Abstract
BACKGROUND/OBJECTIVES:
Improving glycemic control in older African Americans with diabetes and mild cognitive impairment (MCI) is important as the population ages and becomes more racially diverse.
DESIGN:
Randomized controlled trial.
SETTING:
Recruitment from primary care practices of an urban academic medical center. Community-based treatment delivery.
PARTICIPANTS:
Older African Americans with MCI, low medication adherence, and poor glycemic control (N = 101).
INTERVENTIONS:
Occupational therapy (OT) behavioral intervention and diabetes self-management education.
MEASUREMENTS:
The primary outcome was a reduction in hemoglobin A1c level of at least 0.5% at 6 months, with maintenance effects assessed at 12 months.
RESULTS:
At 6 months, 25 of 41 (61.0%) OT participants and 22 of 46 (48.2%) diabetes self-management education participants had a reduction in hemoglobin A1c level of at least 0.5%. The model-estimated rates were 58% (95% confidence interval [CI] = 45%−75%) and 48% (95% CI = 36%−64%), respectively (relative risk [RR] = 1.21; 95% CI = 0.84–1.75; P = .31). At 12 months, the respective rates were 21 of 39 (53.8%) OT participants and 24 of 49 (49.0%) diabetes self-management education participants. The model-estimated rates were 50% (95% CI = 37%−68%) and 48% (95% CI = 36%−64%), respectively (RR = 1.05; 95% CI = 0.70–1.57; P = .81).
CONCLUSION:
Both interventions improved glycemic control in older African Americans with MCI and poor glycemic control. This result reinforces the American Diabetes Associationʼs recommendation to assess cognition in older persons with diabetes and demonstrates the potential to improve glycemic control in this high-risk population.
Keywords: adult-onset diabetes, African Americans, cognition, hemoglobin A1c, medication adherence
INTRODUCTION
African Americans have higher rates of diabetes and worse glycemic control than whites.1–3 These disparities reflect differences in biological (ie, glucose–hemoglobin A1c relationship) and psychosocial factors (eg, access to care, socioeconomic resources, and health beliefs).4,5 Negative beliefs about medications, for example, predict poor glycemic control in African Americans with mild cognitive impairment (MCI), which, in turn, contributes to why they may be more likely than whites to develop diabetes complications.6,7 African Americans are more likely than whites to develop MCI, which is often characterized by deficits in memory and executive function and can interfere with diabetes self-care and worsen glycemic control.8,9
Two systematic reviews of culturally tailored diabetes self-management education interventions for ethnic minorities found that these interventions improve quality of care and glycemic control. Few of these interventions, however, have targeted persons with MCI, who require interventions that address impaired cognition.10,11 Occupational therapists (OTs) are uniquely trained to develop strategies to compensate for cognitive impairment and optimize environments to support function, and they can deliver diabetes education while recognizing the health beliefs of patients.12,13 Whether OTs can achieve positive treatment effects beyond culturally tailored diabetes self-management education interventions is uncertain. In this randomized controlled trial (RCT), we compared the efficacy of an OT behavioral intervention vs community health worker (CHW)–delivered diabetes self-management education to improve glycemic control in African Americans with MCI, low medication adherence, and poor glycemic control.
METHODS
Trial Design and Oversight
This RCT (clinicaltrials.gov NCT02174562) was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Institutional Review Board approval was obtained, and all participants provided written informed consent. The inclusion criteria were: (1) aged 60 years and older; (2) African American race; (3) type 2 diabetes; (4) hemoglobin A1c of 7.5% or greater; (5) MCI14; and (6) 80% or less adherence to an oral hypoglycemic medication or insulin (ie, number of days that a prescribed dose was taken within 3 hours of the prescribed time divided by number of days), as documented during a 2 week run-in phase using a Medication Event Monitoring System (MEMS). The exclusion criteria were: (1) dementia15; (2) Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition–defined psychiatric disorder other than depressive disorders; (3) end-stage renal disease requiring dialysis; and (4) hearing, vision, or motor impairment that precluded research participation.
Recruitment and Enrollment Procedures
The electronic medical record (EMR) system was used to identify potentially eligible patients who were treated in primary care practices of Thomas Jefferson University, Philadelphia, PA. Patients were mailed an introductory letter, and a race-concordant research assistant telephoned them to explain the study. For interested patients, the research assistant administered trial 1 (immediate recall) of the Hopkins Verbal Learning Test-Revised over the telephone.16 Patients who recalled fewer than 5 of 12 words had a comprehensive visit to obtain informed consent and administer the baseline assessment.
Sample Size Calculation, Randomization, and Masking
The prespecified primary outcome was an absolute reduction in hemoglobin A1c level of at least 0.5% from baseline to 6 months. The primary hypothesis was that 55% of OT-treated participants and 25% of CHW-treated participants would attain the primary outcome. Previous studies supported these estimated treatment effects.17–21 Forty participants in each treatment group would yield 80% power to detect this treatment difference using a two-sided test with α = .05. The study statistician randomized eligible participants using a random-numbers table, sealed envelopes containing treatment assignments, and developed a fixed randomization scheme with a 1:1 allocation ratio stratified by baseline hemoglobin A1c level (7.5%−9% vs ≥9%). The RCT was double masked in that all participants received an active intervention and outcome assessors were masked to treatment assignment.
Study Treatments
Both the OT behavioral intervention and the CHW-delivered diabetes self-management education intervention were designed to consist of five 90-minute in-home treatment sessions over the first 3 months of the study, and three 90-minute in-home booster sessions over the next 9 months. To discuss ongoing cases, B.W.R., N.W., R.J.C., and C.V.P. met with the OTs twice monthly, and B.W.R., N.W., and R.J.C. met separately with the CHW twice monthly.
OT Behavioral Intervention
Two white OTs delivered standardized OT treatment and diabetes education tailored to participantsʼ cognitive abilities. The educational materials included the American Association of Diabetes Educators-7 and 4 Steps to Control Your Diabetes for Life. 22,23 The OTs used the Diabetes and Your Heart Facts & Information Patient Workbook to increase awareness of clinical targets for hemoglobin A1c, blood pressure, and cholesterol; and they administered the Allen Cognitive Level Screen (A) and Allen Diagnostic Module (B) to characterize a participantʼs learning potential and needs (eg, following directions, cueing) and the Performance Assessment of Self-Care Skills-Medication Management Task to characterize physical and cognitive aspects of medication taking (eg, efficiency, safety awareness).24–26 The OTs and participants reviewed dosing instructions of all medications, and used five intervention approaches to increase medication adherence: (1) activity simplification (eg, breaking down complex activities into component tasks); (2) environmental modification (eg, reducing clutter, placing medications strategically); (3) adaptive device use (eg, pill organizers); (4) memory compensation strategies (eg, notes, telephone alarms); and (5) discussing the link between medication adherence and glucose levels.27 The OTs used behavioral activation techniques (ie, defining a goal and taking steps to achieve it) to reinforce action plans to increase medication adherence. For example, morning medications were placed by a coffee maker and embedded within the morning routine (eg, “I turn on the coffee maker, take my pills, and mark the check sheet on the refrigerator.”). If pills needed to be transferred to a pill organizer, the OTs supervised the transfer based on the medication prescription. Over time, OTs modified action plans as necessary and developed action plans for other diabetes self-care activities. An action plan for diet might include reducing soft drink consumption, eating three vegetables/day, and using the plate method to balance food portions. Via the EMR, the OTs sent primary care physicians (PCPs) information on participantsʼ functional capacities and treatment goals, and queried physicians as needed to obtain information to guide treatment or answer participant questions. Although OTs were able to include the MEMS medication into a general adherence plan, they did not advise on MEMS use.
Diabetes Self-Management Education
This intervention consisted of CHW-delivered diabetes self-management education. The CHW, who was African American, used supportive techniques (eg, encourage personal expression, convey empathy) to create an accepting treatment environment, and delivered an accurate understanding of diabetes that accorded with the American Association of Diabetes Educatorsʼ position statement on CHWs.28 This intervention was designed to match the OT intervention in visit frequency and duration, educational materials, and delivery characteristics (reducing attrition and unmasking) but did not include the OT assessments, intervention approaches to increase medication adherence, action plans, or communication with PCPs.
Treatment Fidelity
All treatment sessions were audiotaped. Coinvestigators N.W., R.J.C., and C.V.P. (for the OT intervention) and N.W. and R.J.C. (for the CHW intervention) reviewed the tapes of 30% of (randomly selected) participants. Reviewed tapes included all initial sessions, a random selection of sessions 2 to 5, and one randomly selected booster session, and were rated on adherence to the respective treatment protocols. Each session was rated on establishing rapport; reviewing the interventions; and providing general diabetes education. The means (SDs) for the experimental and control treatments were, respectively: 5.0 (0.2) and 5.0 (0); 4.6 (0.8) and 5.0 (0); and 4.9 (0.5) and 5.0 (0). The OTs were also rated on the quality of OT treatment delivery; on a scale of 0 (no competency) to 2 (high competency), scores ranged from 1.88 (0.3) (assess participantʼs understanding of strategies) to 2.0 (0) (uses appropriate speech; provides feedback). The OTs were also rated on delivery of behavioral activation; on a scale from 0 to 2, scores ranged from 1.1 (0.7) (describes behavioral activation accurately) to 1.9 (0.2) (uses motivational interviewing techniques). The CHW interventionist was rated on aspects of treatment; on a 7-point scale ranging from 1 (not at all) to 7 (very much), mean ratings ranged from 6.6 (0.8) (refrains from goal setting) to 7.0 (0) (conveys understanding; demonstrates commitment to the intervention; and refrains from providing medical advice).
Study Measures
An African American outcome assessor masked to treatment assignment conducted in-home assessments using a standardized protocol to obtain the following data.
Baseline Measures
The baseline measures included age, sex, education, marital status, weight, and list of chronic medical conditions. The Diabetes Self-Care Inventory-Revised was used to measure self-reported adherence to 12 diabetes self-care behaviors (eg, exercise, diet) on a scale from 0 to 100, with higher scores indicating better adherence.29 Cognitive function was assessed using the Mini-Mental State Examination; Logical Memory, immediate and delayed recall; Trail-Making Tests A and B; and Digit Symbol Coding.30,31 A dementia expert (B.W.R.) provided extensive training to and ongoing supervision of the outcome assessor to ensure accuracy of neuropsychological test administration and scoring.
Outcome Measures (Baseline and Months 6 and 12)
Hemoglobin A1c (Primary Outcome)
Hemoglobin A1c levels were measured using the DCA Vantage point-of-care A1c analyzer (Siemens Healthineers). The primary outcome was a reduction in hemoglobin A1c of at least 0.5% at 6 months (short-term effect) and at 12 months (maintenance effect).
Medication Adherence
This was assessed objectively using a MEMS (Aardex Group).32 The MEMS measured daily bottle openings continuously over 12 months to assess adherence to insulin or an oral hypoglycemic agent. Participants were considered adherent if they took at least 80% of the MEMS-measured medication each month.
Statistical Analyses
Continuous baseline demographic and clinical characteristics were summarized using means and SDs, and categorical variables were summarized using counts and percentages. The primary analysis was performed on the modified intent-to-treat population that included all available data from all participants with at least one follow-up visit. Poisson regression with robust SEs (generalized estimating equation [GEE]) was used to jointly model dichotomous improvement (ie, decline in hemoglobin A1c ≥0.5%) at 6 and 12 months by treatment group. The model included time, treatment, and time by treatment interaction terms as well as baseline hemoglobin A1c level (categorized as ≤9.0% vs >9.0%), age, and run-in adherence rate. A compound symmetric structure was assumed for the working correlation structure. The primary hypothesis test estimated the relative risk (RR) for improvement in the OT vs diabetes self-management education intervention at 6 months. Mixed effects linear regression was used to jointly model hemoglobin A1c levels at baseline, 6 months, and 12 months by treatment group. The model included time, treatment, and time by treatment interaction terms as well as baseline hemoglobin A1c level (categorized as ≤9.0% vs >9.0%), age, and run-in adherence rate. A first-order autoregressive correlation structure was assumed for the residual errors. The same analysis was applied to other secondary continuous outcomes.
The GEE model is valid under the assumption that missing data are missing completely at random (MCAR). To assess sensitivity to the MCAR assumption, missing hemoglobin A1c data at months 6 and 12 were imputed for all randomized participants under the missing at random mechanism using the full conditional specification method in SAS PROC MI. A total of 200 data sets were imputed, and the same GEE model was applied to these 200 complete data sets with estimates summarized using SAS PROC MIANALYZE.
Poisson regression with robust SEs was also used to model monthly MEMS medication adherence outcomes. The model included time (grouped into periods as 1–3, 4–6, 7–9, and 10–12 months), treatment, time by treatment interaction, baseline hemoglobin A1c, age, and percentage of run-in doses taken. The RR of adherence for the OT vs diabetes self-management education intervention was calculated for each 3-month period. A compound symmetric structure was assumed for the working correlation structure. Statistical analysis was performed using SAS 9.4 (SAS Institute).
RESULTS
From March 2015 to October 2017, 391 individuals had baseline assessments. Of them, 290 (74.2%) were ineligible, most often because hemoglobin A1c level was 7.5% or less (170; 58.7%), MCI criteria were not met (44; 15.2%), or medication adherence exceeded 80% (43; 14.8%). A total of 101 participants with MCI were randomized to the OT behavioral intervention (n = 50) or the CHW-delivered diabetes self-management education (n = 51). Participants in the two treatment groups had similar baseline demographic, clinical, and neuropsychological characteristics (Table 1 and Supplementary Table S1). The average age of participants was 68.4 (6.4) years (range = 60–85 years), and 62% were women.
Table 1.
Baseline Characteristics by Treatment Group
| Characteristics | Total Sample (N = 101) | Occupational Therapy (n = 50) | Diabetes Education (n = 51) |
|---|---|---|---|
| Age, mean ± SD, y | 68.4 ± 6.4 | 68.2 ± 6.1 | 68.7 ± 6.7 |
| Female sex, No. (%) | 63 (62) | 31 (62) | 32 (63) |
| Married, No. (%) | 30 (30) | 18 (36) | 12 (24) |
| Education, mean±SD, y | 12.3 ± 2.1 | 12.3 ± 2.0 | 12.3 ± 2.2 |
| Chronic medical conditions, mean±SD | 5.6 ± 2.3 | 5.5 ± 2.3 | 5.7 ± 2.4 |
| Mini-Mental State Examination score, mean±SDa | 25.4 ± 2.6 | 25.7 ± 2.8 | 25.2 ± 2.4 |
| Hemoglobin A1c, mean±SD, % | 9.3 ± 1.6 | 9.2 ± 1.4 | 9.4 ± 1.7 |
| Diabetes Self-Care Inventory, mean±SDb | 57.2 ± 14.7 | 57.4 ± 14.2 | 57.0 ± 15.2 |
| Morisky medication adherence, mean±SDc | 1.2 ± 1.2 | 1.2 ± 1.1 | 1.3 ± 1.3 |
| Medication Event Monitoring System adherence, mean±SDd | 37.3 ± 30.4 | 39.0 ± 30.8 | 35.7 ± 30.3 |
Range is 0 to 30; higher score indicates better global cognition.
Range is 1 to 100; higher score is better self-reported diabetes self-management.
Range is 0 to 4; lower score is better self-reported medication adherence.
Percentage of days during run-in phase that diabetes medication was taken within 3 hours of recommended time.
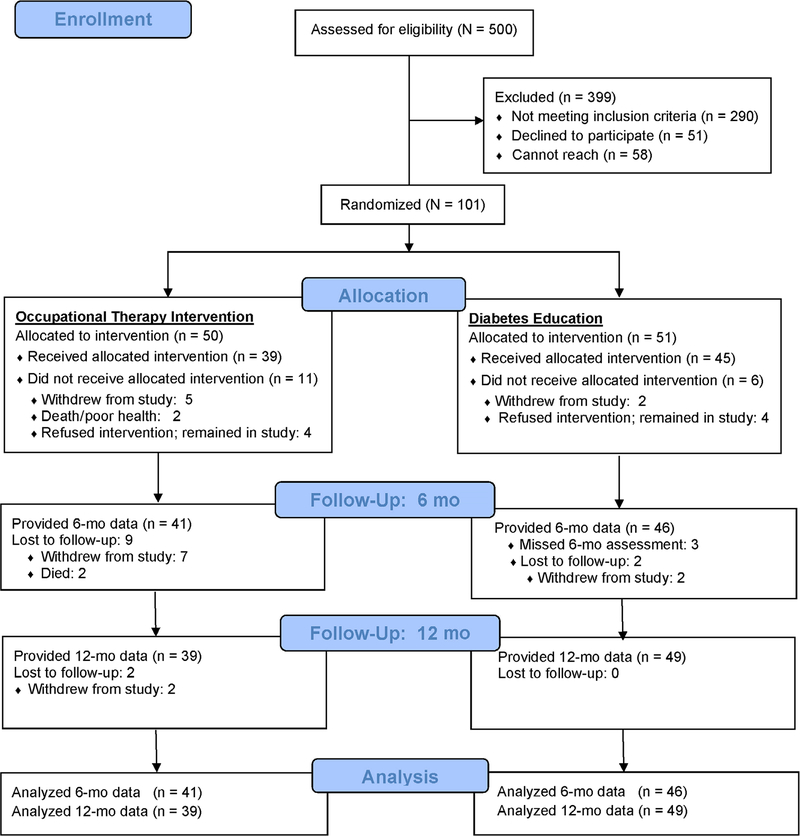
The CONSORT figure (Figure 1) shows participant completion rates. At 6 months, there was no statistically significant difference between the two treatment groups (Fisherʼs exact P = .148). At 12 months, fewer OT vs diabetes self-management education participants provided follow-up data (P = .007). There were no statistically significant differences in the baseline demographic or clinical characteristics of participants who provided follow-up data at 6 and 12 months (ie, the modified intent-to-treat population) vs those with no 6- or 12-month follow-up data (ie, withdrew or died before the 6- or 12-month follow-up). OT participants had significantly fewer initial treatment sessions than diabetes self-management education participants (mean = 4.2 [1.7] vs 5.3 [1.6], respectively; F [1, 87] = 8.8; P = .004) but a similar number of booster sessions from 6 to 12 months (mean = 2.0 [1.4] vs 2.6 [1.0], respectively; F [1, 87] = 0.28; P = .599). For the OT-treated participants, PCPs responded to 29 of 69 (42%) OT queries.
Figure 1.
CONSORT chart.
At 6 months (primary study end point) and at 12 months (maintenance treatment effects), there were no statistically significant treatment group differences in reductions in hemoglobin A1c level (Table 2). Multiple imputation analyses results were similar to the primary analyses (6 months: RR = 1.20; 95% confidence interval [CI] = 0.83–1.74; P = .32; 12 months: RR = 1.02; 95% CI = 0.68–1.52; P = .92). When the analysis was stratified by baseline hemoglobin A1c levels, greater rates of improvement were observed in participants in the higher hemoglobin A1c stratum. The RR of improvement between treatment groups, however, did not differ by stratum (Table 2). Also, no demographic or baseline clinical variables were significantly associated with the primary outcome (data not shown).
Table 2.
Reductions in HbAlc by Treatment Group at Months 6 and 12
| Time Point | OT | Diabetes Education | RR (95% CI) | P Value |
|---|---|---|---|---|
| Month 6 | ||||
| Raw rate, No./total (%)a | 25/41 (61.0) | 22/46 (47.8) | ||
| Model-estimated rate (95% CI)b | 0.58 (0.45 to 0.75) | 0.48 (0.36 to 0.64) | 1.21 (0.84 to 1.75) | .31 |
| HbAlc ≥ 9.0% (n = 43): model-estimated rate (95% CI)b | 0.74 (0.57 to 0.96) | 0.60 (0.43 to 0.84) | 1.24 (0.83 to 1.87) | .29 |
| HbAlc < 9.0% (n = 44): model-estimated rate (95% CI)b | 0.46 (0.28 to 0.74) | 0.40 (0.24 to 0.66) | 1.15(0.58 to 2.31) | .68 |
| Mean change in HbAlc from baseline to 6 mo (95% CI)b | −0.78 (−1.23 to −0.33) | .0008 | ||
| Mean change in HbAlc from baseline to 6 mo (95% CI)b | −0.44 (−0.87 to −0.004) | .0480 | ||
| Treatment group difference in mean change in HbAlc, mean (95% CI) (OT − diabetes education) | −0.34 (−0.97 to 0.28) | .2785 | ||
| Month 12 | ||||
| Raw rate, No./total (%)a | 21/39 (53.9) | 24/49 (49.0) | ||
| Model-estimated rate (95% CI)b | 0.50 (0.37 to 0.68) | 0.48 (0.36 to 0.64) | 1.05 (0.70 to 1.57) | .81 |
| HbAlc ≥ 9.0% (n = 44): model-estimated rate (95% CI)b | 0.66 (0.48 to 0.92) | 0.57 (0.41 to 0.80) | 1.16 (0.74 to 1.83) | .51 |
| HbAlc < 9.0% (n = 44): model-estimated rate (95% CI)b | 0.36 (0.20 to 0.65) | 0.41 (0.25 to 0.67) | 0.89 (0.41 to 1.91) | .75 |
| Mean change in HbAlc from baseline to 12 mo (95% CI)b | −0.91 (−1.45 to −0.37) | .0011 | ||
| Mean change in HbA1c from baseline to 12 mo (95% CI)b | −0.54 (−1.04 to −0.036) | .0358 | ||
| Treatment group difference in mean change in HbAlc, mean (95% CI) (OT − diabetes education) | −0.37 (−1.11 to 0.37) | .3273 | ||
Abbreviations: CI, confidence interval; HbAlc, hemoglobin A1c; OT, occupational therapy; RR, relative risk.
Decrease in HbAlc by 0.5%.
Adjusted for baseline HbAlc, age, and run-in adherence rate.
When hemoglobin A1c level was considered as a continuous variable in the modified intent-to-treat population, both treatment groups had statistically significant declines in hemoglobin A1c level from baseline to 6 and 12 months (Table 2). The change was larger in the OT intervention, but there were no statistically significant treatment group differences at 6 or 12 months.
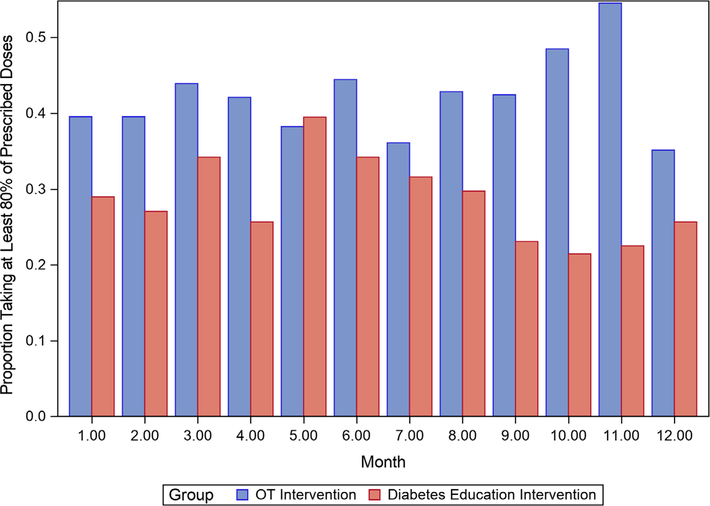
Figure 2 shows the percentage of participants who took 80% or more of MEMS-measured medications each month by treatment group. OT participants tended to have higher adherence rates than diabetes self-management education participants, but treatment group differences were not statistically significant (data not shown). A mixed effect model analyzing change in mean scores on the Diabetes Self-Care Inventory-Revised revealed no statistically significant between-group differences from baseline to 12 months (1.75; 95% CI = −4.72 to 8.21; P = .59). Serious adverse events (ie, hospitalizations, deaths) occurred in 18 OT-treated participants (39 events; two deaths) and in 16 CHW-treated participants (37 events; no deaths).
Figure 2.
Proportion of participants taking at least 80% of prescribed doses by treatment group. OT indicates occupational therapy.
DISCUSSION
Both an OT-delivered behavioral intervention and CHW-delivered diabetes self-management education improved glycemic control in approximately 50% of older African Americans with MCI, low medication adherence, and a hemoglobin A1c level of 7.5% or greater. This result indicates that targeted interventions can meaningfully help this high-risk group of patients, who are commonly seen in clinical practice and who frequently often have difficulty controlling to control their diabetes. Although a hemoglobin A1c level of 7.5% may be appropriate for some older adults, improving diabetes self-care to bring the level closer to or at goal is nevertheless clinically reasonable.33 How best to accomplish this in older African Americans with impaired memory was the focus of this RCT.
The OT behavioral intervention had a strong conceptual foundation, drew from best clinical practices, was successfully implemented, and aimed to engage PCPs. Interventions like this, with multiple treatment activities, are considered complex interventions and cannot easily be disaggregated into distinct components.34 Nevertheless, the PCP component was suboptimal. Physicians responded to less than half of OT queries, potentially diminishing treatment efficacy. Multiple obstacles to collaborative care exist, including acceptance of care fragmentation, lack of financial incentive, immediate clinical demands, and uncertain effectiveness. Collaborative care models are emerging in the literature, including OT-based interventions for patients with diabetes; however, we are unaware of any comparable interventions for patients with diabetes and MCI. The diabetes self-management education intervention was associated with a reasonable expectation of benefit. We delivered this credible intervention as a control treatment to meet ethical responsibilities, establish equipoise, and maximize retention. Two systematic reviews of similar interventions for African Americans reported reductions in mean hemoglobin A1c levels from 0.69% to 0.83%.20,21 In this RCT, the reductions were slightly lower (0.44% at 6 months and 0.54% at 12 months), perhaps reflecting participantsʼ impaired cognition. Diabetes education is readily available but needs to be relatively intensive to be effective. Previous studies have shown that cultural tailoring, one-on-one delivery by community educators, and greater than 6-month treatment duration confer better efficacy.10 This RCT uniquely adds to this literature by demonstrating that patients with MCI can benefit from diabetes self-management education.
Both study interventions had treatment features that conferred cultural relevance. Both interventions were delivered in home to increase access, both provided referrals to social service agencies as needed, and both used educational materials that African Americans find acceptable. For example, “4 Steps to Control Your Diabetes for Life” includes images of racially diverse older persons engaged in diabetes self-management activities. Also, the two treatments were matched on social contact, which may benefit cognition and glycemic control. Some features of the two interventions, however, differed in cultural relevance. The OT behavioral intervention uniquely included self-selected treatment goals, active treatment strategies, consideration of spirituality, family, and health beliefs, and guidance to obtain needed devices (eg, glucometers); while diabetes self-management education was delivered by African American CHWs, white OTs delivered the OT behavioral intervention. Some aspects of these treatment differences may have been more culturally salient than others and may have differentially influenced retention rates and treatment outcomes. This clinical trial was not designed to disentangle these effects, but it clearly highlights the need for additional study.
The primary outcome of this RCT was a decline of at least 0.5% in hemoglobin A1c level. A reduction of this magnitude across all hemoglobin A1c levels is clinically significant and reduces healthcare costs.35–37 At 6 months (primary study end point), the model-adjusted rate of 58% of OT-treated participants who achieved this outcome slightly exceeded the hypothesized 55% response rate. The response rate in CHW-treated participants, however, substantially exceeded the hypothesized rate (48% vs 25%, respectively), and accounts for the lack of a statistically significant treatment group difference. The greater than expected efficacy of the CHW intervention may reflect the higher number of initial treatment visits, compared to the OT intervention, and, as noted, the race concordance of the CHW interventionist and control participants. At 12 months, treatment effects declined slightly in the OT intervention (50%) and remained stable (48%) in diabetes self-management education participants, attesting to the overall maintenance of treatment effects. There was no evidence of heterogeneity of treatment effects by age, sex, baseline cognitive function, or education. We also found no treatment group differences in the secondary outcome measures of medication adherence (measured objectively with MEMS) or self-reported diabetes self-care (which was subject to recall bias in this sample of participants with MCI).
The strengths of this study include systematic recruitment of a minority population that is often underrepresented in clinical trials, the randomized active-control design, community-based treatment delivery, masked outcome assessments, adequate power, low attrition, high adherence to two standardized interventions, and the use of reliable and valid outcome measures. The studyʼs limitations include uncertain generalizability, racial discordance in the active treatment but race concordance in the control treatment, and the absence of a usual care control. Participants in both treatment groups received attention, but it is unlikely that attention alone or the passage of time accounts for the observed treatment effects.
Patients with diabetes and MCI remain at high risk for poor outcomes. For example, PCPs may wrongly attribute poor glycemic control to inadequate treatment rather than forgetfulness, and may intensify treatment unnecessarily. This risk is particularly high in African Americans because physicians and family members often fail to detect MCI in this population.8,38 Meanwhile, the number of older African Americans with diabetes is predicted to double, from 1 million today to 2 million by 2030.1 The demographic change will increase the burden of impaired cognition in this population, especially because diabetes also increases the risk of cognitive decline.39 By demonstrating a positive treatment effect, this RCT reinforces the American Diabetes Associationʼs recommendation to screen for cognitive impairment in all older persons with diabetes, and demonstrates the potential to improve glycemic control in older persons with impaired cognition.40
Supplementary Material
Supplementary Table S1: Neuropsychological Test Scores (Baseline) by Treatment Group
ACKNOWLEDGMENTS
Financial Disclosure: This randomized controlled trial (clinicaltrials.gov NCT02174562) was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grant R01 DK102609-01).
Sponsorʼs Role: The sponsor played no role in the design, methods, subject recruitment, data collection, analysis, or preparation of article.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes in African Americans. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health; 2005. NIH Publication No. 02–3266. [Google Scholar]
- 2.Centers for Disease Control and Prevention. CDC Health Disparities and Inequalities Report—United States, 2013. http://www.cdc.gov/minorityhealth/CHDIReport.html. Accessed January 16, 2020.
- 3.Parrinello CM, Rastegar I, Gobino JG, Miedema MD, Matsushita K, Selvin E. Prevalence of racial disparities in risk factor control in older adults with diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2015;38:1290–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mann DM, Ponieman D, Leventhal H, Halm EA. Misconceptions about diabetes and its management among low-income minorities with diabetes. Diabetes Care. 2009;32:591–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piccolo RS, Subramanian SV, Pearce N, Florez JC, McKinlay JB. Relative contributions of socioeconomic, local environmental, psychosocial, lifestyle/behavioral, biophysiological, and ancestral factors to racial/ethnic disparities in type 2 diabetes. Diabetes Care. 2016;39:1208–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rovner BW, Casten RJ. Health beliefs and medication adherence in blacks with diabetes and mild cognitive impairment. Am J Geriatr Psychiatry. 2018; 26:812–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hazel-Fernandez L, Li Y, Nero D, et al. Racial/ethnic and gender differences in severity of diabetes-related complications, health care resource use and costs in a Medicare population. Popul Health Manag. 2014;18(2):115–122. [DOI] [PubMed] [Google Scholar]
- 8.Alzheimerʼs Association. 2010 Alzheimerʼs disease facts and figures. Alzheimers Dement. 2010;6:158–194. [DOI] [PubMed] [Google Scholar]
- 9.Campbell NL, Boustani MA, Skopelja EN, Gao S, Unverzagt FW, Murray MD. Medication adherence in older adults with cognitive impairment: a systematic evidence-based review. Am J Geriatr Pharmacother. 2012; 10(3):165–177. [DOI] [PubMed] [Google Scholar]
- 10.Glazier RH, Bajcar J, Kennie NR, Willson K. A systematic review of interventions to improve diabetes care in socially disadvantaged populations. Diabetes Care. 2006;29(7):1675–1688. [DOI] [PubMed] [Google Scholar]
- 11.Magwood GS, Zapka J, Jenkins C. A review of systematic reviews evaluating diabetes interventions: focus on quality of life and disparities. Diabetes Educ. 2008;34(2):242–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pyatak E. The role of occupational therapy in diabetes self-management interventions. Occup Participation Health. 2011;31(2):89–86. [Google Scholar]
- 13.Sanders MJ, Van Oss T. Using daily routines to promote medication adherence in older adults. Am J Occup Ther. 2013;67(1):91–99. [DOI] [PubMed] [Google Scholar]
- 14.Benedict RHB, Brandt J. Manual: Hopkins Verbal Learning Test-Revised/Brief Visuospatial Memory Test Revised. Lutz, FL: Psychological Assessment Resources; 1998. [Google Scholar]
- 15.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimerʼs disease: recommendations from the National Institute on Aging-Alzheimerʼs Association workgroups on diagnostic guidelines for Alzheimerʼs disease. Alzheimers Dement. 2011;7(3):270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimerʼs disease: recommendations from the National Institute on Aging-Alzheimerʼs Association workgroups on diagnostic guidelines for Alzheimerʼs disease. Alzheimers Dement. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gary TL, Genkinger JM, Guallar E, Peyrot M, Brancati FL. Meta-analysis of randomized educational and behavioral interventions in type 2 diabetes. Diabetes Educ. 2003;29(3):488–501. [DOI] [PubMed] [Google Scholar]
- 18.Bogner HR, de Vries HF. Integrating type 2 diabetes mellitus and depression treatment among African Americans: a randomized controlled pilot trial. Diabetes Educ. 2010;36(2):284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nam S, Janson SL, Stotts NA, Chesla C, Kroon L. Effect of culturally tailored diabetes education in ethnic minorities with type 2 diabetes: a meta-analysis. J Cardiovasc Nurs. 2012;27(6):505–518. [DOI] [PubMed] [Google Scholar]
- 20.Ricci-Cabello I, Ruiz-Perez I, Nevot-Cordero A, Rodriguez-Barranco M, Sordo L, Goncalves DC. Health care interventions to improve the quality of diabetes care in African Americans: a systematic review and meta-analysis. Diabetes Care. 2013;36(3):760–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peek ME, Cargill A, Huang ES. Diabetes health disparities: a systematic review of health care interventions. Med Care Res Rev. 2007;64:101S–156S. 10.1177/1077558707305409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beck J, Greenwood DA, Blanton L, Bollinger ST, Butcher MK, et al. 2017 National standards for diabetes self-management education and support. Diabetes Care. 2017;40:1409–1419. [DOI] [PubMed] [Google Scholar]
- 23.National Diabetes Education Program. 4 Steps to Control Your Diabetes for Life. NIH Publication No. 11–5492. 2011. http://ndep.nih.gov/media/4_steps.pdf. Accessed February 25, 2015. [Google Scholar]
- 24.Hills-Briggs F, Lazo M, Renosky R, Ewing C. Usability of a diabetes and cardiovascular disease education module in an African American, diabetic sample with physical, visual, and cognitive impairment. Rehabil Psychol. 2008;53(1):1–8. [Google Scholar]
- 25.Allen CK, Blue T. Cognitive disabilities model: creating a fit between functional cognitive abilities and cognitive activity demands. In: Katz N, Toglia J, eds. Cognition, Occupation, and Participation Across the Lifespan. 4th ed. Bethesda, MD: American Occupational Therapy Association; 2018:225–280. [Google Scholar]
- 26.Chisholm D, Toto P, Raina K, Holm M, Rogers J. Evaluating capacity to live independently and safely in the community: performance assessment of self-care skills. Br J Occup Ther. 2014;77(2):59–63. 10.4276/030802214X13916969447038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Occupational Therapy Association. Occupational therapy practice framework: domain and process (3rd ed). Am J Occup Ther. 2014;68(suppl 1):S1–S48. 10.5014/ajot.2014.682006. [DOI] [Google Scholar]
- 28.American Association of Diabetes Educators. Diabetes community health workers. Diabetes Educ. 2003;29(5):821–824. [DOI] [PubMed] [Google Scholar]
- 29.Weinger K, Butler HA, Welch GW, La Greca AM. Measuring diabetes self-care: a psychometric analysis of the self-care inventory-revised with adults. Diabetes Care. 2005;28(6):1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 31.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20(4):210–216. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez JS, Schneider HE. Methodological issues in the assessment of diabetes treatment adherence. Curr Diab Rep. 2011;11(6):472–479. [DOI] [PubMed] [Google Scholar]
- 33.American Diabetes Association. Improving care and promoting health in populations: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(suppl 1):S7–S12. 10.2337/dc19-S001. [DOI] [PubMed] [Google Scholar]
- 34.Campbell NC, Murray E, Darbyshire J, et al. Designing and evaluating complex interventions to improve health care. BMJ. 2007;334:455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The Diabetes Control and Complications Trial Research Group. The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes. 1996;45(10):1289–1298. [PubMed] [Google Scholar]
- 37.Menzin J, Langley-Hawthorne C, Friedman M, Boulanger L, Cavanaugh R. Potential short-term economic benefits of improved glycemic control: a managed care perspective. Diabetes Care. 2001;24(1):51–55. [DOI] [PubMed] [Google Scholar]
- 38.Rovner BW, Casten RJ, Arenson C, Salzman B, Kornsey EB. Racial differences in the recognition of cognitive dysfunction in older persons. Alzheimer Dis Assoc Disord. 2012;26(1):44–49. [DOI] [PubMed] [Google Scholar]
- 39.Mayeda ER, Karter AJ, Huang ES, Moffet HH, Haan MN, Whitmer RA. Racial/ethnic differences in dementia risk among older type 2 diabetic patients: the diabetes and aging study. Diabetes Care. 2014;37:1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American Diabetes Association. 12. Older adults: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(suppl 1):S139–S147. 10.2337/dc19s012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Neuropsychological Test Scores (Baseline) by Treatment Group