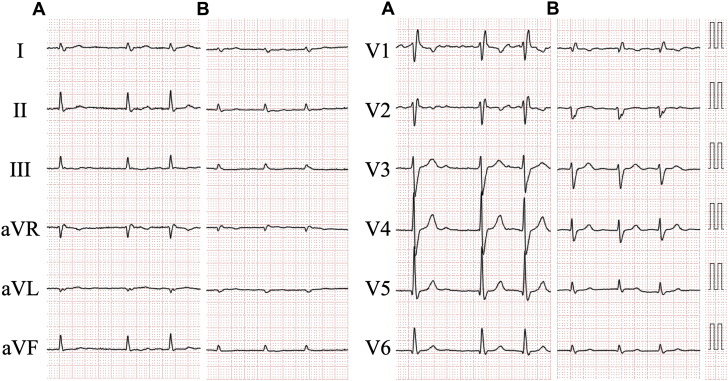
Four years prior, she had been admitted due to acute decompensated heart failure (ADHF). An electrocardiogram revealed atrial fibrillation and complete right bundle branch block (Figure 1A). The echocardiogram demonstrated severe mitral regurgitation (MR) due to chordal rupture, a dilated left ventricle (LV) with end-diastolic dimension of 59.5 mm, dilated left atrium with volume of 88.1 ml/m2, and normal interventricular septal wall thickness of 9.3 mm (Figure 2A). A mildly reduced LV ejection fraction of 46%, and slightly abnormal global LV longitudinal strain of −15.3% with apical-to-basal longitudinal strain ratio of 1.2 were observed. The patient had been diagnosed with ADHF due to severe MR resulting from chordal rupture, complicated by atrial fibrillation. Surgical repair of MR was not performed. She was discharged on 5 mg apixaban, 30 mg azosemide, 8 mg candesartan cilexetil, 25 mg spironolactone, and 2.5 mg amlodipine besylate daily.
Figure 1.
Electrocardiograms Recorded Before and at Admission
Electrocardiogram recorded 4 years before (A) and at admission (B).
Figure 2.
Transthoracic Echocardiogram
Echocardiograms recorded 4 years before (A) and at admission (B to E). (C) The bull’s eye map (with apex at the center of the color-coding map) illustrates segmental longitudinal left ventricular (LV) peak systolic strain values of 16-segment model, which were semiautomatically generated by speckle-tracking analysis of 2-dimensional LV images acquired from apical 2-, 3-, and 4-chamber views, and shows a reduced global longitudinal LV peak systolic strain value, calculated as the mean of these 16 values, of −12.4% (marked with an asterisk) with an apical-to-basal strain ratio, calculated as apical septal longitudinal strain divided by average basal septal (anteroseptal and inferoseptal) longitudinal strain, of 2.2 indicating apical sparing (>2.1). Two separate jets of mitral regurgitation (MR), of which one directed toward the center of the left atrium due to atrial functional MR (small arrow) and another directed eccentrically in connection with the aorta due to chordal rupture (arrowheads), are shown. Asterisks and large arrows indicate pericardial effusion and torn chordae of the mitral valve, respectively.
Differential Diagnosis
The differential diagnosis included ADHF due to worsening MR or due to MR and another heart disease.
Investigations
Blood tests revealed a high-sensitivity cardiac troponin I level of 58.9 pg/mL and a brain natriuretic peptide level of 210.9 pg/mL, over the reference range (≤16.0 pg/mL and ≤18.4 pg/mL, respectively). The estimated glomerular filtration rate was 43 ml/min/1.73 m2. A chest radiograph displayed cardiomegaly with pulmonary congestion and massive pleural effusion. An electrocardiogram revealed atrial fibrillation with low voltage, right axis deviation, and incomplete right bundle branch block (Figure 1B). The height in R-waves was decreased in all leads compared with that 4 years prior (Figure 1A). The echocardiogram demonstrated a dilated LV with end-diastolic dimension of 55.5 mm, dilated left atrium with volume of 95.5 ml/m2, and an increase in the interventricular septum of 12.3 mm (Figure 2B). Mildly hypokinetic wall motions in the entire LV with an ejection fraction of 43% was accompanied by global LV longitudinal strain of −12.4% with apical-to-basal longitudinal strain ratio of 2.2 (Figure 2C). Ruptured chordae of the mitral valve with severe MR and pericardial effusion were also detected (Figures 2B, 2D, and 2E). The peak velocity across the tricuspid valve was 3.63 m/s, which is suggestive of pulmonary hypertension.
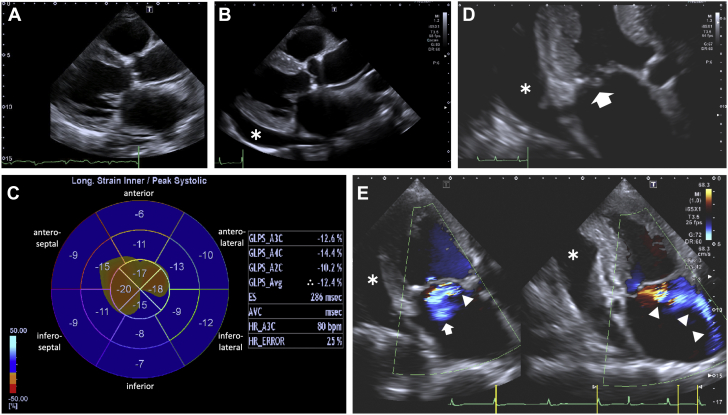
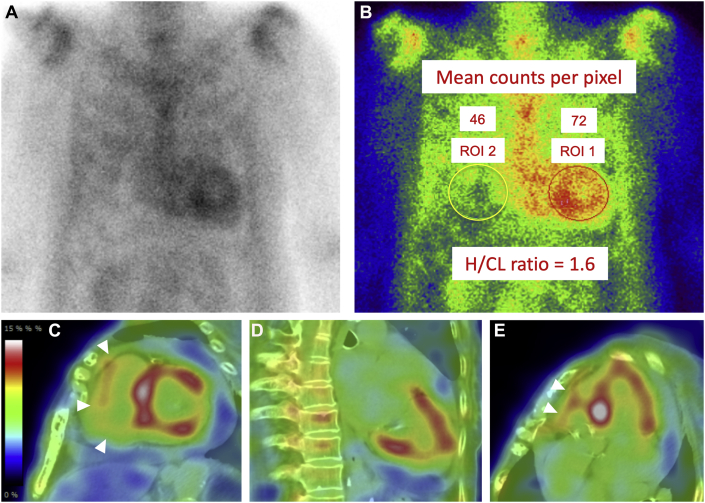
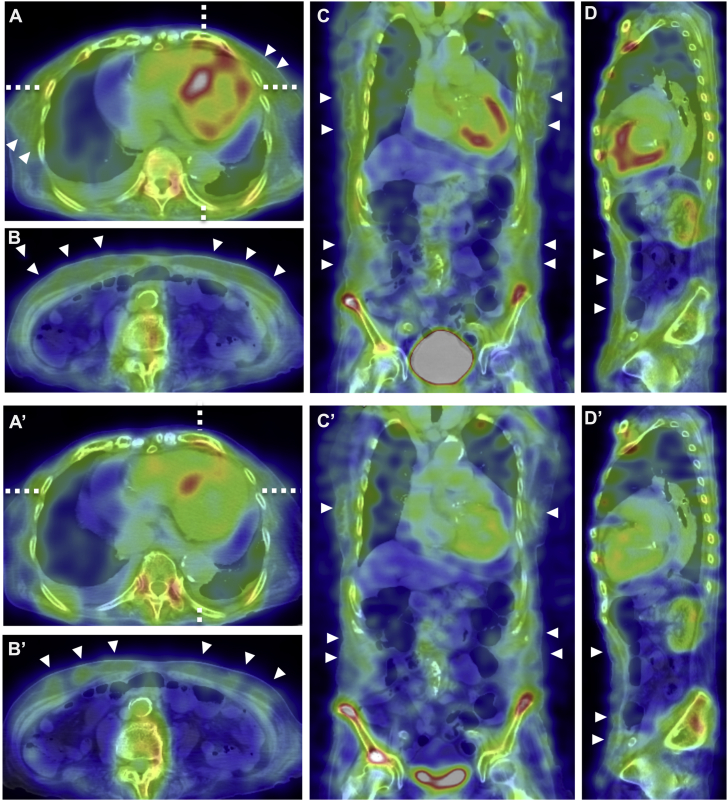
Because cardiac amyloidosis was suspected, technetium-99m (99mTc)-pyrophosphate (PYP) scintigraphy was performed. Chest-centered planar and single photon emission computed tomography (SPECT) images with fusion to computed tomography (CT) images were obtained 2 hours after intravenous injection of 20 mCi of the radiotracer. The acquisition protocol for both studies was as follows: end-expiratory breath-hold for CT imaging versus free-breathing for 99mTc-PYP SPECT imaging. A 99mTc-PYP scan showed grade 3 myocardial uptake with a heart/contralateral lung ratio of 1.6 (Figure 3). Marked accumulation of the tracer in the extracardiac sites, such as chest and abdominal walls, was observed (Figure 4). Due to this unexpected result in the 99mTc-PYP scan, SPECT images were obtained again 4 hours post intravenous injection of the radiotracer; most faded out, although some remained.
Figure 3.
99mTc-PYP Scintigrams Showing Biventricular Myocardial Uptake of the Tracer
Technetium-99m–pyrophosphate (99mTc-PYP) planar imaging (anteroposterior view) (A), semi-quantitative heart-to-contralateral lung (H/CL) ratio on planar imaging (B), and single photon emission computed tomographic images with fusion to computed tomographic images—short-axis view (C), vertical long-axis view (D), horizontal long-axis view (E)—obtained 2 h after injection of the radiotracers. ROI 1 and ROI 2 indicate the regions of interest positioned to maximize the coverage of the heart without including the adjacent lung and to minimize the overlap with the sternal or focal rib uptake, respectively. Arrowheads indicate the uptake of 99mTc-PYP in the right ventricular myocardium.
Figure 4.
SPECT Images of 99mTc-PYP Scintigrams
Single photon emission computed tomographic (SPECT) images of technetium-99m-pyrophosphate (99mTc-PYP) scintigrams with fusion to computed tomographic (CT) images obtained 2 h (A to D) and 4 h (A′ to D′) after injection of the radiotracers. Images show horizontal plane at the level of the heart and at the level of the umbilicus, respectively (A/A′, B/B′); coronal plane (C/C′); sagittal plane (D/D′). Horizontal lines in A/A′ indicate the level of the coronal slice shown by C/C′, and the vertical lines in A/ A′ indicate the sagittal plane shown by D/D′, respectively. There is some misregistration of SPECT/CT fusion images resulting from patient movement and respiration. However, the impact on image interpretation due to this misregistration is minimal. Arrowheads indicate abnormal accumulation of 99mTc-PYP in chest and abdominal walls.
Serum and urine immunofixation tests revealed no immunoglobulin monoclonal proteins. However, serum free light chain kappa/lambda ratio of 2.01 was slightly elevated (reference range: 0.26 to 1.65). Subsequently, we performed an abdominal fat pad fine-needle aspiration biopsy with positive results for transthyretin amyloidosis. However, a gene sequencing test to establish wild-type transthyretin cardiac amyloidosis (ATTR-CM) versus variant-type ATTR-CM was not performed because the patient was unable to provide informed consent for the test.
Management
The patient was diagnosed with ADHF, of which the etiology was both ATTR-CM and severe MR mainly due to chordal rupture, complicated by atrial fibrillation. Daily intravenous injection of furosemide (40 mg) and oral tolvaptan (15 mg) did not show sufficient improvement. Thus, daily intravenous injection of potassium canrenoate (100 mg), followed by oral spironolactone (75 mg) were administered, leading to decreased congestion and symptomatic relief. Tafamidis meglumine was not administered because differentiation between the wild-type and the variant ATTR by genetic testing is a prerequisite for the drug to be prescribed in Japan.
Discussion
Radionuclide imaging plays a critical role in the diagnosis and identification of ATTR-CM. Three tracers—99mTc-PYP, -3,3-diphosphono-1,2-propanedicarboxylic acid (DPD), and -hydroxymethylene diphosphonate (HMDP)—have been evaluated for imaging ATTR-CM, although these are not universally available (1,2). 99mTc-PYP is available in the United States, whereas 99mTc-DPD, and -HMDP are accessible in Europe. In Japan, 99mTc-PYP is most used. No direct comparison exists between these tracers. According to available information, they may be employed interchangeably. 99mTc-DPD/-HMDP, predominantly employed for whole body imaging, allows the detection of extracardiac amyloid infiltration. In contrast, whole body imaging for 99mTc-PYP does not provide additional diagnostic value (1). Reports regarding abnormal noncardiac accumulation of 99mTc-PYP, as clearly displayed in the patient, are limited (3). It is unclear why 99mTc-PYP markedly accumulated in extracardiac sites in the patient. However, this could indicate a large amyloid burden in the affected sites.
The precise diagnosis of ATTR-CM requires endomyocardial biopsy to demonstrate amyloid deposition. However, this procedure is relatively invasive and cannot be performed routinely. Sampling of an alternative tissue has minimized its need. For example, fine-needle aspiration of the abdominal fat pad is a safe screening procedure, although it has very low sensitivity because of small amyloid burden in ATTR-CM (4). In our patient, the unexpected result in the 99mTc-PYP scan guided the biopsy of the abdominal fat pad.
Follow-Up
Hypotension occurred as water retention improved. Therefore, amlodipine besylate was terminated and the dosage of candesartan cilexetil was decreased from 8 mg to 4 mg daily. Our patient was not readmitted for ADHF over a 3-month follow-up.
Conclusions
In a patient with ATTR-CM, 99mTc-PYP imaging demonstrated extracardiac amyloid burden. In 99mTc-PYP imaging, whole-body imaging performed concurrently with chest imaging might allow evaluation of multi-organ systemic amyloid involvement and a substantial additional benefit of radionuclide evaluation of cardiac amyloidosis.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The authors thank Editage for English language editing.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Singh V., Falk R., Di Carli M.F., Kijewski M., Rapezzi C., Dorbala S. State-of-the-art radionuclide imaging in cardiac transthyretin amyloidosis. J Nucl Cardiol. 2019;26:158–173. doi: 10.1007/s12350-018-01552-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorbala S., Ando Y., Bokhari S. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: part 1 of 2—evidence base and standardized methods of imaging. J Nucl Cardiol. 2019;26:2065–2123. doi: 10.1007/s12350-019-01760-6. [DOI] [PubMed] [Google Scholar]
- 3.Sperry B.W., Gonzalez M.H., Brunken R., Cerqueira M.D., Hanna M., Jaber W.A. Non-cardiac uptake of technetium-99m pyrophosphate in transthyretin cardiac amyloidosis. J Nucl Cardiol. 2019;26:1630–1637. doi: 10.1007/s12350-017-1166-7. [DOI] [PubMed] [Google Scholar]
- 4.Quarta C.C., Gonzalez-Lopez E., Gilbertson J.A. Diagnostic sensitivity of abdominal fat aspiration in cardiac amyloidosis. Eur Heart J. 2017;38:1905–1908. doi: 10.1093/eurheartj/ehx047. [DOI] [PMC free article] [PubMed] [Google Scholar]