Abstract
Acute mesenteric ischemia (AMI) is a severe condition associated with poor prognosis, ultimately leading to death due to multiorgan failure. Several mechanisms may lead to AMI, and non-occlusive mesenteric ischemia (NOMI) represents a particular form of AMI. NOMI is prevalent in intensive care units in critically ill patients. In NOMI management, promptness and accuracy of diagnosis are paramount to achieve decisive treatment, but the last decades have been marked by failure to improve NOMI prognosis, due to lack of tools to detect this condition. While real-life diagnostic management relies on a combination of physical examination, several biomarkers, imaging, and endoscopy to detect the possibility of several grades of NOMI, research studies only focus on a few elements at a time. In the era of artificial intelligence (AI), which can aggregate thousands of variables in complex longitudinal models, the prospect of achieving accurate diagnosis through machine-learning-based algorithms may be sought. In the following work, we bring you a state-of-the-art literature review regarding NOMI, its presentation, its mechanics, and the pitfalls of routine work-up diagnostic exams including biomarkers, imaging, and endoscopy, we raise the perspectives of new biomarker exams, and finally we discuss what AI may add to the field, after summarizing what this technique encompasses.
Keywords: Mesenteric ischemia, Biomarkers, Critically ill, Machine learning, Artificial intelligence
Core Tip: In this review we focus on non-occlusive mesenteric ischemia and discuss the challenges of a reliable diagnosis, which requires several simultaneous elements, including physical examination, biomarkers, and imaging elements. While taken individually these elements do not provide sufficient diagnostic accuracy, a multimodal approach relying on artificial intelligent algorithms may increase speed and accuracy in recognizing this rare but severe condition.
INTRODUCTION
Acute mesenteric ischemia (AMI), due to inadequacy between oxygen demand and supply in the digestive tract, is a life-threatening emergency[1]. This term encompasses several entities that differ regarding their initial trigger of gut ischemia but ultimately converge towards digestive and systemic complications such as tissue necrosis, perforation, bacterial translocation, and eventually, death due to multiorgan failure. Contrary to most conditions, AMI is associated with a poor prognosis, which has not improved in the last decades. Mortality ranges around 80% and mostly depends on early diagnosis and adequate treatment.
Diagnosis of AMI secondary to large vessels occlusion mainly relies on imaging, including contrast-enhanced abdominal computed tomography (CT) scan, allowing the identification of the occluded vessel (or vessels) in order to choose between different revascularization options (interventional, surgical, or medical treatment).
While obstructive AMI has been reported at length, and their management is supported by evidence-based guidelines[2], AMI occurring in the absence of major vascular occlusion, non-occlusive mesenteric ischemia (NOMI), frequently raises diagnostic and therapeutic challenges. Indeed, NOMI often occurs as the consequence of a critical condition[3]. The diagnosis is often suspected in the intensive care unit (ICU) in the context of a patient’s clinical condition worsening after a prior episode of profound and acute circulatory failure, such as a successfully resuscitated cardiac arrest, cardiopulmonary bypass surgery, as well as septic, hypovolemic, or cardiogenic shock. Reported mortality rates are extremely high, and time to diagnosis represents a key factor for improving its associated prognosis[4,5].
Several leads have been pursued to achieve this goal, including the development of new biomarkers as well as new multimodal tools. In the last decade, the advent of artificial intelligence (AI) allowed the facilitation of complex diagnoses relying on imaging.
In the following work, we bring you a state-of-the-art literature review regarding NOMI, its presentation, its mechanics, the pitfalls of routine work-up diagnostic exams, and perspectives in new biomarker exams and finally discuss what AI may add to the field. For brevity, we did not cover therapeutic management.
EPIDEMIOLOGY, MECHANISMS, AND MANAGEMENT OF NOMI
In contrast to AMI secondary to large vessels occlusion, NOMI was initially poorly understood. Nearly 80 years ago, first reports of NOMI described intestinal gangrene secondary to low cardiac output but without evidence of either arterial or venous occlusion[6,7]. As of today, only case-series and retrospective cohort studies report these severe events and in a selected population. One exception reports epidemiological data in a general population[8]. This Swedish population-based study was performed between 1970 and 1982 and suggested a population-based incidence of fatal NOMI of 2/100000 person-years. From 23446 systematic autopsies, 62 fatal NOMI cases were identified. After clinical data records were investigated, these patients were more likely to have suffered from fatal cardiac failure, atrial fibrillation, and recent surgery. Of note, necroptic examination often showed concomitant infarction of other visceral organs such as liver, spleen, and kidneys suggesting a state of global organ hypoperfusion. Through non-recent retrospective monocentric surgical case-series, NOMI ranges between 4% and 60% of AMI causes, depending on the case-mix[9,10].
Several other smaller cohorts also reported hemodialysis as a setting associated with a risk of NOMI[11]. In a retrospective study of 57 cases occurring in the first 12 h after the last hemodialysis session, all cases were preceded by an episode of hypotension during hemodialysis, and investigations found diffuse (≥ 3) ischemic areas, in 20% of cases[12]. Vasculitis was also reported as an occasional cause of NOMI, especially polyarteritis nodosa[13].
In the ICU, while described for decades, interest in NOMI is growing; as shown by an increase in reporting in the last few years[2,3,14,15]. To date, the largest retrospective multicenter study gathered 780 AMI diagnoses in ICU patients, reporting an in-ICU mortality of 58%[15]. Of note, the occlusive or non-occlusive origin of AMI was not investigated. When AMI occurs in the ICU, NOMI appears prevalent: 91% of cases in a study of 101 AMI patients, with similar rates in other cohorts[4,16]. This increased prevalence in ICU may be explained by the fact that many conditions leading to ICU admission may be associated with a NOMI onset.
Several studies reported NOMI as a complication of cardiopulmonary bypass surgery, occurring in less than 1% of patients, often in patients with peripheral artery disease[4,17-19]. As a result, NOMI should be suspected in patients suffering from multiple organ failure after cardiac surgery; as suggested by Guillaume et al[4] in a cohort study of 320 patients in which NOMI rate was 10%[4]. In this study, the incidence of NOMI was not immediate: The authors reported a median of 7 d between cardiac surgery and NOMI diagnosis.
NOMI may also occur in patients admitted for successfully resuscitated cardiac arrest[5,20]. According to a recent report of a cardiac arrest center, NOMI may affect 2.5% to 6% of patients after cardiac arrest, mortality being 96%[5]. Factors reflecting the severity of the ischemia-reperfusion syndrome, such as higher admission lactate, low flow > 17 min, and higher inotropic score, were associated with NOMI diagnosis. Furthermore, NOMI represents a cause of secondary worsening in septic shock. Investigating the cause of death in septic shock according to time since ICU admission, Daviaud et al[21] identified NOMI respectively as the second and third causes of early (≤ 3 d) and late (> 3 d) death.
Mechanisms of AMI
The pathophysiological mechanical concept of AMI relies on an imbalance between oxygen supply and demand of the intestinal tissues. Ischemic lesions first begin to appear in the intestinal mucosa and subsequently may progress to irreversible transmural necrosis[22,23]. Complications include intestinal perforation, peritonitis, bacteriemia due to rupture of the gut barrier, inflammation leading to further non-mesenteric organ dysfunction, and shock. An essential contribution to the field was the historic work from Chiu et al[24] demonstrating how decreased mesenteric flow generates mucosal lesions. In an animal model, superior mesenteric artery blood flow was modulated, serial biopsies of the small intestine were performed, and ischemic intestinal mucosal lesions were detailed. The authors observed two observations of high importance. First, mucosal lesions appeared very early after the start of the experience. Second, the rapidity and the severity of mucosal lesions were correlated with the importance of decrease in blood flow. This experience highlights how urgent it is to make the diagnosis of AMI and proceed to treatment since vital and functional complications evolve quickly. A parallel can be drawn with acute myocardial infarction and stroke. Hence, intestinal stroke centers allowing early multimodal management have been suggested, and first reports showed increased survival[25].
Although the experiments performed by Chiu et al[24] strongly support the hypothesis of a supply-demand imbalance as a primary step towards NOMI, other complex processes may be involved, ultimately leading to the progression towards intestinal necrosis. These processes include the promotion of remote multiorgan failure through complex inflammatory pathways after a first insult in the form of transient hypoperfusion of the main mesenteric arteries[3,22,26]. Other mechanisms include impaired tissue perfusion responsible for gut barrier failure and endotoxin translocation, endothelial dysfunction and ischemia-reperfusion injury with increased local cytokine production, which may vary according to the primary cause of intestinal hypoxia[3,27-29].
In septic shock, tissue perfusion may be altered at the microcirculation level; despite seemingly optimized global hemodynamic parameters and these microcirculatory abnormalities are directly linked to organ failure[30]. Notably, Dubin et al[31] demonstrated persistence of altered intestinal microcirculation disorders in deceased animals after correction of arterial hypotension in a model of septic shock[31]. Moreover, in sepsis, other mechanisms may participate to tissue dysoxia: Cellular and metabolic disorders[32,33]. Lobo et al[34] showed possible “cytopathic hypoxia” without impairment of oxygen delivery in the development of gut mucosal injury during endotoxic shock[34]. Therefore, a primary transient main mesenteric arteries hypoperfusion may not be a mandatory step in NOMI related to sepsis.
Although NOMI is thought to represent the worst stage of acute gastrointestinal injury in critically ill patients[35], the exact pathophysiology is still poorly understood, and the definition of this concept remains unclear[36]. According to a working group of the European Society of Intensive Medicine, NOMI is one of the possible facets of acute gastrointestinal dysfunction. In a recent update, acute gastrointestinal dysfunction is outlined as the consequence of a multitude of interacting pathophysiological mechanisms, resulting in other life-threating conditions such as Ogilvie’s syndrome, sepsis, gastrointestinal tract perforation or bleeding, and acute compartment syndrome[36].
Additionally, deleterious therapeutic interventions may add to the incidence of NOMI by worsening tissue dysoxia in ICU patients. Experimental and observational studies suggest that the use of vasopressors such as norepinephrine and epinephrine might result in impaired mucosal perfusion[37-39]. Other pharmacological agents such as vasopressin and digoxin[3] as well as acute profound hypovolemia could also worsen ischemic lesions. Lastly, the role of enteral nutrition in critically ill patients is controversial and depends on several factors such as the dose of enteral nutrition, the metabolic phase, and the severity of the patients. In the recent randomized controlled trial “NUTRIREA 2”[40], enteral nutrition was compared to parenteral nutrition with a normocaloric target (i.e. 20-25 kcal/kg per day) during the first days of admission (i.e. catabolic phase) in mechanically ventilated patients with shock. Mortality did not differ between the two groups, but a significantly higher rate of bowel ischemia was reported in the enteral group [19 (2%) patients vs 5 (< 1%) patients]. However, an ancillary study focused on citrulline and intestinal-fatty acid binding protein (I-FABP) biomarkers showed possible protective effects of enteral nutrition on enterocyte mass, raising an interest for further investigation[41]. In particular, some hypothesized that a lower dose of enteral nutrition in these patients may yield a protective effect[42].
Compared with occlusive AMI, NOMI reported mortality is higher, ranging between 70% and 100% depending on the series[4,5,16]. While complications of NOMI are similar to those of occlusive AMI (including necrosis, perforation with peritonitis, bacteremia secondary to digestive translocations, acute compartment syndrome, vasoplegic shock, multi organ failure leading to death), their prognosis is indeed different[2]. In survivor patients, late AMI complications classically include short bowel syndrome, undernourishment, and need of total parental nutrition[43,44]. Of note, late outcomes in NOMI patients (e.g., long-term mortality, quality of life) are currently unknown and should be investigated.
Several reasons may explain this poor prognosis in NOMI as compared to occlusive AMI. To start, in the former, patients are in a critically state, due to an earlier severe aggression, and NOMI represents a “second-hit” added on top of the reason for ICU admission. Secondly, treatment options do not allow a rapid reversal of the causal insult (as opposed to a revascularization of an occluded vessel), leading to late treatment and thus worse outcomes. Thirdly, surgical treatment is complex because of the lack of clear delimitation between viable and necrotic tissue: Lesions are often diffuse or patchy and extensive resections are then performed, when deemed relevant, which often is not the case after laparoscopic evaluation. Lastly, diagnosis is complex and requires multimodal approaches, leading to delays, as compared to obstructive AMI causes[3,45].
DIAGNOSTIC PITFALLS AND CHALLENGES FOR FUTURE RESEARCH
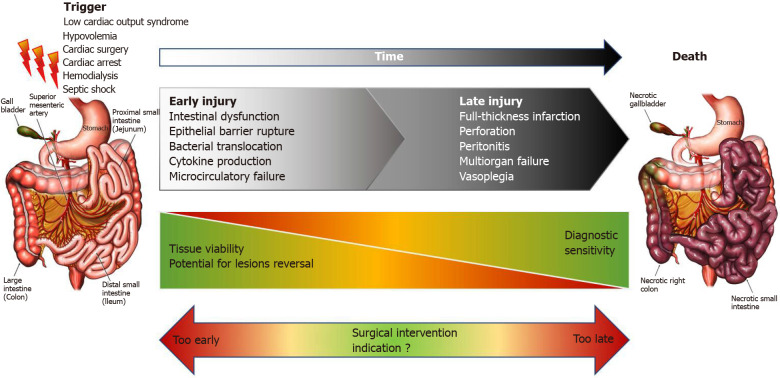
Facing a rapidly evolving disorder, the diagnostic process should provide answers to two important issues. First of all, diagnosing AMI early is essential to avoid progression to transmural necrosis and the associated complications[3,46]. Secondly, reliable information on the presence or absence of intestinal necrosis, and ideally on the intestinal location involved, is needed to guide decision regarding surgical treatment, according to the location and indication of surgery and patients’ condition. The importance of this issue is summarized in Figure 1.
Figure 1.
Timing of events in non-occlusive mesenteric ischemia.
As of today, these vital questions remain unanswered in the setting of NOMI and are often diagnosed too late. At time of diagnosis in NOMI studies, severity biological parameters are usually markedly high, and necrosis is frequently observed in comparison to recent occlusive AMI cohorts[4,5,16,47]. This severity, possibly associated with diagnostic delay, could partially explain the discrepancies between observed mortality rates.
Clinical examination
NOMI has always been presented as a challenging diagnosis due in particular to clinical signs considered to be non-specific[45,46]. Diagnosis is usually suspected in front of novel or worsening of circulatory failure in evocative contexts (e.g., cardiac surgery or after cardiac resuscitation) and is discussed as a differential diagnosis of secondary infections[3]. Digestive signs, similar to that of an occlusive AMI presentation, might then evoke NOMI. These signs include possibly “brutal” abdominal pain, diarrhea, upper or lower digestive hemorrhage, and vomiting. However, a notable difference is that the beginning of occlusive AMI is often brutal allowing to pinpoint the exact onset time; in contrast, in the ICU setting, patients may be sedated and curarized, abdominal exam may not yield much, even if other non-specific digestive signs might suggest NOMI (abnormal gastric residual volume under enteral nutrition, ileus, increase of abdominal perimeter, increased intra-abdominal pressure).
Biomarkers
Biomarkers represent additive tools to this difficult diagnosis. Routine biomarkers, among those reflecting tissue ischemia, plasma lactate, lactate dehydrogenase (LDH), and aspartate aminotransferase (AST), can reinforce NOMI suspicion[45,48].
D-dimers have also been reported as highly sensitive for AMI diagnosis with plausible negative predictive value[49-51]. However, no study focused on a selected NOMI population in which it is likely to find multiple concurring causes for an increase in D-dimer levels, resulting in low specificity, and its dosage is not recommended[2]. Similarly, this conclusion can be drawn with LDH levels and leukocyte count[16,52,53].
After cardiac surgery, there has been interest in AST[53,54]. A value ≥ 100 IU/L was independently associated with AMI diagnosis in patients with multi organ failure[4]. Interestingly, AST was also associated with mortality in 780 AMI patients from various causes[15]. Thus, despite obvious lack of specificity, elevation of AST may reinforce clinical suspicion.
Procalcitonin has also been shown to be associated with mortality in AMI patients[55]. A threshold value of 2.47 ng/mL was suggested in a monocentric retrospective study of 128 AMI patients[56]. However, in a NOMI subgroup, procalcitonin may be less accurate given the high prevalence of acute renal failure and infections[57].
Serum lactate, a long-time marker of tissue ischemia, is usually associated with mortality in the AMI setting[15]. Despite a lack of specificity, lactate could be useful to predict necrosis when associated with other parameters. A prospective study of 67 selected patients with AMI identified three parameters associated with necrosis: Presence of organ failure, serum lactate levels > 2 mmol/L, and bowel dilation on computed tomography (CT) imaging[47]. When all three parameters were present, necrosis requiring surgical resection was highly likely. However, there was only one patient with NOMI in this cohort, and, in the setting of NOMI, an increase in plasma lactate levels is consistent with numerous possible etiologies.
Yet, clinical exam and routine laboratory tests are of only little value to make an early reliable diagnosis and to differentiate suspicion from confirmed NOMI[1,16,58].
Perspectives in biomarkers
Research is in progress to identify candidate AMI biomarkers. One of the most promising is I-FABP. Preliminary studies suggest a potential interest in I-FABP, a small cytosolic protein specific to small bowel released in the context of intestinal ischemia[59]. Experimental studies demonstrated early increase of I-FABP after onset of gut ischemia[60]. Thuijls et al[52] studied plasma and urinary I-FABP accuracy in 46 patients with a suspicion of AMI, of which 22 AMI cases were finally confirmed[52]. The area under the receiver-operating curve (AUC) for urinary I-FABP was 0.93, performing better than plasma I-FABP (AUC = 0.70). Notably, the increase in I-FABP was greater in patients with ischemia of the ileum, which is the main source of I-FABP production. However, in critical illness and particularly in NOMI, acute renal failure is highly prevalent, and urine samples might not be available. Further studies are needed to refine plasma I-FABP accuracy.
Interestingly, Matsumoto et al[53] found an AUC of 0.88 for AMI diagnosis including 15 cases of NOMI and 9 arterial occlusions[53]. The authors also highlighted that I-FABP is increased in various non-vascular intestinal ischemia etiologies such strangulated bowel obstruction, incarcerated hernia, and volvulus. While promising, the integration of plasma I-FABP to the routine monitoring of intestinal ischemia is probably too early at this point and should be further explored. In adults with septic shock, Sekino et al[61] measured daily plasma I-FABP in a monocentric observational study and found a higher incidence of NOMI when I-FABP levels were superior to a threshold of 19.0 ng/mL[61]. Importantly, I-FABP thresholds for AMI diagnosis are not consensual[29], and differences in accuracy of I-FABP dosage according to enzyme-linked immunosorbent assay kits lead to further limitations[62].
Plasmatic citrulline, an amino acid synthesized from glutamine by small bowel enterocytes and metabolized into arginine by the kidney, reflects functional enterocyte mass and has been proposed as a marker of acute intestinal failure in critically ill patients[63]. However, the high prevalence of acute renal failure in the ICU population may lead to high plasma citrulline concentrations despite a reduction of enterocyte mass[64]. Further studies are needed to precise its performance in critical illness and NOMI diagnosis. To a lesser extent, the ability of endothelin-1 to predict NOMI has been investigated in 78 post cardiac surgery patients and revealed high specificity (94%) but poor sensitivity (51%)[65].
Imaging
From clinical suspicion of NOMI to certitude, diagnosis relies on imaging. Historically, angiography was considered pivotal by some experts, as it was considered an efficient treatment for NOMI[66,67]. Angiographic observations of NOMI included the visualization of absence of large artery occlusion and vasoconstriction of small intestinal arteries. Subsequently, angiography enabled the in situ administration of a continuous infusion of vasodilatory drugs like papaverine. Small cohort studies reported efficacy, suggesting this treatment may be associated with fewer progression to necrosis, and improved survival[26]; the effectiveness of this strategy may not be warranted if NOMI is diagnosed at the stage of intestinal necrosis requiring surgical treatment. Moreover, tolerance of vasodilatory drugs in hemodynamically unstable patients is unclear, and given the low availability of the technique, it remains reserved for expert centers. Hence, evidence diagnostic and therapeutic angiography interventions in NOMI remain low, for now.
On the other hand, while contrast-enhanced abdominal CT scan plays a central role in occlusive AMI[68], indicating the occluded vessel and eventually guiding revascularization possibilities, its performance in NOMI is disappointing. A monocentric study compared the classical CT signs evoking AMI of 75 patients with NOMI, with 39 patients in which NOMI was suspected but subsequently ruled out, when compared to macroscopic diagnosis considered as reference[16]. Portal venous gas, pneumatosis intestinalis, and abnormal contrast-induced bowel wall enhancement exhibited good specificities (respectively 95%, 85%, and 71%) but were poorly sensitive to the point, that one quarter of patients exhibited mesenteric ischemia without any suggestive radiological signs.
Abdominal ultrasound has been recently proposed for the investigation of acute gastrointestinal injury, emphasizing the possibilities to measure gastrointestinal diameter, mucosal thickness, peristalsis, and blood flow[69]. As of today, data on ultrasound performance for NOMI diagnosis are scarce, despite the evident advantage of being performed at the bedside non-invasively and the ability to diagnose bowel dilation, intramural, or portal venous gas[70,71].
Endoscopy
Finally, endoscopy is widely used in the ICU setting and presents the advantages of direct visualization of intestinal mucosa at the bedside. Given the relatively low negative predictive value of CT imaging, endoscopy is frequently performed and allows to diagnose a significant number of NOMI cases in the ICU. In post cardiac arrest patients, hemorrhagic or necrotic lesions are likely to be found during gut endoscopy in the presence of clinical signs of gastrointestinal dysfunction[72]. However, its disadvantages are numerous: A large part of the intestines (i.e. small bowel) are inaccessible, the observed mucosal necrosis does not always correspond to transmural necrosis, there exists an inherent risk of perforation in weakened tissues, and availability is dependent on the operators.
Hence, given the numerous pitfalls of the current diagnostic approach for the diagnosis of NOMI, a high index of suspicion is required in populations at risk, such as post cardiac or aortic surgery, hemodialyzed patients, and critically ill patients[23,73]. Research is encouraged to identify or validate new biomarkers and imagery tools and increase knowledge on the pathophysiological understanding of NOMI genesis, especially in critically ill patients[36]. Specific accuracy of these new biomarkers should be further evaluated in the future. However, well designed studies are incredibly difficult due to numerous issues. Importantly, the low incidence of NOMI requires an appropriate selection of the study population with consideration of the pre-test probability. Additionally, patients in which NOMI has been ruled out are difficult to define given the low negative predictive value of CT imaging. Methodological difficulties originate from the lack of knowledge of the physiopathology and the important variability of NOMI time course due to differences in the intensity and duration of the aggression at the origin of NOMI. Furthermore, a working group of the European Society of Intensive Care Medicine stated the need for a consensus definition of NOMI in order to improve the current knowledge, study epidemiology and suggest interventions[36].
Overview of AI in healthcare
AI is a vague term reflecting the use of computers to perform tasks that are thought to require unique skills, often in ways that are hard to pin-point and that evolve with time. For example, although basic game algorithms such as those initially developed for chess, were considered as such 50 years ago, they are now part of every personal computer, and we know how to break them down into discrete steps and feel we understand them[74].
Later, AI encompassed the field of image recognition[75]. Although we humans perform this task naturally, we often cannot articulate exactly how this is done. This lack of supervision is one of the features of machine learning (ML), a subset of AI. It is the study of algorithms that learn from experience without being explicitly programmed for their task. ML incorporates a broad range of statistical methods ranging from linear regression to support vector machines, decision trees, or neural networks that make use of new datapoints to update the function they approximate[76].
As introduced, this field is subdivided into supervised methods, which learn from labeled samples, and unsupervised methods that attempt to find patterns in the data themselves[76,77]. The main applications of supervised models are classification, in other words, choosing to which predefined class an observation belongs, and regression, in which a value is derived from given observations. In medical imagery, these two applications often amount to diagnosis (classification) and prognostication (regression). Clustering, in which observations are grouped in classes that are not pre-defined, and dimensionality reduction used for data structuring or visualization are the most common applications of unsupervised learning. Finally, in reinforcement learning, a model interacts with its environment, performs actions, and learns in a trial-and-error fashion. The main applications of reinforcement learning lie in decision support tools and autonomous agents[78].
During the development of a ML system, the parameters of a model, termed weights, are gradually adjusted to fit a training dataset[79]. In a second step, the model is validated on a separate dataset. An evaluation on the initial dataset would result in overly optimistic results, dubbed the overfitting effect. This common phenomenon occurs when the ML model adapts itself too much to the training dataset and then fails to generalize on other datasets. In other terms, the model remembers the examples seen in the training phase but does not learn any relevant features that are applicable to future observations.
The simplest way to derive a model from a set of observations is variable thresholding. When combining multiple features, linear and logistic regression are the most frequently used techniques in healthcare but require the assumption of normality[80]. Methods capable of using non-linear discriminant functions such as support vector machines as well as methods relying on multiple linear boundaries such as decisions trees have been elaborated. While simple to implement, these models are limited in their ability to process raw data, such as images or time course data, as they struggle to model the relationships of large amounts of variables in multiple dimensions[81]. Instead, the elaboration of such models often needs to rely on considerable domain expertise to extract relevant traits from the raw data, yet, arbitrarily selected features and the underlying physiologic assumptions may fail to capture a specific individual’s response. This is especially true for the analysis of medical images, in which every voxel represents an individual variable influenced by location, tissue type, and surrounding structures as well as time-sensitive data such as those recorded in standardized electronic healthcare records (EHRs).
Specific ML algorithms that are built in a multi-layered fashion, termed deep learning (DL) algorithms, circumvent this limitation by automatically encoding multiple levels of inner representations of relevant features. This is achieved by composing simple non-linear units that sequentially transform the representation, starting from the raw data, into a slightly more abstract representation at a deeper level. Visually, when analyzing images, the first two layers often represent edges and particular arrangements of edges. Subsequent deeper layers then assemble the motifs encoded in the prior layers into larger combinations representing parts of patterns featured in the raw data. The main advantage of this process is that the features are learnt by a general-purpose learning algorithm without any direct human intervention. This allows for the rapid development of models able to discover intricate features in multi-dimensional data. In the last decade, DL has led to major improvements in performance in the fields of computer vision[75,82] and natural language processing[83,84]. The main model architectures used in these domains are convolutional neural networks, recurrent neural networks (RNNs), auto-encoders, and transformers. Even if DL methods have been able to produce spectacular results, it is important to realize that these methods are still in their early days, and their performance does not always exceed that of conventional techniques using hand-selected features[85]. DL works well with large datasets but often requires specific computational infrastructure for the training process, whereas conventional ML methods have advantages for smaller datasets and can be created with classical processors.
The advantages brought by DL-powered data analyses have rapidly been taken over into the medical domain with first translations to radiology[86], ophthalmology[87] and pathology. Although the implementation of such algorithms in a clinical setting remains challenging[74], this progression has culminated in the approval of the first insurance reimbursement for AI augmented medical care[88] for the CT-based detection of large vessel occlusion in stroke. Modern ICUs generate vast streams of data stored in EHRs and current in-silico research has yielded successful DL tools to improve the prediction of mortality[89-93] and to guide clinical decisions[94]. A major focus has been the prediction of sepsis, which, analogously to NOMI, lacks a distinctive marker for an accurate and timely diagnosis. In recent years, multiple ML methods have emerged to diagnose sepsis in real-time or to predict its occurrence. The most prominent models relied on RNNs[95,96], custom hazard models[97] or a combination of multiple models, known as ensembles. Although clinical validation studies are often still lacking, these automated methods offer new possibilities for the early detection of sepsis based on objective variables extracted from EHR data[98,99]. Similarly, the lack of a gold standard non-invasive definition of NOMI and the need for rapid detection make for an excellent opportunity for the application of ML.
Diagnostic approaches in NOMI
Likewise, the diagnosis and management of NOMI highly depends on information obtained from imaging studies, clinical variables, and biological findings. Yet, no single marker allows for the accurate detection of intestinal ischemia. The expertise of gastroenterologists, intensivists, radiologists, and surgeons remains mandatory, but their availability and the time needed to process all these complex data may delay timely surgical intervention.
In NOMI prediction, multivariate logistic regressions models have been described several times[16,100-102]. When applying a threshold to a linear combination of weighted clinical variables, these have been used for the prediction of NOMI in 865 patients after cardiac surgery, of which 78 were angiographically confirmed to have developed mesenteric ischemia[19]. According to the authors, this linear discriminant analysis yielded a sensitivity of 76.9% and specificity of 93.8%. The interpretation of these results remains, however, limited as variables and weights were derived and tested in the same cohort. A follow-up logistic regression model used preoperative, intraoperative, and postoperative risk factors derived from 4449 patients after cardiac surgery to predict the occurrence of NOMI[103]. The authors report an AUC of 0.91 in their control cohort (n = 4299). Although these are encouraging results, the evaluation of these models suffers from methodological flaws as derivation and validation datasets of the model weights were not distinct.
Future models may benefit from more advanced algorithms such as those employed for the prediction of sepsis as discussed above. Furthermore, using continuous data streams instead of single timepoints as input would result in models with closer resemblance to clinical and physiologic reality. Long short-term memory networks in particular, a specific form of RNN, have shown promising results on temporal sequences sampled from ICU EHRs[93,95,96]. The so-called transformer, a successor model to long short-term memory networks integrating the concept of selective attention, has since emerged from the natural language processing domain[104]. Although the application of transformer models to medical EHRs is only beginning[105,106], it is possible model architecture will be prominent in the coming years.
Abdominal CT findings can reveal intestinal ischemia, although inter-rater agreement often remains limited. A multivariate combination of radiological signs has been identified through a logistic regression model in a cohort of 68 patients requiring cardiopulmonary bypass during surgery[107]. The resulting model was not accurately validated but performed well on the training cohort (AUC = 0.84). A model for the detection of transmural intestinal infarction confirmed on laparotomy has been elaborated on CT scans of 207 patients with superior mesenteric venous thrombosis[108]. A follow-up validation on an external cohort (n = 89), demonstrated satisfying performance (AUC = 0.84) and led to the development of a nomogram. Although this model has been developed in a different patient population, it remains one of the most accurately validated models for the detection of intestinal ischemia.
The use of image-based models could strongly improve performance and usability as they do not depend on the detection of a few selected findings and may integrate holistic imaging features, using convolutional neural networks such as those used in abdominal CT scans for the detection of acute appendicitis[109,110]. It is of note that although feature-based models (such as presence of pneumatosis intestinalis or abnormal bowel wall enhancement) for the detection of intestinal ischemia developed on patients with occlusion may translate to patients without occlusion, the features used by image-based models are often hidden to the user and will inadvertently rely on findings extracted from the site of occlusion. Much attention should therefore be paid to a careful selection of the study population and a clear restriction of use-cases for the developed models.
Perspectives in NOMI prediction
The availability of diverse and complex data points makes the use of AI for the detection and prognostication of NOMI in the ICU a valuable clinical opportunity. Currently developed ML models show encouraging results but lack rigorous statistical validation. Moreover, the use of state-of-the-art DL methods is likely to benefit model performance. As such, algorithms can encode an inner representation of relevant patterns and can approximate more complex non-linear functions, their use would forego the need of handcrafted features. This is especially relevant for the analysis of temporal sequences extracted from EHRs and abdominal CT scans. Indeed, although many signs of bowel ischemia have been identified, their performance[16] and inter-rater agreement remain limited[107,111]. Recent advances in fusion models leveraging both imaging and EHR data have performed well in the detection of pulmonary embolism[112] and may be tested in NOMI. To fast-forward the development of future models there is a clear need for the collection and release of datasets incorporating patients with suspicion and definite diagnosis of NOMI. The diagnosis of NOMI in such datasets should ideally be verified pathologically or surgically, be it via laparotomy or laparoscopy, to obtain a clean target definition for model development. Moreover, it is essential to obtain clinical validation through prospective studies of not only the performance of developed models but also the ease of implementation into the ICU setting and their clinical utility following recently published guidelines[113,114]. While most attention is often directed to increasing predictive performance, future AI solutions should account for their predictions to lead to wider clinical applicability and acceptance. Ideally, future AI systems should therefore strive to achieve ease-of-use, interpretability, and diagnostic performance.
It is likely that the results achieved by ML will continue to improve as the computational power at disposition increases, collected datasets grow, and more performant and adequate algorithms are developed. When applied to EHR data and medical imaging with statistical rigor, ML models could refine the accuracy and speed of diagnosis of NOMI in critically ill patients. Used appropriately, this emerging technology could further be leveraged to identify and explore new disease mechanisms and single-out yet unrealized connections between datapoints, paving the way for a deeper understanding of the intricated interactions leading to NOMI in the ICU.
CONCLUSION
NOMI is associated with poor prognosis due to lack of accurate diagnostic tools. While taken individually, several biomarkers and imagery modalities exist, their combination and the study of their variation through time, which requires sheer computational power that may be provided by artificial intelligent tools, is bound to increase diagnostic performance in NOMI and improve therapeutic management.
ACKNOWLEDGEMENTS
We thank Pr Alain Cariou for participating in the inception of this work.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest regarding this work.
Manuscript source: Invited manuscript
Peer-review started: January 28, 2021
First decision: March 7, 2021
Article in press: June 18, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shirai T, Wang Y S-Editor: Fan JR L-Editor: Filipodia P-Editor: Liu JH
Contributor Information
Simon Bourcier, Department of Intensive Care Medicine, University Hospital of Geneva, Geneva 1201, Switzerland.
Julian Klug, Department of Internal Medicine, Groupement Hospitalier de l’Ouest Lémanique, Nyon 1260, Switzerland.
Lee S Nguyen, Department of Intensive Care Medicine, CMC Ambroise Paré, Neuilly-sur-Seine 92200, France. nguyen.lee@icloud.com.
References
- 1.Clair DG, Beach JM. Mesenteric Ischemia. N Engl J Med. 2016;374:959–968. doi: 10.1056/NEJMra1503884. [DOI] [PubMed] [Google Scholar]
- 2.Björck M, Koelemay M, Acosta S, Bastos Goncalves F, Kölbel T, Kolkman JJ, Lees T, Lefevre JH, Menyhei G, Oderich G Esvs Guidelines Committee. Kolh P, de Borst GJ, Chakfe N, Debus S, Hinchliffe R, Kakkos S, Koncar I, Sanddal Lindholt J, Vega de Ceniga M, Vermassen F, Verzini F, Document Reviewers, Geelkerken B, Gloviczki P, Huber T, Naylor R. Editor's Choice - Management of the Diseases of Mesenteric Arteries and Veins: Clinical Practice Guidelines of the European Society of Vascular Surgery (ESVS) Eur J Vasc Endovasc Surg. 2017;53:460–510. doi: 10.1016/j.ejvs.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Oldenburg WA, Lau LL, Rodenberg TJ, Edmonds HJ, Burger CD. Acute mesenteric ischemia: a clinical review. Arch Intern Med. 2004;164:1054–1062. doi: 10.1001/archinte.164.10.1054. [DOI] [PubMed] [Google Scholar]
- 4.Guillaume A, Pili-Floury S, Chocron S, Delabrousse E, De Parseval B, Koch S, Samain E, Capellier G, Piton G. Acute Mesenteric Ischemia Among Postcardiac Surgery Patients Presenting with Multiple Organ Failure. Shock. 2017;47:296–302. doi: 10.1097/SHK.0000000000000720. [DOI] [PubMed] [Google Scholar]
- 5.Paul M, Bougouin W, Legriel S, Charpentier J, Jaubert P, Savary G, Bourcier S, Pène F, Dumas F, Grimaldi D, Cariou A. Frequency, risk factors, and outcomes of non-occlusive mesenteric ischaemia after cardiac arrest. Resuscitation. 2020;157:211–218. doi: 10.1016/j.resuscitation.2020.09.028. [DOI] [PubMed] [Google Scholar]
- 6.Ende N. Infarction of the Bowel in Cardiac Failure. N Engl J Med . 1958;258:879–881. doi: 10.1056/NEJM195805012581804. [DOI] [PubMed] [Google Scholar]
- 7.Wilcox MG, Howard TJ, Plaskon LA, Unthank JL, Madura JA. Current theories of pathogenesis and treatment of nonocclusive mesenteric ischemia. Dig Dis Sci. 1995;40:709–716. doi: 10.1007/BF02064966. [DOI] [PubMed] [Google Scholar]
- 8.Acosta S, Ogren M, Sternby NH, Bergqvist D, Björck M. Fatal nonocclusive mesenteric ischaemia: population-based incidence and risk factors. J Intern Med. 2006;259:305–313. doi: 10.1111/j.1365-2796.2006.01613.x. [DOI] [PubMed] [Google Scholar]
- 9.Sitges-Serra A, Mas X, Roqueta F, Figueras J, Sanz F. Mesenteric infarction: an analysis of 83 patients with prognostic studies in 44 cases undergoing a massive small-bowel resection. Br J Surg. 1988;75:544–548. doi: 10.1002/bjs.1800750614. [DOI] [PubMed] [Google Scholar]
- 10.Endean ED, Barnes SL, Kwolek CJ, Minion DJ, Schwarcz TH, Mentzer RM Jr. Surgical management of thrombotic acute intestinal ischemia. Ann Surg. 2001;233:801–808. doi: 10.1097/00000658-200106000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeier M, Wiesel M, Rambausek M, Ritz E. Non-occlusive mesenteric infarction in dialysis patients: the importance of prevention and early intervention. Nephrol Dial Transplant. 1995;10:771–773. [PubMed] [Google Scholar]
- 12.Quiroga B, Verde E, Abad S, Vega A, Goicoechea M, Reque J, López-Gómez JM, Luño J. Detection of patients at high risk for non-occlusive mesenteric ischemia in hemodialysis. J Surg Res. 2013;180:51–55. doi: 10.1016/j.jss.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Hatemi I, Hatemi G, Çelik AF. Systemic vasculitis and the gut. Curr Opin Rheumatol. 2017;29:33–38. doi: 10.1097/BOR.0000000000000344. [DOI] [PubMed] [Google Scholar]
- 14.Björck M, Wanhainen A. Nonocclusive mesenteric hypoperfusion syndromes: recognition and treatment. Semin Vasc Surg. 2010;23:54–64. doi: 10.1053/j.semvascsurg.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Leone M, Bechis C, Baumstarck K, Ouattara A, Collange O, Augustin P, Annane D, Arbelot C, Asehnoune K, Baldési O, Bourcier S, Delapierre L, Demory D, Hengy B, Ichai C, Kipnis E, Brasdefer E, Lasocki S, Legrand M, Mimoz O, Rimmelé T, Aliane J, Bertrand PM, Bruder N, Klasen F, Friou E, Lévy B, Martinez O, Peytel E, Piton A, Richter E, Toufik K, Vogler MC, Wallet F, Boufi M, Allaouchiche B, Constantin JM, Martin C, Jaber S, Lefrant JY. Outcome of acute mesenteric ischemia in the intensive care unit: a retrospective, multicenter study of 780 cases. Intensive Care Med. 2015;41:667–676. doi: 10.1007/s00134-015-3690-8. [DOI] [PubMed] [Google Scholar]
- 16.Bourcier S, Oudjit A, Goudard G, Charpentier J, Leblanc S, Coriat R, Gouya H, Dousset B, Mira JP, Pène F. Diagnosis of non-occlusive acute mesenteric ischemia in the intensive care unit. Ann Intensive Care. 2016;6:112. doi: 10.1186/s13613-016-0213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Björck M, Bergqvist D, Troëng T. Incidence and clinical presentation of bowel ischaemia after aortoiliac surgery--2930 operations from a population-based registry in Sweden. Eur J Vasc Endovasc Surg. 1996;12:139–144. doi: 10.1016/s1078-5884(96)80098-0. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson J, Hansson E, Andersson B. Intestinal ischemia after cardiac surgery: analysis of a large registry. J Cardiothorac Surg. 2013;8:156. doi: 10.1186/1749-8090-8-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groesdonk HV, Klingele M, Schlempp S, Bomberg H, Schmied W, Minko P, Schäfers HJ. Risk factors for nonocclusive mesenteric ischemia after elective cardiac surgery. J Thorac Cardiovasc Surg. 2013;145:1603–1610. doi: 10.1016/j.jtcvs.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Wurm R, Cho A, Arfsten H, van Tulder R, Wallmüller C, Steininger P, Sterz F, Tendl K, Balassy C, Distelmaier K, Hülsmann M, Heinz G, Adlbrecht C. Non-occlusive mesenteric ischaemia in out of hospital cardiac arrest survivors. Eur Heart J Acute Cardiovasc Care. 2018;7:450–458. doi: 10.1177/2048872616687096. [DOI] [PubMed] [Google Scholar]
- 21.Daviaud F, Grimaldi D, Dechartres A, Charpentier J, Geri G, Marin N, Chiche JD, Cariou A, Mira JP, Pène F. Timing and causes of death in septic shock. Ann Intensive Care. 2015;5:16. doi: 10.1186/s13613-015-0058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reilly PM, Wilkins KB, Fuh KC, Haglund U, Bulkley GB. The mesenteric hemodynamic response to circulatory shock: an overview. Shock. 2001;15:329–343. doi: 10.1097/00024382-200115050-00001. [DOI] [PubMed] [Google Scholar]
- 23.Reintam Blaser A, Acosta S, Arabi YM. A clinical approach to acute mesenteric ischemia. Curr Opin Crit Care. 2021;27:183–192. doi: 10.1097/MCC.0000000000000802. [DOI] [PubMed] [Google Scholar]
- 24.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 25.Corcos O, Castier Y, Sibert A, Gaujoux S, Ronot M, Joly F, Paugam C, Bretagnol F, Abdel-Rehim M, Francis F, Bondjemah V, Ferron M, Zappa M, Amiot A, Stefanescu C, Leseche G, Marmuse JP, Belghiti J, Ruszniewski P, Vilgrain V, Panis Y, Mantz J, Bouhnik Y. Effects of a multimodal management strategy for acute mesenteric ischemia on survival and intestinal failure. Clin Gastroenterol Hepatol 2013; 11: 158-65. :e2. doi: 10.1016/j.cgh.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 26.Klotz S, Vestring T, Rötker J, Schmidt C, Scheld HH, Schmid C. Diagnosis and treatment of nonocclusive mesenteric ischemia after open heart surgery. Ann Thorac Surg. 2001;72:1583–1586. doi: 10.1016/s0003-4975(01)03179-4. [DOI] [PubMed] [Google Scholar]
- 27.Grimaldi D, Guivarch E, Neveux N, Fichet J, Pène F, Marx JS, Chiche JD, Cynober L, Mira JP, Cariou A. Markers of intestinal injury are associated with endotoxemia in successfully resuscitated patients. Resuscitation. 2013;84:60–65. doi: 10.1016/j.resuscitation.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Piton G, Cypriani B, Regnard J, Patry C, Puyraveau M, Capellier G. Catecholamine use is associated with enterocyte damage in critically ill patients. Shock. 2015;43:437–442. doi: 10.1097/SHK.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 29.Piton G, Capellier G. Biomarkers of gut barrier failure in the ICU. Curr Opin Crit Care. 2016;22:152–160. doi: 10.1097/MCC.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 30.Ait-Oufella H, Bourcier S, Lehoux S, Guidet B. Microcirculatory disorders during septic shock. Curr Opin Crit Care. 2015;21:271–275. doi: 10.1097/MCC.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 31.Dubin A, Edul VS, Pozo MO, Murias G, Canullán CM, Martins EF, Ferrara G, Canales HS, Laporte M, Estenssoro E, Ince C. Persistent villi hypoperfusion explains intramucosal acidosis in sheep endotoxemia. Crit Care Med. 2008;36:535–542. doi: 10.1097/01.CCM.0000300083.74726.43. [DOI] [PubMed] [Google Scholar]
- 32.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abraham E, Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit Care Med. 2007;35:2408–2416. doi: 10.1097/01.ccm.0000282072.56245.91. [DOI] [PubMed] [Google Scholar]
- 34.Lobo SM, De Backer D, Sun Q, Tu Z, Dimopoulos G, Preiser JC, Nagy N, Vray B, Vercruy V, Terzi RG, Vincent JL. Gut mucosal damage during endotoxic shock is due to mechanisms other than gut ischemia. J Appl Physiol (1985) 2003;95:2047–2054. doi: 10.1152/japplphysiol.00925.2002. [DOI] [PubMed] [Google Scholar]
- 35.Reintam Blaser A, Malbrain ML, Starkopf J, Fruhwald S, Jakob SM, De Waele J, Braun JP, Poeze M, Spies C. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med. 2012;38:384–394. doi: 10.1007/s00134-011-2459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reintam Blaser A, Preiser JC, Fruhwald S, Wilmer A, Wernerman J, Benstoem C, Casaer MP, Starkopf J, van Zanten A, Rooyackers O, Jakob SM, Loudet CI, Bear DE, Elke G, Kott M, Lautenschläger I, Schäper J, Gunst J, Stoppe C, Nobile L, Fuhrmann V, Berger MM, Oudemans-van Straaten HM, Arabi YM, Deane AM Working Group on Gastrointestinal Function within the Section of Metabolism. Endocrinology and Nutrition (MEN Section) of ESICM. Gastrointestinal dysfunction in the critically ill: a systematic scoping review and research agenda proposed by the Section of Metabolism, Endocrinology and Nutrition of the European Society of Intensive Care Medicine. Crit Care. 2020;24:224. doi: 10.1186/s13054-020-02889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meier-Hellmann A, Reinhart K, Bredle DL, Specht M, Spies CD, Hannemann L. Epinephrine impairs splanchnic perfusion in septic shock. Crit Care Med. 1997;25:399–404. doi: 10.1097/00003246-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 38.De Backer D, Creteur J, Silva E, Vincent JL. Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: which is best? Crit Care Med. 2003;31:1659–1667. doi: 10.1097/01.CCM.0000063045.77339.B6. [DOI] [PubMed] [Google Scholar]
- 39.Krychtiuk KA, Richter B, Lenz M, Hohensinner PJ, Huber K, Hengstenberg C, Wojta J, Heinz G, Speidl WS. Epinephrine treatment but not time to ROSC is associated with intestinal injury in patients with cardiac arrest. Resuscitation. 2020;155:32–38. doi: 10.1016/j.resuscitation.2020.05.046. [DOI] [PubMed] [Google Scholar]
- 40.Reignier J, Boisramé-Helms J, Brisard L, Lascarrou JB, Ait Hssain A, Anguel N, Argaud L, Asehnoune K, Asfar P, Bellec F, Botoc V, Bretagnol A, Bui HN, Canet E, Da Silva D, Darmon M, Das V, Devaquet J, Djibre M, Ganster F, Garrouste-Orgeas M, Gaudry S, Gontier O, Guérin C, Guidet B, Guitton C, Herbrecht JE, Lacherade JC, Letocart P, Martino F, Maxime V, Mercier E, Mira JP, Nseir S, Piton G, Quenot JP, Richecoeur J, Rigaud JP, Robert R, Rolin N, Schwebel C, Sirodot M, Tinturier F, Thévenin D, Giraudeau B, Le Gouge A NUTRIREA-2 Trial Investigators; Clinical Research in Intensive Care and Sepsis (CRICS) group. Enteral versus parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2) Lancet. 2018;391:133–143. doi: 10.1016/S0140-6736(17)32146-3. [DOI] [PubMed] [Google Scholar]
- 41.Piton G, Le Gouge A, Brulé N, Cypriani B, Lacherade JC, Nseir S, Mira JP, Mercier E, Sirodot M, Rigaud JP, Malaquin S, Soum E, Djibre M, Gaudry S, Thévenin D, Reignier J. Impact of the route of nutrition on gut mucosa in ventilated adults with shock: an ancillary of the NUTRIREA-2 trial. Intensive Care Med. 2019;45:948–956. doi: 10.1007/s00134-019-05649-3. [DOI] [PubMed] [Google Scholar]
- 42.Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, Hiesmayr M, Mayer K, Montejo JC, Pichard C, Preiser JC, van Zanten ARH, Oczkowski S, Szczeklik W, Bischoff SC. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38:48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 43.Huisman-de Waal G, Schoonhoven L, Jansen J, Wanten G, van Achterberg T. The impact of home parenteral nutrition on daily life-a review. Clin Nutr. 2007;26:275–288. doi: 10.1016/j.clnu.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Gupta PK, Natarajan B, Gupta H, Fang X, Fitzgibbons RJ Jr. Morbidity and mortality after bowel resection for acute mesenteric ischemia. Surgery. 2011;150:779–787. doi: 10.1016/j.surg.2011.07.079. [DOI] [PubMed] [Google Scholar]
- 45.van den Heijkant TC, Aerts BA, Teijink JA, Buurman WA, Luyer MD. Challenges in diagnosing mesenteric ischemia. World J Gastroenterol. 2013;19:1338–1341. doi: 10.3748/wjg.v19.i9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howard TJ, Plaskon LA, Wiebke EA, Wilcox MG, Madura JA. Nonocclusive mesenteric ischemia remains a diagnostic dilemma. Am J Surg. 1996;171:405–408. doi: 10.1016/S0002-9610(97)89619-5. [DOI] [PubMed] [Google Scholar]
- 47.Nuzzo A, Maggiori L, Ronot M, Becq A, Plessier A, Gault N, Joly F, Castier Y, Vilgrain V, Paugam C, Panis Y, Bouhnik Y, Cazals-Hatem D, Corcos O. Predictive Factors of Intestinal Necrosis in Acute Mesenteric Ischemia: Prospective Study from an Intestinal Stroke Center. Am J Gastroenterol. 2017;112:597–605. doi: 10.1038/ajg.2017.38. [DOI] [PubMed] [Google Scholar]
- 48.Montagnana M, Danese E, Lippi G. Biochemical markers of acute intestinal ischemia: possibilities and limitations. Ann Transl Med. 2018;6:341. doi: 10.21037/atm.2018.07.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Acosta S, Nilsson TK, Björck M. D-dimer testing in patients with suspected acute thromboembolic occlusion of the superior mesenteric artery. Br J Surg. 2004;91:991–994. doi: 10.1002/bjs.4645. [DOI] [PubMed] [Google Scholar]
- 50.Akyildiz H, Akcan A, Oztürk A, Sozuer E, Kucuk C, Karahan I. The correlation of the D-dimer test and biphasic computed tomography with mesenteric computed tomography angiography in the diagnosis of acute mesenteric ischemia. Am J Surg. 2009;197:429–433. doi: 10.1016/j.amjsurg.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Chiu YH, Huang MK, How CK, Hsu TF, Chen JD, Chern CH, Yen DH, Huang CI. D-dimer in patients with suspected acute mesenteric ischemia. Am J Emerg Med. 2009;27:975–979. doi: 10.1016/j.ajem.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 52.Thuijls G, van Wijck K, Grootjans J, Derikx JP, van Bijnen AA, Heineman E, Dejong CH, Buurman WA, Poeze M. Early diagnosis of intestinal ischemia using urinary and plasma fatty acid binding proteins. Ann Surg. 2011;253:303–308. doi: 10.1097/SLA.0b013e318207a767. [DOI] [PubMed] [Google Scholar]
- 53.Matsumoto S, Sekine K, Funaoka H, Yamazaki M, Shimizu M, Hayashida K, Kitano M. Diagnostic performance of plasma biomarkers in patients with acute intestinal ischaemia. Br J Surg. 2014;101:232–238. doi: 10.1002/bjs.9331. [DOI] [PubMed] [Google Scholar]
- 54.Mitsuyoshi A, Obama K, Shinkura N, Ito T, Zaima M. Survival in nonocclusive mesenteric ischemia: early diagnosis by multidetector row computed tomography and early treatment with continuous intravenous high-dose prostaglandin E(1) Ann Surg. 2007;246:229–235. doi: 10.1097/01.sla.0000263157.59422.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merle C, Lepouse C, De Garine A, Frayssinet N, Leymarie F, Leon A, Jolly D. Surgery for mesenteric infarction: prognostic factors associated with early death within 72 hours. J Cardiothorac Vasc Anesth. 2004;18:734–741. doi: 10.1053/j.jvca.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 56.Cosse C, Sabbagh C, Browet F, Mauvais F, Rebibo L, Zogheib E, Chatelain D, Kamel S, Regimbeau JM. Serum value of procalcitonin as a marker of intestinal damages: type, extension, and prognosis. Surg Endosc. 2015;29:3132–3139. doi: 10.1007/s00464-014-4038-0. [DOI] [PubMed] [Google Scholar]
- 57.Leone M, Lefrant JY, Martin C, Constantin JM. Acute mesenteric ischemia, procalcitonin, and intensive care unit. Intensive Care Med. 2015;41:1378. doi: 10.1007/s00134-015-3867-1. [DOI] [PubMed] [Google Scholar]
- 58.Bourcier S, Combes A. The NUTRIREA-2 study. Lancet. 2019;393:1501–1502. doi: 10.1016/S0140-6736(18)33198-2. [DOI] [PubMed] [Google Scholar]
- 59.Kanda T, Fujii H, Tani T, Murakami H, Suda T, Sakai Y, Ono T, Hatakeyama K. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology. 1996;110:339–343. doi: 10.1053/gast.1996.v110.pm8566578. [DOI] [PubMed] [Google Scholar]
- 60.Schellekens DH, Grootjans J, Dello SA, van Bijnen AA, van Dam RM, Dejong CH, Derikx JP, Buurman WA. Plasma intestinal fatty acid-binding protein levels correlate with morphologic epithelial intestinal damage in a human translational ischemia-reperfusion model. J Clin Gastroenterol. 2014;48:253–260. doi: 10.1097/MCG.0b013e3182a87e3e. [DOI] [PubMed] [Google Scholar]
- 61.Sekino M, Funaoka H, Sato S, Okada K, Inoue H, Yano R, Matsumoto S, Ichinomiya T, Higashijima U, Hara T. Intestinal fatty acid-binding protein level as a predictor of 28-day mortality and bowel ischemia in patients with septic shock: A preliminary study. J Crit Care. 2017;42:92–100. doi: 10.1016/j.jcrc.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 62.Treskes N, Persoon AM, van Zanten ARH. Diagnostic accuracy of novel serological biomarkers to detect acute mesenteric ischemia: a systematic review and meta-analysis. Intern Emerg Med. 2017;12:821–836. doi: 10.1007/s11739-017-1668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piton G, Manzon C, Monnet E, Cypriani B, Barbot O, Navellou JC, Carbonnel F, Capellier G. Plasma citrulline kinetics and prognostic value in critically ill patients. Intensive Care Med. 2010;36:702–706. doi: 10.1007/s00134-010-1751-6. [DOI] [PubMed] [Google Scholar]
- 64.Piton G, Manzon C, Cypriani B, Carbonnel F, Capellier G. Acute intestinal failure in critically ill patients: is plasma citrulline the right marker? Intensive Care Med. 2011;37:911–917. doi: 10.1007/s00134-011-2172-x. [DOI] [PubMed] [Google Scholar]
- 65.Groesdonk HV, Raffel M, Speer T, Bomberg H, Schmied W, Klingele M, Schäfers HJ. Elevated endothelin-1 level is a risk factor for nonocclusive mesenteric ischemia. J Thorac Cardiovasc Surg 2015; 149: 1436-42. :e2. doi: 10.1016/j.jtcvs.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 66.Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology. 2000;118:954–968. doi: 10.1016/s0016-5085(00)70183-1. [DOI] [PubMed] [Google Scholar]
- 67.Trompeter M, Brazda T, Remy CT, Vestring T, Reimer P. Non-occlusive mesenteric ischemia: etiology, diagnosis, and interventional therapy. Eur Radiol. 2002;12:1179–1187. doi: 10.1007/s00330-001-1220-2. [DOI] [PubMed] [Google Scholar]
- 68.ID, Kroeker MA, Greenberg HM. Biphasic CT with mesenteric CT angiography in the evaluation of acute mesenteric ischemia: initial experience. Radiology. 2003;229:91–98. doi: 10.1148/radiol.2291020991. [DOI] [PubMed] [Google Scholar]
- 69.Perez-Calatayud AA, Carrillo-Esper R, Anica-Malagon ED, Briones-Garduño JC, Arch-Tirado E, Wise R, Malbrain MLNG. Point-of-care gastrointestinal and urinary tract sonography in daily evaluation of gastrointestinal dysfunction in critically ill patients (GUTS Protocol) Anaesthesiol Intensive Ther. 2018;50:40–48. doi: 10.5603/AIT.a2017.0073. [DOI] [PubMed] [Google Scholar]
- 70.McCarthy E, Little M, Briggs J, Sutcliffe J, Tapping CR, Patel R, Bratby MJ, Uberoi R. Radiology and mesenteric ischaemia. Clin Radiol. 2015;70:698–705. doi: 10.1016/j.crad.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 71.Piton G, Capellier G, Delabrousse E. Echography of the Portal Vein in a Patient With Shock. Crit Care Med. 2016;44:e443–e445. doi: 10.1097/CCM.0000000000001476. [DOI] [PubMed] [Google Scholar]
- 72.L'Her E, Cassaz C, Le Gal G, Cholet F, Renault A, Boles JM. Gut dysfunction and endoscopic lesions after out-of-hospital cardiac arrest. Resuscitation. 2005;66:331–334. doi: 10.1016/j.resuscitation.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 73.Kougias P, Lau D, El Sayed HF, Zhou W, Huynh TT, Lin PH. Determinants of mortality and treatment outcome following surgical interventions for acute mesenteric ischemia. J Vasc Surg. 2007;46:467–474. doi: 10.1016/j.jvs.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 74.Meskó B, Görög M. A short guide for medical professionals in the era of artificial intelligence. NPJ Digit Med. 2020;3:126. doi: 10.1038/s41746-020-00333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krizhevsky A, Sutskever I, Hinton GE. ImageNet Classification with Deep Convolutional Neural Networks. Adv Neural Inf Process Syst. 2012;25:1097–1105. [Google Scholar]
- 76.Mitchell TM. Machine Learning. McGraw-Hill, 2003. [Google Scholar]
- 77.Hinton G, Sejnowski TJ. Unsupervised Learning: Foundations of Neural Computation. MIT Press, 1999. [Google Scholar]
- 78.Andrew G, Barto RSS. Reinforcement Learning: An Introduction. A Bradford Book, 1998. [Google Scholar]
- 79.Hinton G. Deep Learning-A Technology With the Potential to Transform Health Care. JAMA. 2018;320:1101–1102. doi: 10.1001/jama.2018.11100. [DOI] [PubMed] [Google Scholar]
- 80.Schmidt AF, Finan C. Linear regression and the normality assumption. J Clin Epidemiol. 2018;98:146–151. doi: 10.1016/j.jclinepi.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 81.LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436–444. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- 82.Farabet C, Couprie C, Najman L, Lecun Y. Learning hierarchical features for scene labeling. IEEE Trans Pattern Anal Mach Intell. 2013;35:1915–1929. doi: 10.1109/TPAMI.2012.231. [DOI] [PubMed] [Google Scholar]
- 83.Howard J, Ruder S. Universal Language Model Fine-tuning for Text Classification. 2018 Preprint. Available from: arXiv:180106146.
- 84.Peters ME, Neumann M, Iyyer M, Gardner M, Clark C, Lee K, Zettlemoyer L. Deep contextualized word representations. 2018 Preprint. Available from: arXiv:180205365.
- 85.Liu X, Faes L, Kale AU, Wagner SK, Fu DJ, Bruynseels A, Mahendiran T, Moraes G, Shamdas M, Kern C, Ledsam JR, Schmid MK, Balaskas K, Topol EJ, Bachmann LM, Keane PA, Denniston AK. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digit Health. 2019;1:e271–e297. doi: 10.1016/S2589-7500(19)30123-2. [DOI] [PubMed] [Google Scholar]
- 86.Rajpurkar P, Irvin J, Zhu K, Yang B, Mehta H, Duan T, Ding D, Bagul A, Langlotz C, Shpanskaya K, Lungren MP, Ng AY. CheXNet: Radiologist-Level Pneumonia Detection on Chest X-Rays with Deep Learning. 2017 Preprint. Available from: arXiv:171105225.
- 87.De Fauw J, Ledsam JR, Romera-Paredes B, Nikolov S, Tomasev N, Blackwell S, Askham H, Glorot X, O'Donoghue B, Visentin D, van den Driessche G, Lakshminarayanan B, Meyer C, Mackinder F, Bouton S, Ayoub K, Chopra R, King D, Karthikesalingam A, Hughes CO, Raine R, Hughes J, Sim DA, Egan C, Tufail A, Montgomery H, Hassabis D, Rees G, Back T, Khaw PT, Suleyman M, Cornebise J, Keane PA, Ronneberger O. Clinically applicable deep learning for diagnosis and referral in retinal disease. Nat Med. 2018;24:1342–1350. doi: 10.1038/s41591-018-0107-6. [DOI] [PubMed] [Google Scholar]
- 88.Federal Register. Medicare Program; Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long-Term Care Hospital Prospective Payment System and Final Policy Changes and Fiscal Year 2021 Rates; Quality Reporting and Medicare and Medicaid Promoting Interoperability Programs Requirements for Eligible Hospitals and Critical Access Hospitals. [cited 10 January 2021]. Available from: https://www.federalregister.gov/documents/2020/09/18/2020-19637/medicare-program-hospital-inpatient-prospective-payment-systems-for-acute-care-hospitals-and-the .
- 89.Saria S, Rajani AK, Gould J, Koller D, Penn AA. Integration of early physiological responses predicts later illness severity in preterm infants. Sci Transl Med. 2010;2:48ra65. doi: 10.1126/scitranslmed.3001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Johnson AE, Ghassemi MM, Nemati S, Niehaus KE, Clifton DA, Clifford GD. Machine Learning and Decision Support in Critical Care. Proc IEEE Inst Electr Electron Eng. 2016;104:444–466. doi: 10.1109/JPROC.2015.2501978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aczon M, Ledbetter D, Ho L, Gunny A, Flynn A, Williams J, Wetzel R. Dynamic Mortality Risk Predictions in Pediatric Critical Care Using Recurrent Neural Networks. 2017 Preprint. Available from: arXiv:170106675.
- 92.Nielsen AB, Thorsen-Meyer HC, Belling K, Nielsen AP, Thomas CE, Chmura PJ, Lademann M, Moseley PL, Heimann M, Dybdahl L, Spangsege L, Hulsen P, Perner A, Brunak S. Survival prediction in intensive-care units based on aggregation of long-term disease history and acute physiology: a retrospective study of the Danish National Patient Registry and electronic patient records. Lancet Digit Health. 2019;1:e78–e89. doi: 10.1016/S2589-7500(19)30024-X. [DOI] [PubMed] [Google Scholar]
- 93.Thorsen-Meyer HC, Nielsen AB, Nielsen AP, Kaas-Hansen BS, Toft P, Schierbeck J, Strøm T, Chmura PJ, Heimann M, Dybdahl L, Spangsege L, Hulsen P, Belling K, Brunak S, Perner A. Dynamic and explainable machine learning prediction of mortality in patients in the intensive care unit: a retrospective study of high-frequency data in electronic patient records. Lancet Digit Health. 2020;2:e179–e191. doi: 10.1016/S2589-7500(20)30018-2. [DOI] [PubMed] [Google Scholar]
- 94.Komorowski M, Celi LA, Badawi O, Gordon AC, Faisal AA. The Artificial Intelligence Clinician learns optimal treatment strategies for sepsis in intensive care. Nat Med. 2018;24:1716–1720. doi: 10.1038/s41591-018-0213-5. [DOI] [PubMed] [Google Scholar]
- 95.Kam HJ, Kim HY. Learning representations for the early detection of sepsis with deep neural networks. Comput Biol Med. 2017;89:248–255. doi: 10.1016/j.compbiomed.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 96.Lauritsen SM, Kalør ME, Kongsgaard EL, Lauritsen KM, Jørgensen MJ, Lange J, Thiesson B. Early detection of sepsis utilizing deep learning on electronic health record event sequences. Artif Intell Med. 2020;104:101820. doi: 10.1016/j.artmed.2020.101820. [DOI] [PubMed] [Google Scholar]
- 97.Henry KE, Hager DN, Pronovost PJ, Saria S. A targeted real-time early warning score (TREWScore) for septic shock. Sci Transl Med. 2015;7:299ra122. doi: 10.1126/scitranslmed.aab3719. [DOI] [PubMed] [Google Scholar]
- 98.Saria S, Henry KE. Too Many Definitions of Sepsis: Can Machine Learning Leverage the Electronic Health Record to Increase Accuracy and Bring Consensus? Crit Care Med. 2020;48:137–141. doi: 10.1097/CCM.0000000000004144. [DOI] [PubMed] [Google Scholar]
- 99.Fleuren LM, Klausch TLT, Zwager CL, Schoonmade LJ, Guo T, Roggeveen LF, Swart EL, Girbes ARJ, Thoral P, Ercole A, Hoogendoorn M, Elbers PWG. Machine learning for the prediction of sepsis: a systematic review and meta-analysis of diagnostic test accuracy. Intensive Care Med. 2020;46:383–400. doi: 10.1007/s00134-019-05872-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ghosh S, Roberts N, Firmin RK, Jameson J, Spyt TJ. Risk factors for intestinal ischaemia in cardiac surgical patients. Eur J Cardiothorac Surg. 2002;21:411–416. doi: 10.1016/s1010-7940(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 101.Chaudhuri N, James J, Sheikh A, Grayson AD, Fabri BM. Intestinal ischaemia following cardiac surgery: a multivariate risk model. Eur J Cardiothorac Surg. 2006;29:971–977. doi: 10.1016/j.ejcts.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 102.Stahl K, Busch M, Maschke SK, Schneider A, Manns MP, Fuge J, Wiesner O, Meyer BC, Hoeper MM, Hinrichs JB, David S. A Retrospective Analysis of Nonocclusive Mesenteric Ischemia in Medical and Surgical ICU Patients: Clinical Data on Demography, Clinical Signs, and Survival. J Intensive Care Med. 2020;35:1162–1172. doi: 10.1177/0885066619837911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bomberg H, Stroeder J, Karrenbauer K, Groesdonk HV, Wagenpfeil S, Klingele M, Bücker A, Schäfers HJ, Minko P. Establishment of Predictive Models for Nonocclusive Mesenteric Ischemia Comparing 8,296 Control with 452 Study Patients. J Cardiothorac Vasc Anesth. 2019;33:1290–1297. doi: 10.1053/j.jvca.2018.08.194. [DOI] [PubMed] [Google Scholar]
- 104.Vaswani A, Shazeer N, Parmar N, Uszkoreit J, Jones L, Gomez AN, Kaiser L, Polosukhin I. Attention Is All You Need. 2017 Preprint. Available from: arXiv:170603762.
- 105.Li Y, Rao S, Solares JRA, Hassaine A, Ramakrishnan R, Canoy D, Zhu Y, Rahimi K, Salimi-Khorshidi G. BEHRT: Transformer for Electronic Health Records. Sci Rep. 2020;10:7155. doi: 10.1038/s41598-020-62922-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Choi E, Xu Z, Li Y, Dusenberry MW, Flores G, Xue Y, Dai AM. Learning the Graphical Structure of Electronic Health Records with Graph Convolutional Transformer. 2020 Preprint. Available from: arXiv:190604716.
- 107.Barrett T, Upponi S, Benaglia T, Tasker AD. Multidetector CT findings in patients with mesenteric ischaemia following cardiopulmonary bypass surgery. Br J Radiol. 2013;86:20130277. doi: 10.1259/bjr.20130277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jiang M, Li CL, Pan CQ, Lv WZ, Ren YF, Cui XW, Dietrich CF. Nomogram for predicting transmural bowel infarction in patients with acute superior mesenteric venous thrombosis. World J Gastroenterol. 2020;26:3800–3813. doi: 10.3748/wjg.v26.i26.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Park JJ, Kim KA, Nam Y, Choi MH, Choi SY, Rhie J. Convolutional-neural-network-based diagnosis of appendicitis via CT scans in patients with acute abdominal pain presenting in the emergency department. Sci Rep. 2020;10:9556. doi: 10.1038/s41598-020-66674-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rajpurkar P, Park A, Irvin J, Chute C, Bereket M, Mastrodicasa D, Langlotz CP, Lungren MP, Ng AY, Patel BN. AppendiXNet: Deep Learning for Diagnosis of Appendicitis from A Small Dataset of CT Exams Using Video Pretraining. Sci Rep. 2020;10:3958. doi: 10.1038/s41598-020-61055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Copin P, Ronot M, Nuzzo A, Maggiori L, Bouhnik Y, Corcos O, Vilgrain V SURVI team. Inter-reader agreement of CT features of acute mesenteric ischemia. Eur J Radiol. 2018;105:87–95. doi: 10.1016/j.ejrad.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 112.Huang SC, Pareek A, Zamanian R, Banerjee I, Lungren MP. Multimodal fusion with deep neural networks for leveraging CT imaging and electronic health record: a case-study in pulmonary embolism detection. Sci Rep. 2020;10:22147. doi: 10.1038/s41598-020-78888-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cruz Rivera S, Liu X, Chan AW, Denniston AK, Calvert MJ SPIRIT-AI and CONSORT-AI Working Group; SPIRIT-AI and CONSORT-AI Steering Group; SPIRIT-AI and CONSORT-AI Consensus Group. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. Nat Med. 2020;26:1351–1363. doi: 10.1038/s41591-020-1037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu X, Rivera SC, Moher D, Calvert MJ, Denniston AK SPIRIT-AI and CONSORT-AI Working Group. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI Extension. BMJ. 2020;370:m3164. doi: 10.1136/bmj.m3164. [DOI] [PMC free article] [PubMed] [Google Scholar]