Abstract
Background
We present preliminary data from a patient cohort undergoing thoracic endovascular aortic repair for Ishimaru zone 0 and 1 using a novel branched arch endograft.
Methods
This US multicenter early feasibility investigational device exemption clinical trial treated 9 patients with a mean age 72.8 ± 8.0 years (77.8% male). The endograft was designed with a single side branch designed to facilitate aortic coverage proximal to the innominate or left carotid artery while maintaining branch vessel patency. Pathology treated included fusiform (n = 2) or saccular (n = 7) aneurysm, with a maximum aortic diameter of 6.3 ± 0.7 cm. Treatment was into zone 0 in 8 patients, and zone 1 in 1 patient.
Results
All patients underwent initial successful first-stage supra-aortic trunk revascularization using a variety of techniques, without the occurrence of stroke. For the second thoracic endovascular aortic repair stage, median total treatment length was 20 cm. The primary end point of device delivery and branch vessel patency was achieved in 100% of patients, without 30-day mortality or spinal cord ischemia. Cerebrovascular events were observed in 2 patients through 30 days. No type I or III endoleaks were reported and all side branches were patent at 12-month imaging follow-up.
Conclusions
Endovascular repair of Ishimaru zone 0 or 1 arch aortic aneurysms can be achieved with a novel branched arch endograft. Future studies will evaluate the mid-term outcomes with this device in other pathologies and further define the occurrence of postoperative neurologic events.
Key Words: aortic arch, outcomes, endovascular
Abbreviations and Acronyms: ChimPS, chimney, periscope, or snorkel; EEG, electroencephalography; LCC, left common carotid artery; LSA, left subclavian artery; TBE, Gore Thoracic Branch Endoprosthesis; TEVAR, thoracic endovascular aortic repair; WHO, World Health Organization
This is a novel branched endograft implanted for zones 0 and 1 aortic arch pathology.
Central Message.
This multicenter US early feasibility study evaluates the placement of a novel single-branched endograft for use in Ishimaru zones 0 and 1 aortic arch pathology with no periprocedural mortality.
Perspective.
Treatment of aortic arch pathology by endovascular methods is limited by presence of critical branch vessels and lack of available branched endografts. This multicenter US early feasibility study describes successful preliminary results for the use of a novel branched arch endograft for Ishimaru zones 0 and 1 without perioperative mortality and no type I or III endoleak reported through 12 months.
See Commentaries on pages 7 and 9.
Thoracic endovascular aortic repair (TEVAR) has been successfully applied for descending thoracic aortic pathology.1,2 A major limitation for successful application in the aortic arch has been the presence of critical supra-aortic trunk vessels in intended landing zones or treated segments. With the lack of commercially available branched endografts specifically designed for the arch, many groups have described alternatives such as hybrid construction of complex extra-anatomic bypasses with TEVAR or use of chimney, periscope, or snorkel (ChimPS) parallel branch vessel endografts to achieve complete endoluminal solutions.3, 4, 5, 6, 7, 8, 9 The optimal solution, however, would use a branched stent graft design designed for arch pathology. We have recently described early results after use of a novel single-side branch thoracic endograft (Gore Thoracic Branch Endoprosthesis [TBE]; W. L. Gore & Associates, Inc, Flagstaff, Ariz) for Ishimaru zone 2 pathology.10 We now describe preliminary perioperative results of this US early feasibility multicenter investigational device exemption study for pathology requiring treatment into Ishimaru zone 0 and 1 (Clinical Trials Gov Identifier: NCT02264977).
Methods
This is a prospective, nonrandomized multicenter study evaluating the feasibility of the Gore TBE device (W. L. Gore & Associates, Inc) in treating aneurysms involving the arch aorta. The protocol and procedures of this trial were approved at each participating institution by individual institutional review boards. Each site obtained consent for the patients. The device design and delivery has been described previously.10 To summarize in brief, it is a modular system, designed for “off-the-shelf” use. The components are a nitinol-based stent frame with an expanded polytetrafluoroethylene graft. There is a main aortic component with an available extender aortic cuff. An 8- or 12-mm diameter portal sits within the main aortic component and docks a tapered side branch endograft. The side branch is oriented in a retrograde manner to allow for an easier delivery via a femoral artery approach. For zone 0 implants, a 12-mm portal diameter TBE device was mandated. However, for zone 1 implants, either an 8-mm or a 12-mm was allowed, depending on patient anatomy.
The zone 0/1 early feasibility study allowed enrollment for up to 20 patients considered high risk for conventional open repair. The pathologic conditions permitted within the protocol included either fusiform aneurysm >5.5 cm or twice the diameter of normal native aorta or saccular aneurysms of any size. The treatment landing zone requirement was a native aortic segment or surgical graft in either Ishimaru zone 0 or 1. Supra-aortic trunk revascularization was required for all branches and was to be performed at least 24 hours before endograft deployment. In addition, depending on the proximal landing zone, the proximal segment of the left carotid and/or left subclavian artery (LSA) was occluded by suture ligation, endovascular coiling, or plug placement to prevent type II endoleaks. The primary end points for this feasibility study included (1) successful access and deployment of the TBE, and (2) primary patency of the side branch endograft assessed by angiography at the conclusion of the procedure. The secondary end points included a 1-month Core Imaging Laboratory (AortaCore, Madison, Wis) analysis of side branch primary patency and device-related endoleaks.
Data Collection and Statistical Analysis
Data were collected from all 9 enrolled (and treated) patients treated as part of the protocol, and both 1-month and 1-year outcomes (100% complete) are presented here. Subject data were collected using protocol-specific case report forms developed by compiling research forms from sites and evaluation of the imaging by the Core Imaging Laboratory. Stroke in this study was defined with criteria developed by the World Health Organization (WHO).11 A longitudinal assessment of neurologic dysfunction was also obtained with use of modified Rankin Scale stroke score data at the screening, post-extra anatomic revascularization, post-TEVAR, and 1-month time points. Summary data were presented as proportions for categorical variables and averages (means or median) with standard deviations for continuous variables. Follow-up at the time of data export occurred at a mean of 17 ± 7 months.
Results
Early Periprocedural Outcomes
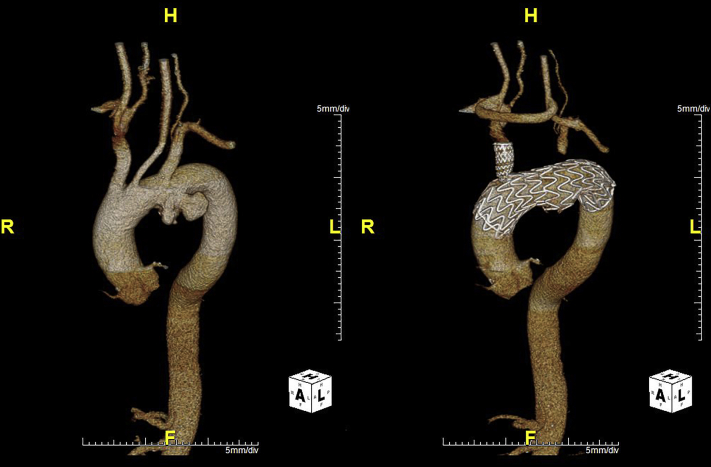
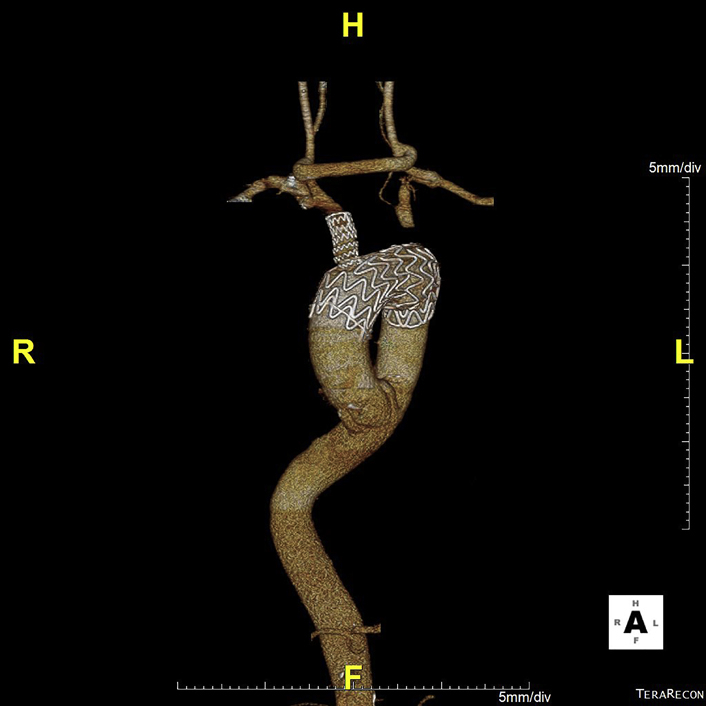
The mean age of the cohort was 72.8 ± 8.0 years (77.8% male). Associated comorbidities are listed in Table 1. Aneurysm morphology was either fusiform (2) or saccular (7) and the maximum aortic diameter was 6.3 ± 0.7 cm (range 5.4-7.6 cm). The proximal landing zone was Ishimaru 0 in 8 patients, with the remaining patient treated into Ishimaru zone 1. An example of a treated patient is shown in Figure 1.
Table 1.
Demographics and comorbidities
| Characteristic | Study cohort (n = 9) |
|---|---|
| Age, y, mean ± standard deviation | 72.8 ± 8.0 |
| Male sex | 7 (77.8%) |
| White race | 7 (77.8%) |
| Body mass index, median (range) | 25.5 (15-34) |
| Hypertension | 9 (100%) |
| Diabetes mellitus | 3 (37.5%) |
| Coronary artery disease | 7 (77.8%) |
| Previous coronary artery bypass grafting | 2 (22.2%) |
| Peripheral artery disease | 1 (11.1%) |
| Previous stroke | 1 (11.1%) |
| Nicotine use | 7 (77.8%) |
| Chronic obstructive pulmonary disease | 6 (66.7%) |
| Prior aortic repair | 1 (11.1%) |
| Preoperative creatinine, mg/dL, mean ± standard deviation | 1.2 ± 0.3 |
| Preoperative ankle-left brachial index, mean ± standard deviation | 1.0 ± 0.2 |
| Preoperative left to right brachial index, mean ± standard deviation | 1.0 ± 0.1 |
Figure 1.
This computed tomography scan was from a 62-old-male patient with multiple comorbidities who presented with an asymptomatic 6.2-cm isolated aortic arch saccular aneurysm requiring extensive treatment into zone 0. In this scenario, the distance between the innominate artery to the aneurysm was approximately 2.1 cm. The lesion length was 5.6 cm, and the proximal aortic diameters were 3.3 to 3.4 cm, whereas the distal landing zone lengths were 2.9 cm. The patient was treated with a right-to-left carotid artery bypass and a left carotid-to-left subclavian artery bypass with subsequent branched thoracic endovascular aortic repair. The postoperative 1-month image is shown as well.
A first-stage procedure was performed in all patients. The single patient treated with a zone 1 implant received an initial left common carotid artery (LCC) to LSA bypass. The remaining patients ultimately treated with zone 0 endograft implants received a combination of extra-anatomic revascularization strategies. These included LSA and LCC double transpositions in 2, LCC transposition with LSA bypass in 3, right common carotid artery to LCC bypass with LSA transposition in 2, and right common carotid artery to LSA bypass with reimplantation of the LCC in 1. No neurologic event was observed after the first-stage procedure.
All 9 patients successfully achieved the primary end point of device deployment and side branch patency at the conclusion of the procedure. Devices were delivered with a transfemoral route using general anesthesia in all patients but one, who required iliac artery access. In the patient treated in zone 1, a through and through left carotid to femoral artery was used to place the side branch endograft. Median total treatment length was 20 cm (range, 15.0-26.5 cm). No extender cuffs were required or placed. Mean contrast volume use was 159.7 mL (range, 40-240 mL). Median estimated blood loss was 200 mL (range, 100-1000 mL). Finally, median procedure duration was 219 minutes (range, 95-378 minutes).
Seven patients received both staged procedures during the same hospitalization, whereas the remaining 2 were discharged and readmitted for the stent graft procedure. The median total length of stay for both procedures was 20 days (range, 3-48 days). There was no in-hospital or 30-day mortality. No patient required dialysis. Spinal cord ischemia was not observed in any patient.
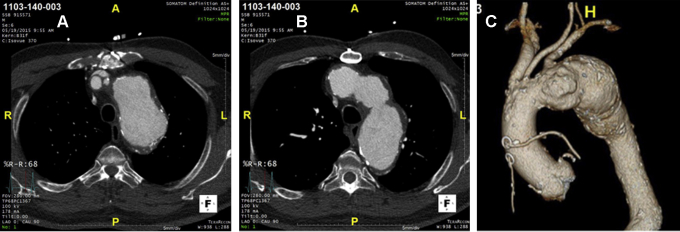
Two patients sustained perioperative strokes meeting WHO criteria. In the first patient, a left middle cerebral artery stroke was identified on the initial TEVAR postoperative day (Rankin score change from 0 to 5 on post-TEVAR day 2). Review of the patient anatomy (Figure 2) and intraoperative echocardiographic imaging identified the presence of arch atheromatous disease that likely served as the source of emboli. The clinical events committee adjudicated this as related to the device and the procedure. This patient had persistent right-sided weakness at 1 month with a modified Rankin score of 4. The second patient sustained a nonserious intraoperative WHO stroke documented with a change in electroencephalography (EEG) in the left frontal lobe. This patient was treated with an initially positioned distal conformable GORE TAG (CTAG) device followed by delivery of a proximal TBE device. The EEG event was noted after the distal CTAG deployment, and the procedure was transiently halted for resolution of EEG changes before proceeding with the TBE deployment. The patient's right-sided hemiparesis resolved within the first 48 hours of surgery (change in Rankin score from 0 to 1 on post-TEVAR day, then 0 at 1 month). This event was adjudicated as nonserious and related to the endovascular procedure.
Figure 2.
Preoperative imaging studies from the single patient who likely sustained an embolic event to his left middle cerebral artery (A and B are separate cross-sectional images, and C is the 3-dimensional aorta reconstruction). Note the aorta with protruding atheroma that likely contributed to this complication.
There was no change in right ankle to brachial index (preoperative 1.2 ± 0.1 and postoperative 1 month 1.2 ± 0.1). No site-reported endoleaks were observed at the 1-month imaging study. Finally, Core Lab evaluation showed 100% side branch patency at 1 month.
Late Results at 12 Months
There were 2 deaths within the first 12 months, and the causes have been reported as hypoxic–ischemic encephalopathy (postoperative day 182) and acute respiratory failure (postoperative day 265). No conversions or reinterventions of the target branch vessel or thoracic aorta have been reported. Neurologic events (stroke only) occurred in 2 patients in late follow-up, both in the patients who also sustained periprocedural strokes. The first patient (who also had the previously described intraoperative EEG event) sustained a cardiac arrest on day 178, developed bilateral cerebral infarctions meeting WHO criteria on day 179 and died from cardiac arrest and respiratory failure on day 182. This event was adjudicated as unrelated to the device and the initial procedure. The second patient (who also had a left-sided periprocedural stroke) sustained another serious WHO stroke in the bilateral frontal lobe and left parietal lobe at 6 months. The clinical events committee adjudicated this an unknown relationship to the device or procedure.
The 1-year postoperative right ankle brachial index was 1.2 ± 0.3. Core Lab evaluation showed a 100% patency of the side branch through 12 months. Three patients were determined to have endoleaks as determined by the Core Lab. One patient had an indeterminate endoleak at 6 months and a type II endoleak at 12 months. Another patient was identified with an indeterminate endoleak at the postprocedure imaging study, and this has persisted through 12 months. Finally, a third patient had an indeterminate endoleak at 12 months. There have been no instances of device migration or aneurysm growth (≥5 mm) at 12 months, as assessed by the Core Lab.
Discussion
Since the introduction of the first commercially available device, the successful application of TEVAR has resulted in a treatment paradigm shift for pathology of the descending thoracic aorta.1,2,12 If descending thoracic aorta pathology extends into the arch aorta, pure endovascular solutions are partly complicated by the need to extend treatment into zones encompassing origins of critical branch vessels. Similar difficulties exist for disease isolated to the arch aorta, although this configuration is less frequently encountered.7 In these settings, endovascular solutions have required extra-anatomic bypass grafts or placement of complex ChimPS side branch endografts to maintain branch vessel patency.3, 4, 5, 6, 7, 8, 9 For Ishimaru zone 0 or 1 treatment, the former strategy involves extensive adjunctive open surgery, whereas the latter approach is prone to failure with development of gutter endoleaks and parallel endograft compression. The optimal solution would include the developing branched endograft technology.
We describe 1-year results of the US early feasibility multicenter study evaluating the Gore TBE for the treatment of Ishimaru Zone 0 and 1 aortic arch pathology. This report extends our work on the use of this device in zone 2.10 The single-side branch construct of the endograft mandated extra-anatomic revascularization for zone 0 coverage before TEVAR. The single patient treated with a zone 1 implantation also underwent adjunctive LSA revascularization before branched TEVAR. Our results support the continued evaluation of this approach for a high-risk elderly patient population presenting predominantly with saccular arch aneurysms. The primary end point of device implantation and side branch patency was successfully achieved in all patients. No early mortality was reported, and no type I or III endoleaks were observed by Core Lab through 1-year follow-up.
The frequency of stroke observed in this study deserves mention. The risk for stroke in this patient population consisting of predominantly saccular pathology was likely high, given the known frequency of atherosclerotic disease in this group.7 One patient sustained the clinically transient event during deployment of a nonbranched distal CTAG component. The second patient likely sustained a permanent event due to an atheromatous burden in the aortic arch. While this was not an exclusion criterion, the study protocol for the pivotal study has now been modified to consider avoiding patient anatomy with this extent of atheroma in the arch. Although not the subject of this report, there have been an additional 10 patients treated with the TBE device in zone 0/1 with Emergency and Compassionate Use (data not shown). There was one periprocedural stroke identified in this patient population, and this cumulative experience may reflect an overall more accurate incidence with better sample size and additional experience with this device.
Published results with the alternative approaches of hybrid TEVAR, ChimPS, or physician-modified endografts have described stroke and mortality ranging from 0.8%-18.8% and 0%-20.8%, respectively.3, 4, 5, 6, 7, 8, 9,13,14 The only published report of total endovascular repair using commercially available branched stent grafts in zone 0 pathology was that by Haulon and colleagues15 describing an initial experience with the Cook inner branched arch endograft (Cook Inc, Bloomington, Ind). They identified a significant impact of a learning curve in reducing rates of death (first 10 patients = 30% vs last 28 = 7.1%) and stroke (first 10 = 30% vs last 28 = 10.7%) after branched TEVAR. None of these studies used a consistent approach with prospective evaluation with validated stroke scales or neurologists. As the experience with aortic valve surgery has suggested, retrospective or non-neurologic adjudicated assessments of stroke rates are largely under-reported.16,17 Thus, comparisons of risk of stroke after TEVAR are difficult between other reports and our study. Given the described experience with aortic valve replacement, it will be most important for future studies to use validated stroke assessment scales, such as the National Institutes of Health Stroke Scale. Potential techniques to reduce incidence of cerebrovascular events could include minimizing deployment sheath rotation particularly in the arch aorta, and then specific deairing or CO2 immersion techniques for sheath and graft preparation.
In conclusion, our study describes preliminary results with a novel branched arch stent graft for the treatment of Ishimaru zone 0 and 1 aortic arch pathology. Our 1-year data suggest this approach can be investigated to determine the safety and efficacy for branched TEVAR in the arch aorta. Future studies with increasing sample sizes should evaluate mid-term outcomes in additional pathologies and further elucidate the incidence and risk factors for postoperative neurologic events.
Conflict of Interest Statement
M.D.D. reported W. L. Gore & Associates Clinical Research and Trial Support and Consultant. J.E.B. reported Medtronic Clinical Research Investigator, W. L. Gore & Associates Clinical Research Investigator, and Member, Scientific Advisory Board. G.O. reported Clinical Research Investigator for W. L. Gore & Associates and Cook. M.F. reported Consultant for W. L. Gore & Associates, Cook, and Endologix. J.S.M. reported Clinical Research Investigator for W. L. Gore & Associates, Abbott, Medtronic, Cook, and Endologix. H.J.P. reported Consultant and Co-patent holder with W. L. Gore & Associates and Consultant for Medtronic. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
Potential bias due to conflicts was managed by independent review and approval of data and manuscript by 2 coauthors who did not disclose any conflict of interest.
Footnotes
Dr Patel was generously supported by the Joe D. Morris Collegiate Professorship, David Hamilton Fund, and the Phil Jenkins Breakthrough Fund in Cardiac Surgery at the University of Michigan Frankel Cardiovascular Center.
Read at the American Association for Thoracic Surgery Aortic Symposium 2016, New York, New York, May 12-13, 2016.
Clinical Trials Gov Identifier: NCT02264977 (Informed consent obtained at each trial site/IRB).
References
- 1.Dake M.D., Miller D.C., Semba C.P., Mitchell R.S., Walker P.J., Liddell R.P. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med. 1994;331:1729–1734. doi: 10.1056/NEJM199412293312601. [DOI] [PubMed] [Google Scholar]
- 2.Patel H.J., Williams D.M., Drews J.D., Dasika N.L., Eliason J.L., Passow M.C., et al. A 20-year experience with thoracic endovascular aortic repair. Ann Surg. 2014;260:691–697. doi: 10.1097/SLA.0000000000000930. [DOI] [PubMed] [Google Scholar]
- 3.Czerny M., Weigang E., Sodeck G., Schmidli J., Antona C., Gelpi G., et al. Targeting landing zone 0 by total arch rerouting and TEVAR: midterm results of a transcontinental registry. Ann Thorac Surg. 2012;94:84–89. doi: 10.1016/j.athoracsur.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Murashita T., Matsuda H., Domae K., Iba Y., Tanaka H., Sasaki H., et al. Less invasive surgical treatment for aortic arch aneurysms in high-risk patients: a comparative study of hybrid thoracic aortic endovascular repair and conventional total arch replacement. J Thorac Cardiovasc Surg. 2012;143:1007–1013. doi: 10.1016/j.jtcvs.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 5.Andersen N.D., Williams J.B., Hanna J.M., Shah A.A., McCann R.L., Hughes G.C. Results with an algorithmic approach to hybrid repair of the aortic arch. J Vasc Surg. 2013;57:655–667. doi: 10.1016/j.jvs.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benedetto U., Melina G., Angeloni E., Codispoti M., Sinatra R. Current results of open total arch replacement vs. hybrid thoracic aortic endovascular repair for aortic arch aneurysm: a meta-analysis of comparative studies. J Thorac Cardiovasc Surg. 2013;145:305–306. doi: 10.1016/j.jtcvs.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Sood V., Patel H.J., Williams D.M., Dasika N.L., Yang B., Deeb G.M. Open and endovascular repair of the nontraumatic isolated aortic arch aneurysm. J Vasc Surg. 2014;60:57–63. doi: 10.1016/j.jvs.2014.01.066. [DOI] [PubMed] [Google Scholar]
- 8.Cao P., De Rango P., Czerny M., Evangelista A., Fattori R., Neinaber C.A. Systematic review of clinical outcomes in hybrid procedures for aortic arch dissections and other arch diseases. J Thorac Cardiovasc Surg. 2012;144:1286–1300. doi: 10.1016/j.jtcvs.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Moulakakis K.G., Mylonas S.N., Dalainas I., Sfyroeras G.S., Markatis F., Kotsis T., et al. The chimney graft technique for preserving supra-aortic branches: a review. Ann Cardiothorac Surg. 2013;2:339–346. doi: 10.3978/j.issn.2225-319X.2013.05.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel H.J., Dake M.D., Bavaria J.E., Singh M.J., Filinger M., Fischbein M.P., et al. Branched endovascular therapy of the distal arch aorta: preliminary results of the feasibility multicenter trial of the Gore Thoracic Branch Endoprosthesis. Ann Thorac Surg. 2016;102:1190–1198. doi: 10.1016/j.athoracsur.2016.03.091. [DOI] [PubMed] [Google Scholar]
- 11.Aho K., Harmsen P., Hatano S., Marguardsen J., Smirnov V.E., Strasser T., et al. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Org. 1980;58:113–130. [PMC free article] [PubMed] [Google Scholar]
- 12.Scali S.T., Goodney P.P., Walsh D.B., Travis L.L., Nolan B.W., Goodman D.C., et al. National trends and regional variation of open and endovascular repair of thoracic and thoracoabdominal aneurysms in contemporary practice. J Vasc Surg. 2011;53:1499–1505. doi: 10.1016/j.jvs.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu J., Dai X., Noininyom P., Luo Y., Fan H., Feng Z., et al. Fenestrated thoracic endovascular aortic repair using physician modified stent grafts in zone 0 and zone 1 for aortic arch diseases. Cardiovasc Intervent Radiol. 2019;42:19–27. doi: 10.1007/s00270-018-2079-9. [DOI] [PubMed] [Google Scholar]
- 14.Kurimoto Y., Maruyama R., Ujihira K., Nishioka N., Hasegawa K., Iba Y., et al. Thoracic endovascular aortic repair for challenging aortic arch diseases using fenestrated stent grafts from zone 0. Ann Thorac Surg. 2015;100:24–32. doi: 10.1016/j.athoracsur.2015.01.071. [DOI] [PubMed] [Google Scholar]
- 15.Haulon S., Greenberg R.K., Spear R., Eagleton M., Abraham C., Lioupis C., et al. Global experience with an inner branched arch endograft. J Thorac Cardiovasc Surg. 2014;148:1709–1716. doi: 10.1016/j.jtcvs.2014.02.072. [DOI] [PubMed] [Google Scholar]
- 16.Messé S.R., Acker M.A., Kasner S.E., Fanning M., Giovannetti T., Ratcliffe S.J., et al. Stroke after aortic valve surgery: results from a prospective cohort. Circulation. 2014;129:2253–2261. doi: 10.1161/CIRCULATIONAHA.113.005084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mack M.J. The harder one looks, the more one finds. J Thorac Cardiovasc Surg. 2016;152:5–6. doi: 10.1016/j.jtcvs.2016.03.041. [DOI] [PubMed] [Google Scholar]