Abstract
The population of patients with hepatocellular carcinoma (HCC) overlaps to a high degree with those for chronic kidney disease (CKD) and end-stage renal disease (ESRD). The degrees of renal dysfunction vary, from the various stages of CKD to dialysis-dependent ESRD, which often affects the prognosis and treatment choice of patients with HCC. In addition, renal dysfunction makes treatment more difficult and may negatively affect treatment outcomes. This study summarized the possible causes of the high comorbidity of HCC and renal dysfunction. The possible mechanisms of CKD causing HCC involve uremia itself, long-term dialysis status, immunosuppressive agents for postrenal transplant status, and miscellaneous factors such as hormone alterations and dysbiosis. The possible mechanisms of HCC affecting renal function include direct tumor invasion and hepatorenal syndrome. Finally, we categorized the risk factors that could lead to both HCC and CKD into four categories: Environmental toxins, viral hepatitis, metabolic syndrome, and vasoactive factors. Both CKD and ESRD have been reported to negatively affect HCC prognosis, but more research is warranted to confirm this. Furthermore, ESRD status itself ought not to prevent patients receiving aggressive treatments. This study then adopted the well-known Barcelona Clinic Liver Cancer guidelines as a framework to discuss the indicators for each stage of HCC treatment, treatment-related adverse renal effects, and concerns that are specific to patients with pre-existing renal dysfunction when undergoing aggressive treatments against CKD and ESRD. Such aggressive treatments include liver resection, simultaneous liver kidney transplantation, radiofrequency ablation, and transarterial chemoembolization. Finally, focusing on patients unable to receive active treatment, this study compiled information on the latest systemic pharmacological therapies, including targeted and immunotherapeutic drugs. Based on available clinical studies and Food and Drug Administration labels, this study details the drug indications, side effects, and dose adjustments for patients with renal dysfunction. It also provides a comprehensive review of information on HCC patients with renal dysfunction from disease onset to treatment.
Keywords: Hepatocellular carcinoma, Chronic kidney disease, End-stage renal disease, Hemodialysis, Cancer prognosis, Cancer therapeutics
Core Tip: The varying degrees of renal dysfunction, from the various stages of chronic kidney disease to dialysis-dependent end-stage renal disease, often affect the choice of treatment and prognosis of patients with hepatocellular carcinoma (HCC). This complicates HCC treatment. This review encompasses the presumptive causes of the high degree of comorbidity of HCC and renal dysfunction, the impact of renal dysfunction on HCC prognosis, and the concerns that are specific to patients with pre-existing renal dysfunction for each stage of HCC treatment.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a common malignancy worldwide and accounts for substantial morbidity and mortality[1,2]. However, the etiology, incidence, and mortality of HCC are geographically uneven. Most HCC cases are found in East Asia and Sub-Saharan Africa. Although the incidence rates are relatively lower in Western countries, the mortality rates remain high[1]. The major risk factors for HCC include chronic hepatitis B virus (HBV), chronic hepatitis C virus (HCV), cirrhosis, alcoholic liver disease, and nonalcoholic steatohepatitis (NASH)[3]. Another leading cause of morbidity and mortality worldwide is renal dysfunction, which includes chronic kidney disease (CKD) and end-stage renal disease (ESRD). Caring for patients with renal diseases has greatly burdened health care systems[4]. Notably, patients with renal dysfunction have a higher prevalence of cancer, including liver cancer, compared with the general population[5,6]. Furthermore, such patients—especially those on maintenance dialysis for ESRD—were reported to have a higher prevalence of viral hepatitis compared with the general population[7]. In certain regions where both renal dysfunction and HCC are highly prevalent, the two conditions are highly comorbid[8]. In the present study, we searched and organized the available evidence-based literature to provide a comprehensive review guided by the following research questions: (1) Does any correlation or causality exist between renal dysfunction and the development of HCC? (2) Would renal dysfunction, including CKD and ESRD status, affect the prognosis and treatment outcomes of HCC? And (3) What are the challenges of treating HCC in patients with renal dysfunction in all categories of the Barcelona Clinic Liver Cancer (BCLC) system algorithm?
Despite the lack of a validated international consensus on the management of patients with both renal dysfunction and HCC, we aimed to summarize information that is critical to the development of preventive and therapeutic strategies for this specific population.
ASSOCIATION AND POSSIBLE PATHOPHYSIOLOGICAL MECHANISMS BETWEEN RENAL DYSFUNCTION AND HCC
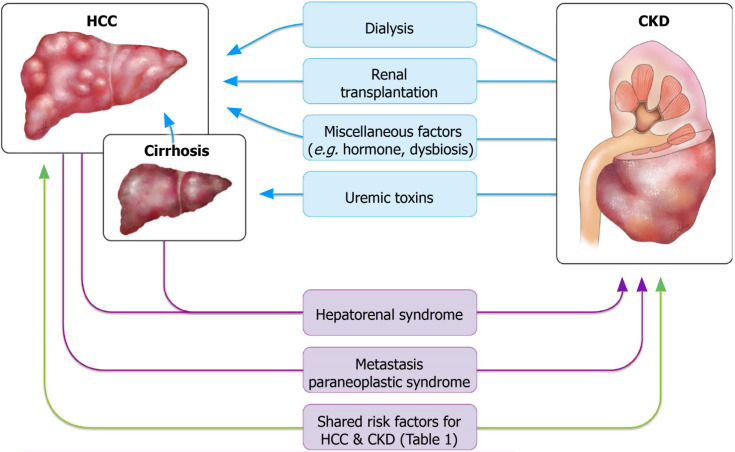
A high incidence of cancer has been reported in patients with renal dysfunction. Both CKD and ESRD were reported to be bidirectionally connected to cancer: Renal dysfunction can serve as a risk factor for cancers including HCC, whereas cancer and related treatments can directly or indirectly lead to or aggravate renal dysfunction[6]. In addition to the mutual relationship between renal dysfunction and cancer, renal dysfunction and HCC share common risk factors that complicate the association between the two diseases. These risk factors can be categorized into vasoactive factors and those related to environmental toxins, viral hepatitis, and metabolic diseases (Table 1). The complex pathways linking renal dysfunction and HCC are depicted in Figure 1. The following subsections summarize the studies that provide evidence for each of these links.
Table 1.
Shared risk factors for hepatocellular carcinoma and chronic kidney disease
|
Risk factors
|
|
| Environmental toxins | Arsenic |
| Cadmium | |
| Aflatoxin | |
| Aristolochic acid | |
| Viral hepatitis | Hepatitis B virus |
| Hepatitis C virus | |
| Metabolic syndrome and related disorders | Non-alcoholic fatty liver disease |
| Nonalcoholic steatohepatitis | |
| Diabetes mellitus | |
| Vasoactive factors | Renin-angiotensin system activation |
Figure 1.
Association between hepatocellular carcinoma and chronic kidney disease. This figure summarizes the confirmed and presumptive links between hepatocellular carcinoma and renal dysfunction. HCC: Hepatocellular carcinoma; CKD: Chronic kidney disease.
Renal dysfunction as a cause of or risk factor for HCC
Cancer risk has been reported to be elevated in patients with renal dysfunction. Numerous studies have demonstrated an increased risk of liver cancer in patients with ESRD on dialysis[9-11]. Limited data are available on whether less advanced CKD, where dialysis is not needed, can increase the risk of liver cancer[12,13]. Several hypotheses have been proposed for these correlations, including a dysregulated immune system, defective DNA repair mechanism, impaired antioxidant defense, accumulation of carcinogenic compounds caused by reduced renal elimination, and the uremia milieu[14]. Because it is well-established that kidney transplant recipients have an increased cancer risk because of the aggressive administration of immunosuppressive agents[15], it is reasonable to have a separate discussion for post-kidney-transplantation status.
CKD without dialysis − accumulation of uremic toxins and elevated serum levels of cytokines: The well-documented phenomenon of patients with impaired renal function having increased cancer risk raises the following question: Could uremic toxin accumulation, one of the direct consequences of renal dysfunction, be carcinogenic?
P-Cresyl sulfate (PCS), a protein-bound uremic toxin prototype that cannot be efficiently removed through routine dialysis procedures, has been found to be fibrogenic in the kidney and vascular system of mice through epithelial-to-mesenchymal transition (EMT)[16-18]. EMT is an irreversible process through which epithelial cells lose their cell polarity and cell–cell adhesion and acquire migratory properties to become mesenchymal cells. EMT has also been implicated in the development of liver fibrosis and cirrhosis[19-22]. It has been widely accepted that transforming growth factor-beta (TGF-β) plays a crucial role in hepatic EMT through stellate cell activation and excessive matrix synthesis[19,20,23,24]. The sequential progression from chronic liver fibrosis to cirrhosis culminates in the development of HCC, which is a major cause of death in patients with compensated cirrhosis[25,26]. A study in Taiwan found that PCS increased the incidence of liver fibrosis in people with HBV and HCV[27]. Although limited data exist on whether the PCS can directly induce EMT in the liver, we postulate that PCS accumulation secondary to renal dysfunction influences liver fibrosis, cirrhosis, and eventually the risk of HCC. Hwang et al[28] conducted a population-based study to examine the mechanism behind the high incidence of HCC in ESRD, and they found PCS to be positively correlated with HCC occurrence[28]. However, in that study, ESRD was no longer associated with a higher incidence of HCC than in the general population after matching was conducted for hepatitis and liver cirrhosis. Those authors concluded that the high incidence of HCC in patients with ESRD was caused by a high viral hepatitis rate rather than by uremia per se[28]. This does not violate our aforementioned assumption, despite appearing to do so, that PCS indirectly contributes to HCC occurrence through liver inflammation and cirrhosis. Further studies are warranted to clarify the link between PCS and HCC.
Cytokines constitute another topic worthy of discussion. Renal dysfunction is also known to increase the level of cytokines in the body, such as interleukin (IL)-1, IL-6, and tumor necrosis factor-α (TNF-α)[29-32]. Several studies have reported that IL-6, TNF-α, and other cytokines are associated with more severe problems in liver necrosis, tissue repair and regeneration, and the accumulation of mutations caused by aberrant cell proliferation, thus increasing the transformation potential of hepatocytes and the risk of HCC[33,34]. More studies focusing on the underlying pathophysiology are required to determine whether these cytokines lead to hepatic carcinogenesis, either directly or indirectly. In addition to proinflammatory properties, uremia has been recognized to compromise normal immune response by enhancing the apoptosis of activated immune cells[35-37]. The immune alterations associated with uremia possibly contribute to cancer occurrence. However, data on site-specific cancers such as HCC are lacking, and further studies are required to delineate the relationships between uremia, immune dysfunction, and HCC.
Effect of ESRD on chronic dialysis status: Studies have suggested that the overall cancer risk in chronic dialysis patients is significantly higher than that in the general population, for both HCC and cancers of other primary sites[10,12,13]. Whether such carcinogenic effects originate from the dialysis procedure itself or other ESRD-related factors remains to be determined[38]. Wong et al[39] found an increased dialysis time to be a significant risk factor for common solid organ cancers regardless of age. They demonstrated that a dose-dependent relationship exists between the duration of maintenance dialysis and overall cumulative cancer risk, and this relationship was independent of the dialysis modality. The findings were attributed to the immunodeficient and chronic inflammatory status in uremia and to the substances the patient was exposed to during dialysis, including nitrites, chloramines, and other unknown elements[39]. Another study on the pattern of excess cancer in dialysis and transplantation reported that dialysis was associated with a small increase in immune deficiency–related cancers, including liver cancer; however, the risk of liver cancer was not particularly high in patients on dialysis [standardized incidence ratio (SIR) 2.2, 95% confidence interval (CI): 1.2-3.7] relative to other types of immune deficiency–related cancers. Moreover, the overall findings for such cancers would be unchanged if liver cancer was excluded from the group[5]. Because both HBV and HCV are prevalent in patients on hemodialysis[40-42], studies have attributed HCC incidence in part to exposure to oncogenic virus infection in dialysis populations through blood transfusion and contamination. Furthermore, a nationwide study in Taiwan revealed that liver cancer was the second most common cancer found in patients receiving dialysis and that the SIR of liver cancer in chronic dialysis patients was also higher than that of their healthy counterparts[9]. This result is inconsistent with the relatively lower frequency of liver cancer found in an international collaborative study[10]. A high frequency of liver cancer among the dialysis population might be explained by HBV and HCV infection being endemic to Taiwan[9]. In the aforementioned studies, except for the effects of infection-related factors, whether the dialysis procedure itself increases HCC risk remains inconclusive.
Kidney transplantation: Kidney transplantation is known to be associated with a marked increase in cancer risk at various sites[12]. In the late 1960s, the immunosuppressive agents administered to patients who underwent a transplant were discovered to increase the risk of cancer; compared with that in recipients of a cardiac or hepatic transplant, the aforementioned risk is a major outcome factor in recipients of a kidney transplant because of their longer survival owing to dialysis being widely available[43]. However, studies that have discussed HCC separately have reported mixed results on whether kidney transplant increases the incidence, reporting either no trend[5] or only a moderately increased risk[12,44]. Therefore, studies have provided limited support for the theory that kidney transplantation and the related application of immunosuppressive agents increases the risk of HCC.
Miscellaneous factors: HCC is more prevalent in men than in women. Both androgen and estrogen sex steroids can contribute to the gender disparity in HCC prevalence, where their effects are distinct to each sex[45]. Higher levels of androgen signaling are associated with an increased risk of HBV-related HCC[46,47], whereas higher estrogen pathway activity plays a protective role in female hepatocarcinogenesis. The estrogen axis is critical for maintaining a lower serum IL-6 level, thus reducing liver cancer risk in women[48,49]. A large cohort study[50] found CKD to increase liver cancer mortality in women and, to a lesser extent, in men; the gender disparity is likely explained by CKD-related hypogonadism, but this remains to be examined. The following sections discuss other possible risk factors shared by HCC and CKD, including environmental toxins, metabolic diseases, and genetic factors and their connections with CKD. However, the causal effects between these factors with CKD and HCC are still being debated.
Dysbiosis, which refers to the qualitative and quantitative alteration of gut microbiota, has been commonly observed in CKD patients. The imbalance of pathogenic flora and symbiotic flora was also implicated in the progression of CKD, increased cardiovascular risk, uremic toxicity, and inflammation[51]. An enhanced permeability of the intestinal barrier, allowing the passage of endotoxins and other bacterial products into the blood, was also reported in CKD[52]. Notably, dysbiosis is another possible risk factor for HCC that has been identified in recent years. Enterohepatic circulation is accompanied by low-grade exposure to gut microbiota–derived metabolites and products, often termed microbiota-associated molecular patterns (MAMPs)[53]. Changes in the intestinal barrier cause leakiness, leading to hepatic exposure to MAMPs. Accumulating evidence from the last decade, mostly from animal studies in rodents, suggests a key role of gut microbiota in the progression of chronic liver disease and in the development of HCC. The HCC risk induced by several types of carcinogens has been found to be profoundly reduced in gut-sterilized mice[54-56]. In a study exploring the differences between the gut microbiota of patients with nonalcoholic fatty liver disease (NAFLD)-related cirrhosis with and without HCC and in healthy controls, gut microbiota profile and systemic inflammation were significantly correlated and can occur together in the process of hepatocarcinogenesis[57]. It remains unclear whether chronic inflammation driven by the translocation of MAMPs from a leaky gut is the dominant contributor to HCC or whether the carcinogenic effect is limited to specific cases such as NAFLD, as does whether dysbiosis could serve as a causal link bridging CKD to HCC[58]. Further studies targeting dysbiosis may elucidate the association between CKD and HCC.
HCC and associated comorbidities causing renal dysfunction
A study found a significant prevalence of CKD in patients with cancer, particularly a higher rate of hematologic malignancy and liver cancer[59]. Hepatorenal syndrome (HRS), either with or without cirrhosis, is a major cause of CKD in patients with HCC. Direct invasion of the renal parenchyma by tumor cells is a rare cause but has been reported in the literature.
HRS: HRS is a unique type of kidney failure that usually occurs in advanced cirrhosis. HRS is characterized by functional impairment of the kidneys caused by vasoconstriction of the renal arteries in the absence of tubular dysfunction, proteinuria, or other histologic changes in the kidneys[54]. The exact mechanism of HRS is not completely understood, but its hallmark is severe vasodilation of the splanchnic arteries owing to portal hypertension, which compromises the effective arterial blood volume and arterial pressure[60]. HRS has two subtypes, which differ in terms of disease course and the presence of detectable precipitating factors[61]. Type 1 HRS is characterized by the rapid progression of renal failure, with the serum creatinine value increasing to greater than 2.5 mg/dL within 2 wk. It is often triggered by a precipitating event, such as bacterial infection, hypotension, or multiple organ failure. By definition, the renal dysfunction caused by type 1 HRS often falls into the category of acute kidney injury (AKI) or an acute deterioration of CKD termed acute-on-chronic kidney injury. By contrast, type 2 HRS is associated with gradual or insidious renal failure with a moderate rise in serum creatinine to 1.5-2.5 mg/dL. One of the major clinical manifestations of type 2 HRS is refractory ascites, for which a specific trigger is often lacking. With a median survival of 6 mo, Type 2 HRS has a superior prognosis compared with type 1 HRS, which has a median survival of less than 2 wk. The relatively moderate disease course is more consonant with the present article’s focus on CKD.
Advanced cirrhosis, a critical precursor lesion of liver cancer, can cause portal hypertension, which may subsequently lead to HRS and result in kidney function deterioration[62]. In a 49-year-old man with HCC, ascites, and measured portal hypertension but no cirrhosis of the liver, the hypertension was secondary to microscopic invasion of the central and small portal veins[63]. Therefore, isolated liver cancer with high tumor burden has also been found to cause portal hypertension and HRS regardless of the presence of comorbid cirrhosis.
Direct tumor metastasis to the kidney: Renal metastasis of HCC is exceedingly rare. A literature search yielded only a few cases of renal metastasis from HCC[64]. Most renal metastases are small, bilateral, and multifocal; however, large and solitary metastatic tumors do occur. These tumors may cause difficulty in diagnosis because they often have no specific radiologic findings to distinguish them from primary renal neoplasms[65]. In some cases, metastatic tumors do not necessarily result in declined renal function. Nevertheless, in one case of HCC metastasis to the kidney mimicking renal cell carcinoma, a prolonged elevated serum creatinine level of 2.24 mg/dL was observed[66]. Therefore, direct invasion of the renal parenchyma by metastatic tumor cells still constitutes a differential diagnosis of renal dysfunction that should not be ignored in patients with HCC.
Paraneoplastic syndrome: Paraneoplastic syndromes arise from the tumor secretion of hormones, peptides, or cytokines or from immune cross-reactivity between malignant and normal tissues. These disorders may affect diverse organ systems, most notably the endocrine, nervous, dermatological, rheumatological, and hematological systems[67,68]. HCC may present with a wide range of paraneoplastic phenomena, which may precede local manifestations of the tumor, including hypercholesterolemia, erythrocytosis, hypoglycemia, and hypercalcemia. Hypercalcemia is a well-known paraneoplastic metabolic condition associated with numerous malignancies. In HCC, hypercalcemia accounts for 7.8% of paraneoplastic syndromes, and it mainly occurs as a terminal event[69]. Most malignancies associated with hypercalcemia have been verified to be caused by parathyroid hormone (PTH)-related peptide. The metastasis of malignancies to the bone can also cause osteolysis and lead to hypercalcemia. In rare cases, hypercalcemia may result from ectopic PTH production by tumors. Patients with hypercalcemia typically present with volume depletion, which might lead to a reduction in glomerular filtration rate (GFR) and calcium clearance[70]. Hypercalcemia may also provoke AKI or hypertension, or aggravate the tubular necrosis frequently found in cases of AKI[71]. Case reports on HCC-induced hypercalcemia have been published, but little information about renal function has been reported in these studies[72]. A case of combined HCC and neuroendocrine carcinoma with ectopic secretion of PTH was documented[73]; the authors observed impaired renal function (creatinine = 2.16 mg/dL), and continuous renal replacement therapy was applied to treat acute renal failure induced by hypercalcemia. However, the patient died during the study period. It is relatively certain that HCC may cause AKI through the paraneoplastic effect of hypercalcemia; nevertheless, more clinical observations and studies are warranted to determine whether HCC-related hypercalcemia causes sustained, even irreversible, renal dysfunction.
Risk factors shared by HCC and renal dysfunction
In the investigation of the relationship between CKD and HCC, some common risk factors have been found. The overlap of these risk factors leads to a high degree of comorbidities between HCC and renal dysfunction. These risk factors may cause the two diseases separately; however, little evidence exists for whether these factors serve as a causal link from HCC to CKD or vice versa. Hence, in this article we attempt to list these risk factors to provide clinicians and researchers with a useful summary. These risk factors can be further divided into several categories, including those of toxic, infectious, metabolic, and vascular origins (Table 1).
Environmental toxins: According to epidemiological and animal studies, several environmental toxins, including arsenic, cadmium, mycotoxins, and aristolochic acid (AA), are associated with both renal impairment and liver cancer[50].
In renal proximal tubules, arsenic or cadmium can cause the depletion of intracellular glutathione stores. This leads to the incremental production of free radicals and results in inflammation and apoptosis[74,75]. A high arsenic level in drinking water was discovered to be a cause for ESRD, independent of other documented risk factors[76]. Continual cadmium exposure can also progress to renal Fanconi syndrome and ultimately CKD[75]. By contrast, arsenic and cadmium carcinogenesis targets the liver[77]. Dimethylarsinic acid and trimethylarsine oxide, the organic metabolites of inorganic arsenic, have been found to cause oxidative DNA damage and enhance cell proliferation in rats[78,79]. In humans, arsenic exposure has also been potentially linked to HCC and other liver tumors or paraneoplastic lesions; for example, hepatomegaly, hepatoportal sclerosis, fibrosis, or cirrhosis often occurs after chronic arsenic exposure[80-82]. According to an in vitro experiment, cadmium is specifically internalized by Kupffer cells, which could lead to the release of various proinflammatory cytokines such as IL-6 and TNF-α[83,84]. Studies have also examined the potential effects of long-term cadmium exposure on the expression of cytochrome P450 (CYP) enzymes in the liver and its impact on the activation and clearance of therapeutic drugs, alcohol, and environmental substances. Under chronic cadmium exposure, DNA adducts associated with CYP-mediated metabolism are produced; they accumulate in liver cells and result in mutations, altered gene expression, and eventually carcinogenesis[85-87]. In epidemiological studies, elevated blood and urine cadmium levels have been found to play a role in HCC, although a direct effect has not been confirmed[77,88].
Aflatoxins (AFs) are highly toxic secondary metabolites that are synthesized by Aspergillus flavus and Aspergillus parasiticu[89]. Afs are the most toxic of all mycotoxins, causing considerable health problems and economic loss through the contamination of food and animal feed. Cereal crops, oil crops, and dairy products are frequently contaminated. Afs can be divided into AFB1 and AFB2, which emit blue fluorescence, and AFG1 and AFG2, which emit green fluorescence under chromatographic and fluorescence analysis[89]. Similar to cadmium, aflatoxin is metabolized by CYP enzymes into aflatoxin-8,9-exo-epoxide. The exo-epoxide can form derivatives with DNA, RNA, and proteins, including the p53 tumor suppressor gene. Moreover, the exo-epoxide can bind DNA to form the predominant promutagenic 8,9-dihydro-8-(N7 guanyl)-9-hydroxy AFB1 adduct (AFB1-N7-Gua), which may secondarily form the more mutagenic AFB1-formamidopyrimidine. These derivatives generate a risk of malignancy over time[89,90]. A review of the epidemiological evidence also indicated that AF is a critical contributor to the high incidence rates of HCC in Asia and Sub-Saharan Africa[91]. In vitro and in vivo studies have revealed that AFB1 and AFM1 cause kidney toxicity through oxidative stress by altering the expression of proline dehydrogenase and L-proline levels, leading to downstream apoptosis[89]. More population-based research is warranted to verify whether Afs are associated with renal dysfunction in humans. However, theoretically aflatoxin is likely to be a common risk factor shared by CKD and HCC.
Another factor worthy of discussion is AA. AA is traditionally known as the main culprit of Chinese herb nephropathy, a type of rapidly progressive renal failure characterized by severe anemia, glycosuria, leukocyturia, mild hypertension, and asymmetric kidneys[92]. Apart from being responsible for renal toxicity, AA has also been implicated in the genesis of urothelial carcinoma. AA-derived DNA adducts and TP53 mutations have been found in ureteric tissues, indicating the carcinogenic potential of AA on the urothelium[93,94]. AA can also result in significant DNA adduct formation and mutation in the liver, albeit at a lower level than in the kidneys[95]. Several epidemiological studies have implicated AA in the development of HCC in Asia, but more data are required to evaluate the impact of AA exposure on HCC occurrence worldwide[96,97].
Viral hepatitis: Chronic HBV and HCV infections are known to be dominant risk factors for HCC. HBV is the most frequent underlying cause of HCC. Case-control studies have demonstrated that chronic HBV carriers have a five- to fifteen-fold increased risk of HCC compared with the general population[98]. Approximately 70% to 90% of HBV-related HCCs develop in patients with cirrhosis, but HBV can also cause HCC in the absence of cirrhosis[99]. Generally, two processes are involved in the hepatocarcinogenesis of HBV infection. Direct mechanisms of hepatocyte transformation include a role for HBV DNA integration, virus mutations, transcriptional activation of growth regulatory genes by HBV-encoded proteins as well as effects on apoptosis, cellular signaling, and DNA repair. The progression of chronic hepatic disease and its associated inflammation, regenerative hyperplasia, and transcriptional deregulation to neoplasia contribute to the indirect pathogenesis of HCC[100,101]. By contrast, the mechanisms underlying HCV-associated carcinogenesis are mainly indirect effects of virus-deregulating host cellular processes, including virus-induced inflammation, oxidative stress, and host immune responses; the resulting genomic instability and mitochondrial damage; and the accompanying increased hepatocyte proliferation and steatosis[102].
In addition, HBV and HCV infection are also established risk factors for CKD. According to epidemiological studies, hepatitis B surface antigen positivity in serum is associated with higher risks of CKD and proteinuria[103]. HBV-related nephropathies include membranous glomerulonephritis, polyarteritis nodosa, and membranoproliferative glomerulonephritis (MPGN)[104]. Moreover, a clinical study reported that HBV causes apoptosis in renal tubular Fas upregulation[105]. HCV has been associated with the development of MPGN and cryoglobulinemia, and it has also been found to increase the risk of CKD[106,107]. Taken together, the aforementioned studies have indicated that both HBV and HCV can be considered critical shared factors in the high comorbidity of HCC and CKD. However, more research is required to verify whether these two infections are causally linked with HCC and CKD.
Metabolic diseases: Abundant epidemiological evidence suggests a correlation between noninsulin-dependent diabetes mellitus (NIDDM, or type 2 diabetes mellitus) and cancers, including HCC[108-110]. Several mechanisms likely explain such an association. Insulin or its precursors may stimulate mitogenesis or carcinogenesis in hepatocytes[111]. Augmented inflammation as measured by TNF-α and IL-6 levels has been found in diabetes[112,113]. Diabetes may also increase the risk of HCC through the development of NASH. Up to 40% of patients with type 1 or type 2 diabetes develop diabetic nephropathy, which is the leading cause of CKD in patients starting renal replacement therapy in developed countries[114]. Consequently, NIDDM is a non-negligible factor contributing to the high comorbidity of HCC and CKD. Likewise, fatty liver disease is a common risk factor for both HCC and CKD. The prevalence of NAFLD is 10%–30% in adults and tends to be higher in developed countries because of the prevalence of obesity and metabolic syndrome[115,116]. NASH belongs to the spectrum of NAFLD and is characterized by hepatic inflammation. In a study conducted to clarify the etiology of non-B, non-C HCC, a total 1374 patients with HCC were enrolled from 1995 to 2009. NASH was noted to be a critical risk factor for HCC, and cirrhosis was detected in 65% of NASH-HCC cases[117]. Studies have defined various factors involved in the necroinflammatory response of NASH, including cytokines, hormones, and neurotransmitters[118]. Rodent animal studies have demonstrated that NASH induced by a high-fat diet is associated with elevated TNF-α and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) with hepatocyte proliferation[113,119]. Hypoadiponectinemia possibly participates in NASH-HCC carcinogenesis, as verified by a adiponectin knock-out mice model[120]. Intriguingly, NAFLD was also found to be a driver of CKD[121]. The presence and severity of NAFLD were noted to be strongly and positively correlated with the prevalence and incidence of CKD, independent of obesity, hypertension, NIDDM, or other common risk factors[122]. Targher et al[123] found that higher levels of patatin-like phospholipase domain–containing protein 3 GG genotype are independently associated with a lower estimated GFR (eGFR) and increased 24-h proteinuria in patients with NAFLD. This single nucleotide polymorphism may be useful for identifying those patients with NAFLD who are also prone to developing CKD[123].
Vasoactive factors − activation of the renin–angiotensin system: The systemic renin–angiotensin system (RAS) regulates blood pressure and maintains normal kidney function. In addition to the traditionally known circulating RAS, scientists have uncovered the existence of a local angiotensin-generating system in several tissues, including the heart, liver, and kidney. The tissue RAS can act locally as a paracrine or autocrine factor to meet the needs of individual tissues, independently of or in cooperation with the circulating counterpart[124]. The crucial role of the RAS in the pathogenesis of CKD has been well documented since the 1980s in experimental and clinical studies[125]. An activated RAS aggravates both systemic and glomerular capillary hypertension, causing hemodynamic injury to the vascular endothelium and glomerulus. Angiotensin II and the downstream product, aldosterone, also exert direct proinflammatory and profibrotic actions, which may promote kidney damage[126,127]. Angiotensin-converting-enzyme inhibition has exhibited considerable therapeutic efficacy in the control of systemic hypertension and the prevention of progressive kidney injury[128,129].
An increasing body of evidence has suggested that the RAS also contributes to liver fibrosis and hepatocarcinogenesis, although probably less so than it does for the kidneys[130]. The main source of the RAS comes from hepatocytes and Kupffer cells, but it has also been found in the bile duct epithelium of the liver[54]. In the liver, angiotensin II regulates cell growth, inflammation, and fibrosis. The expression of angiotensin receptors was found to increase activated hepatic satellite cells (HSCs) following injury. By acting through angiotensin receptors, angiotensin II can be mitogenic for human-activated HSCs, elicit a marked dose-dependent increase in intracellular calcium levels, and induce cell contraction. Angiotensin II also stimulates DNA synthesis and cell proliferation[131]. Angiotensin receptor blockers block the profibrotic and proinflammatory effects of angiotensin II on HSCs, including the expressions of inflammatory cytokines and growth factors (such as TGF-β1, IL-1β, NF-κB, and connective tissue growth factor) and the production of the extracellular matrix[132,133]. RAS might participate in the development of HCC because of the aforementioned proliferative and profibrotic effects. Moreover, angiotensin II was found to enhance vascular endothelial growth factor (VEGF), a potent angiogenic factor that plays an essential role in tumor growth and metastasis[134]. All these findings suggest that the RAS becomes involved in not only kidney injury but also HCC development.
EFFECT OF RENAL DYSFUNCTION ON THE OVERALL PROGNOSIS OF PATIENTS WITH HCC
Few studies have investigated the impact of comorbid renal dysfunction on the prognosis of patients with HCC. However, several studies have examined the influences of CKD or ESRD on specific treatment outcomes and prognosis, which are summarized in the next section. Before we discuss each of these topics in detail, we first provide a concise review of the literature on how renal dysfunction affects the prognosis of patients with HCC.
CKD increases the risk of death in cancer patients. A retrospective study investigating the association between CKD and mortality in cancer patients found an inverse relationship between eGFR and adjusted hazard ratios (HRs)[59]. A single-center study that recruited 440 patients with both CKD and HCC reported that survival from stage 4 and stage 5 CKD was inferior to that of stages 1 and 2. In a prospective population-based analysis, CKD was related to increased cancer-related mortality in liver, kidney, and urinary tract malignancies, with adjusted HRs of 1.74, 3.3, and 7.3, respectively[50]. However, in that study, the percentage of cancer-related mortality decreased, whereas the percentage of cardiovascular mortality markedly increased in patients in more advanced CKD stages. Taken together, we infer from these findings that CKD negatively affects both overall and cancer-related mortality in liver cancer, but some heterogeneity is possible in the etiology of mortality among different stages.
In terms of the prognosis of HCC patients with ESRD on long-term dialysis, studies have reported inconsistent results. In a single-centered observational study comparing the mortality rates of 1298 patients with HCC who were (n = 172) or were not (n = 1126) on long-term hemodialysis, those on hemodialysis had a 2.036-fold greater chance of death than did patients not on hemodialysis. However, cancer-related mortality was not reported and that study was limited by its retrospective nature and short follow-up duration[135]. In another single-center study including 2500 patients with HCC, with only a minority group (1.2%) having ESRD on maintenance dialysis, no significant overall survival difference between dialysis and nondialysis patients was found, although those receiving dialysis had a significantly higher serum bilirubin level, lower serum sodium level, more ascites, and worse performance status[136]. Because 63% of patients undergoing dialysis in that study had undergone nonpalliative management [resection, local ablation, or transarterial chemoembolization (TACE)] for HCC, the authors attributed the unexpectedly good outcomes of the dialysis group to early and aggressive treatment. The authors further concluded that dialysis per se does not predict poor outcomes in patients with HCC and should not be considered a contraindication for active anticancer treatment. In summary, dialysis should not hamper the indicated group from receiving anticancer therapy according to currently available data, and whether dialysis affects the prognosis of HCC remains to be determined.
CHALLENGES OF TREATING HCC IN PATIENTS WITH RENAL DYSFUNCTION
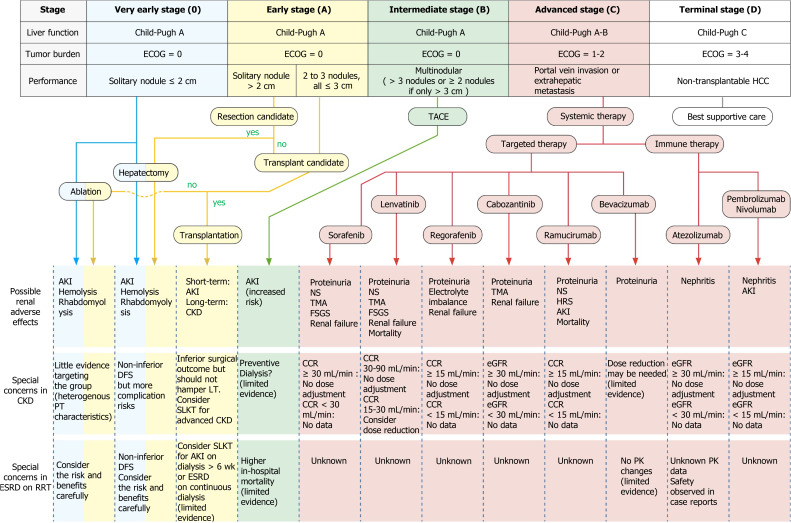
Most patients with HCC have concomitant liver diseases such as chronic hepatitis or cirrhosis. Therefore, the benefits of treating the tumor must be weighed against the potential damage to liver function. This complexity in the management of HCC calls for a multidisciplinary approach, including expertise in hepatology, hepatobiliary surgery, pathology, oncology, radiology, and specialized nursing[137]. The BCLC algorithm classifies patients into one of five stages, taking not only the tumor burden but also the extent of liver dysfunction and the patients’ performance status into consideration[138]. The tumor burden is quantified according to the number and size of nodules, along with the presence or absence of macrovascular tumor invasion or extrahepatic spread. The traditional Child–Turcotte–Pugh (CTP) score provides a subjective assessment of liver function but does not adequately capture the hepatic functional reserve. Alternatives include the Model for End-Stage Liver Disease (MELD) score and the albumin–bilirubin grade[139]. The algorithm then provides treatment recommendations for each stage. Ever since its release in 1999, the BCLC algorithm has been a widely used scoring strategy for HCC. In the very early (0) and early stage (A), patients with a solitary lesion or with up to three nodules less than 3 cm in diameter (without macrovascular invasion or extrahepatic spread) and with preserved liver function are suitable for radical therapies—namely resection, transplantation, or percutaneous treatment. Patients in the intermediate stage (B) do not exhibit symptoms but have large, multifocal tumors without vascular invasion or any spread beyond the liver. If liver function is preserved, these patients could be candidates for TACE. Patients at the advanced stage (C) have symptomatic tumors [grades 1 and 2 according to the Eastern Cooperative Oncology Group (ECOG) Performance Status] or an invasive tumoral pattern of vascular invasion/extrahepatic spread. This group of patients may benefit from systemic medical treatment, which can be categorized into targeted therapy and immunotherapy depending on which of the various pharmacological mechanisms are at work. Finally, patients with terminal disease (D) have poor liver function or marked cancer-related symptoms (ECOG Performance Status > 2). These patients have an extremely poor prognosis and require palliative care[140].
As mentioned in the previous section, CKD was reported to be an independent risk factor for the survival of cancer patients[59]. Treating HCC is difficult in patients with CKD because renal impairment may limit therapeutic options when effective therapy is sought[137]. Currently, perhaps because of the paucity of data regarding HCC outcomes in patients with renal dysfunction, no international treatment consensus exists for this specific population. In the following subsections, we use the BCLC algorithm as a template to discuss special concerns when treating HCC patients with different stages of renal dysfunction compared with the general population. Through reviewing the available literature, we hope to provide the necessary information for developing a modified BCLC for patients with CKD or ESRD (Table 1).
Liver resection
Liver resection is the treatment of choice in noncirrhotic patients and one of the main curative options for early HCC in selected patients with cirrhosis[141-144]. In the last decades, improved surgical techniques and perioperative management as well as improved patient selection have enabled the indications for liver resection to be expanded[145-148]. In a nationwide study using the National Surgical Quality Improvement Program database to investigate the impact of CKD and ESRD on outcomes following major abdominal surgery, 24572 patients were included, of whom only 149 (0.6%) were on hemodialysis preoperatively. In the dialysis group, 30-d postoperative mortality and the overall complication rate (pneumonia and sepsis particularly) were significantly higher than those in the nondialysis group. Furthermore, any degree of preoperative renal impairment, even mild or asymptomatic disease, was associated with clinically significant increases in 30-d postoperative mortality and morbidity following major abdominal surgery[149]. Therefore, the safety and outcomes of liver resection in HCC patients with abnormal renal function deserve a detailed investigation.
Liver resection for HCC in patients with CKD: Few studies have reported on the efficacy and safety of hepatectomy for HCC patients with renal dysfunction. Toshima et al[150] retrospectively reviewed the clinical features of 722 patients with HCC undergoing curative hepatectomy between 1986 and 2009. Seventeen patients (2.4%) with preoperative serum creatinine levels > 2.0 mg/dL were defined as the renal dysfunction group. Clinicopathological characteristics and postoperative outcomes were compared between the renal dysfunction group (n = 17) and the nonrenal dysfunction group (n = 705). Overall survival (P = 0.177) and disease-free survival (P = 0.942) after hepatectomy did not differ significantly. The incidence rates of massive ascites (35.3% vs 14.3%; P = 0.034) and pleural effusion (52.9% vs 17.6%; P = 0.001), defined as massive effusion (ME), were significantly higher in the renal dysfunction group than in the nonrenal dysfunction group. Hypoalbuminemia (≤ 2.8 g/dL; P = 0.031), heavy blood loss (≥ 1000 mL; P = 0.012), and intraoperative blood transfusion (P = 0.007) were risk factors for ME. The authors concluded that preoperative improvement of anemia and reduction of blood loss by meticulous surgical techniques may prevent major complications in patients with renal dysfunction who require hepatectomy for HCC. In another study, data from 735 patients undergoing primary liver resection for HCC between 2002 and 2014 were analyzed[151]. Short- and long-term outcomes were compared between a renal dysfunction group, defined by a preoperative eGFR of < 45 mL/min/1.73 m2, and a nonrenal dysfunction group. The incidence rates of postoperative pleural effusion (24% vs 11%; P = 0.007) and major complications (31% vs 15%; P = 0.003) were significantly higher in the 62 patients with renal dysfunction compared with the nonrenal dysfunction group. In patients with renal dysfunction with CTP score A, the 90-d mortality rate (1.9%) and median survival time (6.11 years) were comparable to those of patients without renal dysfunction. By contrast, patients with renal dysfunction with CTP score B had a very high 90-d mortality rate (22.2%), and a significantly shorter median survival time compared with patients without renal dysfunction (1.19 vs 4.84 years; P = 0.001). The authors concluded that liver resection is safe for CTP-A patients with renal dysfunction, who have comparable oncological outcomes to patients without renal dysfunction; however, liver resection for CTP-B patients with renal dysfunction should be subject to stricter consideration. These findings jointly indicate that CKD status may not necessarily affect overall survival but may lead to more surgical complications. The safety and efficacy of hepatectomy for HCC in patients with CKD could be acceptable if the appropriate patient group is carefully selected, along with judicious pre- and postoperative care.
Liver resection for HCC in patients with ESRD on dialysis: Compared with studies on the CKD population, studies on HCC patients with ESRD on dialysis undergoing hepatic resection are more abundant, probably because these patients’ characteristics are well defined and more effectively targeted. To clarify the role of liver resection in treating HCC in patients with ESRD, Cheng et al[152] conducted a retrospective study to compare the clinicopathological characteristics and operative results of 12 patients with ESRD receiving resection for HCC with those of the other 456 patients without ESRD[152]. The 5-year disease-free survival rates for ESRD and non-ESRD groups were 35.0% and 34.2% (P = 0.31), whereas the 5-year overall survival rates were 67.8% and 53.3% (P = 0.54), respectively. The author commented that liver resection for HCC is justified in select patients with ESRD. In another retrospective study comparing the clinical features of 26 patients with ESRD and HCC with 1198 HCC patients without ESRD undergoing liver resection[153], elevated BUN and creatinine were the only two main independent factors differentiating patients with ESRD and HCC from their counterparts with HCC, and overall and disease-free survival rates were similar between the two groups. Lee et al[136] conducted a retrospective matched-control trial to compare long-term survival between patients with HCC (n = 2472) who were undergoing (n = 30) vs not undergoing dialysis[136]. The patients undergoing dialysis had dual HBV and HCV infection, lower serum α-fetoprotein level (AFP), worse performance status, and higher MELD scores than did the matched controls and patients not undergoing dialysis. No significant difference existed in long-term survival when patients undergoing dialysis were compared with patients who were not or with the matched controls (P = 0.684 and 0.373, respectively). Yeh et al[154] used Taiwan’s National Health Institute Research Database to compare the disease-free survival, overall survival, and perioperative complications between 596 nonuremic controls and 149 patients with uremia and HCC who were also undergoing liver resection. The survival outcomes were comparable between the uremia–HCC cohort and controls, regardless of the extent of hepatic resection. However, the aforementioned had a higher risk of postoperative infections requiring invasive interventions as well as an increased risk of life-threatening heart-associated complications relative to the controls. In summary, ESRD on dialysis does not seem to exert a particular influence on the survival outcomes of patients receiving liver resection for HCC. With careful operative techniques and perioperative care, comparable overall and disease-free survival can be achieved in select patients with ESRD and HCC undergoing liver resection. ESRD on dialysis is not expected to be an obstacle to hepatectomy in the indicated patient group.
Transplantation
Liver transplantation (LT) is considered the gold standard surgical therapy for early-stage HCC co-occurring with cirrhosis or chronic liver disease. The Milan criteria function as the most reliable border for transplantation feasibility both in Western and Asian HCC guidelines[155]. The expected 5-year survival rates of LT for HCC that meets the conventional Milan criteria (single tumor ≤ 5 cm or multiple tumors ≤ 3 nodules ≤ 3 cm in size, without vascular invasion) are 65%-80%, and patients meeting the Milan criteria have a significant survival advantage over patients who do not. LT is recommended as the first-line option for HCC within the Milan criteria but is unsuitable for resection. However, given the distinguished clinicopathological features of patients with renal dysfunction, whether the survival advantage of LT can be extended to this specific population is a more complicated matter. Can patients with renal dysfunction receive LT similar to the general population? How should one assess the feasibility of simultaneous liver kidney transplantation (SLKT)? The following paragraphs address these questions.
LT carries the risk of complications, which occur both immediately after transplantation and in the long term[156]. The main complications in the immediate postoperative period are related to graft dysfunction and rejection and to the surgical technique, infections, and dysfunction involved in the pulmonary, renal, or neurological systems. In the long term, complications are typically a consequence of prolonged immunosuppressive therapy, and they include diabetes mellitus, systemic arterial hypertension, de novo neoplasia, and organ toxicities[157]. AKI is a main complication of LT, especially in the early postoperative period. The reported incidence of AKI after transplantation varies widely because of the different diagnostic criteria used, ranging from 19.26% to 94%[158-161]. Hemodynamic changes during surgery, blood loss, and other stress may cause prerenal AKI or even acute tubular necrosis immediately after surgery[162,163]. Patients who developed AKI tended to have a markedly higher mortality rates[164,165]. It is unclear whether AKI after LT is the primary driver of poorer mortality outcomes or whether this is merely a correlation[166]. CKD is also a common complication after LT with an incidence ranging between 20% and 80%[167,168]. Numerous observations have implicated calcineurin-inhibitor (CNI) as a major risk factor of CKD in recipients of a transplant[169-171], and some studies have advocated the use of tacrolimus or mycophenolate mofetil instead of cyclosporin to reduce the incidence of chronic renal dysfunction after transplantation[170,172]. However, other studies have been unable to show that CNI fully explained post-transplant renal abnormalities[171,173]. Therefore, the hypothesis that CNI use is a major cause of renal dysfunction after LT remains unverified, and CNI’s effect may be overestimated.
Patients with renal dysfunction have been reported to experience poor surgical outcomes following LT. An early study using the National Institute of Diabetes and Digestive and Kidney Diseases LT Database investigated the effect of renal insufficiency in patients with fulminant hepatic failure or chronic liver disease (cirrhosis); that study found that renal insufficiency in fulminant hepatic failure and renal insufficiency requiring dialysis or SLKT in cirrhosis predicts lower patient and graft survival rates after a transplant[174]. In another study reviewing the postoperative courses of 115 liver transplant recipients for liver cirrhosis, the population was divided into two groups based on the threshold of preoperative serum creatinine < 1.0 mg/dL[175]. Patients with preoperative serum creatinine > 1.0 mg/dL had significantly longer intensive care unit stays, higher rates of acute renal failure requiring dialysis, and a greatly increased mortality rate. In a study comparing the LT outcomes of patients with low and high MELD scores, renal function was the most crucial variable associated with morbidity and length of hospital stay[176]. The data not only called for special attention during the perioperative period of renal dysfunction but also cast doubt on whether patients with renal dysfunction are ideal candidates for LT.
Several early studies have found that SLKT could be feasible in patients who have both advanced hepatic and renal dysfunction. In a study compared 16 patients with SLKT and 32 patients with LT matched by age, sex, date, and indication for transplantation; that study reported that both groups had similar levels of reoperation due to bleeding, bacterial infections, liver rejection, arterial hypertension, and median creatinine levels at the 1st and 3rd years[177]. However, early post-transplant dialysis was higher in SLKT than in LT. Survival rates at the 1st, 3rd, 5th, and 7th years were similar in both groups (87.5%, 74%, 74%, and 66% vs 81%, 75%, 75%, and 75% in LT and LKT, respectively). That author inferred that SLKT is an effective therapeutic option in patients with end-stage liver and kidney disease, with most early and late complications and long-term survival being similar to those observed in LT. In one study evaluating the success of SLKT, 20 patients (aged 14-64 years) received a total of 21 LT and 31 kidney transplantation procedures[178]. SLKT was performed in 14 patients, of whom five required further replacement of one or the other of the grafted organs. That study revealed that patients with liver cirrhosis had a very poor prognosis due to their poor overall clinical state at the time of terminal renal failure, whereas patients without liver cirrhosis were more appropriate candidates for SLKT. The author concluded that in general, the indication for SLKT ought to be considered earlier in this case than in the case of transplantation involving only one organ. Notably, a study found that pretransplantation renal dysfunction and exposure to dialysis might affect SLKT treatment outcomes[179]. Adult recipients receiving LT (n = 2700) or SLKT (n = 1361) with moderate renal insufficiency between 2003 and 2013 were included, and the study cohort was stratified into four groups based on serum creatinine level (Scr < 2 mg/dL vs Scr ≥ 2 mg/dL) and on dialysis status at both listing and transplant. SLKT administration led to a greater decrease in post-transplant mortality compared with LT administration across all four groups, but only reached statistical significance (HR 0.77; 95%CI: 0.62–0.96) in recipients not exposed to dialysis and with Scr ≥ 2 mg/dL at transplant. The study indicated the possible advantage of SLKT in patients with both severe liver disease and renal abnormalities. Some studies have indicated that the liver immunologically protects the kidneys after combined liver–kidney transplantation[180,181]. Therefore, patients with end-stage hepatic and renal anomalies may indeed benefit from SLKT. However, this technique faces limitations in being administered widely among HCC patients with renal dysfunction. For example, significant heterogeneity exists in the criteria for SLKT when it comes to noncirrhotic or compensated liver diseases and when it comes to liver transplant candidates with a moderate-to-severe reduction in GFR. To promote discussion and unify the criteria for the indication of SLKT by liver transplant groups, the Spanish LT Society (La Sociedad Española de Trasplante Hepático) held the 6th Consensus Document Meeting on October 20, 2016, in which experts from the 24 authorized Spanish LT programs participated[182]. According to the consensus, SLKT is recommended in patients with liver transplant criteria plus one of the following: (1) CKD in chronic dialysis or eGFR > 30 mL/min; or (2) CKD with eGFR between 30 and 40 mL/min and some signs of poor renal prognosis — such as proteinuria > 1 g/d (> 3 mo) and/or diabetic nephropathy — and/or histological findings of poor prognosis in renal biopsy (more than 30% glomerulosclerosis or more than 30% interstitial fibrosis). SLKT is also recommended in patients who are candidates for LT with acute kidney disease requiring dialysis for 6 consecutive weeks, either continuously or intermittently.
Several more recent studies have specifically focused on the outcomes of patients receiving SLKT for HCC. A study included 2606 patients (mean age: 53 years) receiving SLKT for primary biliary cirrhosis (PBC, n = 76), primary sclerosing cholangitis (n = 81), HBV (n = 98), HCV (n = 945), alcoholic liver disease (n = 495), alcohol and HCV (n = 152), cryptogenic cirrhosis (n = 289), NASH (n = 221), or HCC (n = 249); that study reported that HCV, NASH, and HCC had worse outcomes for liver graft (72%, 66%, and 72% vs 82%; HR: 2.5-3.1), kidney graft (71, 65%, and 71% vs 80%; HR: 2.3-2.8), and patient survival (74, 69, and 69% vs 82%; HR: 2.4-2.7) compared with PBC[183]. In another retrospective analysis of SLKT from the United Network for Organ Sharing registry[184], the authors compared the outcomes of HCC with other transplant indications. HCC was not associated with post-transplant survival among all patients (HR: 1.15; 95%CI: 0.84-1.58) or the propensity score-matched cohort (HR: 0.97; 95%CI: 0.64-1.47). SLKT-HCC patients had similar rates of acute rejection (13.3% vs 10.5%, P = 0.36) and liver graft failure requiring retransplantation (3.2% vs 2.3%, P = 0.44). The author commented that liver transplant candidates with advanced renal dysfunction and HCC may be considered for SLKT[184]. SLKT seems to be a treatment of choice for HCC patients with advanced renal dysfunction. However, more studies specifically targeting patients with HCC as the main indication for SLKT are warranted to support the safety and efficacy of this treatment.
Radiofrequency ablation
Since the early 1990s, radiofrequency ablation (RFA) has been introduced to clinical practices and has rapidly become the first-choice local treatment for small (≤ 3 cm) HCC lesions. Based on the BCLC staging system, RFA is applied for the treatment of patients having very early (Stage 0) and early stage (Stage A) HCC (Figure 2)[138]. For most appropriate patients selected, this treatment is safe and efficient. However, reports of complications are common. Livraghi et al[185] and Takaki et al[186] reported mortality rates between 0.1% and 0.3%[185,186]. The major complication rate was estimated at 2.2% to 2.8%. The causes of death were bowel perforation, peritonitis, tumor rupture, and liver failure due to biliary stricture. The most frequent major complications were hemorrhage and tumor seeding, followed by liver abscess, bowel perforation, hemothorax, and liver failure. Minor complications included acute skin burn, self-limiting intraperitoneal bleeding, subcapsular or intrahepatic hematoma, arterioportal shunt, biliary portal shunt with hemobilia, transient liver decompensation, and direct renal tissue damage. In less common cases, the procedure may cause renal dysfunction or related side effects. Thermal injury could lead to hemolysis and rhabdomyolysis[187-189], and the extensive breakdown and transcellular shift of potassium may lead to varying (and even life-threatening) degrees of hyperkalemia, either in patients with normal baseline renal function or CKD[190]. This clinical implication is anticipated in case of prolonged ablation, and laboratory monitoring during extensive or prolonged RFA procedures is recommended to detect hemolysis early. Laboratory tests including hematocrit, serum potassium, urine hemoglobin, and serum creatine phosphokinase level should be considered[188]. Hemolysis and rhabdomyolysis could also result in AKI[188,189,191,192]. Most patients experience moderately impacted renal function and a slight increase in serum creatinine without deterioration. However, the hemoglobin-mediated obstruction of renal tubules might cause more severe AKI, oliguria, and sometimes even death. One case report even documented progression to CKD[193].
Figure 2.
Current treatment algorithmsfor patients with hepatocellular carcinoma and chronic kidney disease. This figure is based on the Barcelona Clinic Liver Cancer algorithm, which classifies patients into five stages taking not only the tumor burden but also the extent of liver dysfunction and the patients’ performance status into consideration. Described in the table are the indications for each stage of hepatocellular carcinoma treatment, the treatment-related renal adverse effects, and special concerns for patients with pre-existing renal dysfunction in the applying aggressive treatments and the use of the systemic target and immunotherapy. The disease-free survival is compared to the general population without renal impairment. ECOG: The Eastern Cooperative Oncology Group performance status; HCC: Hepatocellular carcinoma; CKD: Chronic kidney disease; ESRD: End stage renal disease; RRT: Renal replacement therapy; AKI: Acute kidney injury; PT: Patient; LT: Liver transplantation; SLKT: Simultaneous liver kidney transplantation; DFS: Disease-free survival; NS: Nephrotic syndrome; TMA: Thrombotic microangiopathy; FSGS: Focal segmental glomerulosclerosis; CCR: Creatinine clearance (mL/min calculated per the Cockcroft-Gault formula); eGFR: Estimated glomerular filtration rate (mL/min/1.73 m2); PK: Pharmacokinetics.
Few original studies or systematic reviews have discussed whether pre-existing renal dysfunction before RFA is related to treatment outcomes, although much more evidence indicating treatment outcomes in patients with ESRD on dialysis receiving RFA for HCC have been emerging. To examine the efficacy and safety of RFA in treating HCC in patients with HD, a study enrolled 108 HD patients with naïve HCC at 15 institutions between 1988 and 2014[194]. Fifty-eight patients with appropriate indications treated with either hepatectomy (n = 23) or RFA (n = 35) were compared with respect to their clinical features, complications, and prognosis. The two treatments did not significantly differ in their overall survival and disease-free survival rates. The author concluded that RFA had a therapeutic efficacy in HD patients with naïve HCC that is comparable to liver resection. Another study included 14 carefully selected HD patients with HCC (five naïve, nine recurrent) who underwent a total of 19 RFA treatments, and revealed no major complications, suggesting that the safety and effectiveness of RFA were not compromised in this specific population[195]. RFA seems to be a promising option for small HCC in patients undergoing regular HD. By contrast, a study using the Japanese Diagnosis Procedure Combination database compared the treatment outcomes in matched-pair samples of 437 dialyzed and 1345 nondialyzed patients[196]. In-hospital mortality and hemorrhagic complications were significantly higher in dialyzed patients with ESRD than in nondialyzed patients. In patients on HD for ESRD, mortality was significantly lower for those aged ≤ 70 years than for those aged older than that (P = 0.02). Patient age may be a useful indicator when considering RFA for HCC in patients with ESRD on HD. Hyperkalemia was also reported in a patient with ESRD on regular HD after RFA for HCC[197]. Therefore, the indications for RFA in dialysis-dependent patients should be considered carefully.
TACE
Transarterial therapy is a standard treatment for unresectable HCC and patients unfit for surgical resection due to compromised hepatic reserve or nonliver general comorbidities[138,198], following which regular contrast-enhanced imaging for residual disease is recommended. The chemotherapeutic agents used in TACE cause tumor necrosis through the combined effects of targeted chemotherapy and arterial embolization[199]. However, the use of a water-soluble iodinated contrast medium in TACE may induce renal failure, especially in high-risk patients with liver cirrhosis-associated nephropathy[200]. AKI is a common complication found after TACE in patients with HCC, and patients with post-TACE AKI have a higher risk of developing complications such as progression to CKD, ESRD, and death[200-204]. Preoperative CTP score, age, proteinuria, hemoglobin, serum total bilirubin, serum uric acid, aminotransferase level, post-TACE gastrointestinal bleeding, and previous post-TACE AKI history have been reported to be predictors of post-TACE AKI in HCC patients[201,205,206].
Given the nephrotoxicity inherent in the intervention, the application of TACE for HCC in patients with underlying renal dysfunction is challenging. According to a retrospective study that investigated the outcomes of TACE in patients with HCC and CKD, more post-therapy complications, including acute renal failure and sepsis, were found in the CKD group than in the non-CKD group[207]. Overall survival in the CKD group was significantly poor (10.9 ± 8.5 vs 23.5 ± 16.3 mo, P < 0.01). However, in another study conducted to clarify the benefits and risk of TACE in patients with HCC and CKD, 35 patients receiving TACE were enrolled and classified into a CKD group [including nondialysis CKD (NDCKD), n = 10 and ESRD, n = 9], and a non-CKD group (n = 16)[208]. The 2- and 5-year survival rates from initial diagnosis were comparable between the CKD and non-CKD groups. The 2- and 5-year survival rates were also similar in patients with NDCKD and those with ESRD. Of note is the strategy of “preventive HD” adopted in that study: All patients with CKD consulted a nephrologist, and HD was performed within 4 h after 20 of the 32 transarterial therapies in the 10 patients with NDCKD to prevent contrast-induced nephropathy in the CKD group. The authors concluded that TACE can be made feasible in patients with CKD by instituting periprocedural HD with survival rates that are similar to those of patients without CKD.
For patients already on regular hemodialysis for ESRD at the time of TACE, data are lacking because invasive treatment is rarely performed in this specific population. A Japanese pair-matched cohort using a nationwide database was recruited to evaluate the in-hospital mortality and complication rates following TACE in this population[209]. A total of 1551 dialyzed and 5585 nondialyzed patients with ESRD were enrolled. The complication rates did not differ between dialyzed and nondialyzed patients, but the in-hospital mortality rate was, at 2.2%, twice as high in dialyzed patients. Among the dialyzed patients, the mortality rate was not significantly associated with sex, age, or Charlson Comorbidity Index. The author concluded that indications for TACE in HD-dependent patients should be considered cautiously by weighing the benefits against the risks.
In summary, the available data regarding TACE in patients with pre-existing renal dysfunction are limited. More studies are warranted before we can definitely determine the safety and feasibility of TACE in patients with CKD or ESRD. Patients with advanced renal dysfunction may benefit from perioperative preventive HD, but further investigations are required to confirm the efficacy and safety of the measure. Because both the CKD and ESRD groups have reported worse prognoses after TACE compared with HCC patients without renal dysfunction, caution should be taken during the treatment planning process, and patients should be well-informed of the risks and complications involved.
Systemic therapy
If HCC is diagnosed at an early stage, a wide array of treatment options that increase overall survival and improve quality of life are available. However, because late diagnosis is common, 70% to 80% of advanced HCC cases will not benefit from tumor resection[3], and only one-third of patients are eligible for curative therapeutic approaches[210]. Current treatment options for patients with unresectable HCC include TACE and systemic medical treatments. Systemic treatments can generally be divided into two categories according to their mechanism of action: Targeted therapy [mainly tyrosine kinase inhibitor (TKI)] and immunotherapy. The following subsections concentrate on the mechanism of action, common adverse effects, and points of caution for people with renal dysfunction with respect to two groups of drugs.
Targeted therapy: The key signal transduction pathways participating in the pathogenesis of HCC include the Wnt-β catenin, EGFR-RAS-MAPK, and c-MET pathways as well as the insulin-like growth factor signaling, Akt/mTOR signaling, and VEGF and platelet-derived growth factor receptor signaling cascades[211]. TKIs are small molecules that inhibit the multiple receptor tyrosine kinases involved in tumor growth, angiogenesis, pathologic bone remodeling, drug resistance, and metastatic progression of cancer[204]. In 2007, a multi-kinase inhibitor (MKI) named sorafenib was approved as the first systemic agent for treating advanced unresectable HCC because a SHARP trial had suggested a survival benefit of approximately 3 mo[212,213]. Sorafenib is an oral MKI that blocks tyrosine kinase receptors (VEGFR-2/3, PDGFR-β, c-Kit, FLT-3, and RET) and other targets (c-Raf and B-Raf)[214]. In the kidneys, glomerular podocytes express VEGF and glomerular endothelial cells express VEGF receptors[215,216]. Podocyte-specific deletion of a single VEGF allele caused proteinuria and capillary endotheliosis in rodents, and disrupted glomerular VEGF signaling was strongly implicated in the pathogenesis of human preeclampsia[208]. Sorafenib’s mechanism of action clearly indicates its ability to induce significant adverse effects on the kidneys, including proteinuria, nephrotic syndrome, and preeclampsia-like syndrome[217,218]. Cases of renal failure, thrombotic microangiopathy (TMA), and focal segmental glomerulosclerosis (FSGS) have also been documented[213,219]. In patients on sorafenib with pre-existing renal dysfunction, studies have found no trend in pharmacokinetic parameters for sorafenib or its metabolites among any renal function group[220,221]. Renal impairment appears to have no clinically relevant effect on the pharmacokinetics of sorafenib and its metabolites; therefore, no dose adjustment was indicated[221]. According to the Food and Drug Administration (FDA), the pharmacokinetics of sorafenib have not been thoroughly confirmed in patients on dialysis[222]. However, an Italian retrospective study investigating the safety and efficacy of sorafenib in patients with renal cell carcinoma and ESRD reported no unexpected major side effects, and the author concluded that sorafenib is not contraindicated in HD groups[223]. In a Japanese study, a 63-year-old man with ESRD on HD started sorafenib therapy (200 mg/d) 8 d after TACE[221]. The pharmacokinetic parameters of sorafenib and its active metabolite M-2 were within the reference levels of patients with normal renal function 8 and 9 d after the initiation of sorafenib. The authors concluded that sorafenib was well tolerated at an initial dose of 200 mg/d for a patient with HCC undergoing HD, thus indicating that renal failure is not necessarily a contraindication for sorafenib therapy.
After the success of sorafenib, various clinical trials were designed in the hope to outperform the efficacy of it. Nevertheless, not until in recent decade had some trials demonstrated the comparable efficacy with sorafenib or survival benefits after first-line treatment failure[224]. The notable novel agents include lenvatinib, regorafenib, cabozantinib, ramucirumab, and bevacizumab.
Lenvatinib was approved for first-line therapy in advanced HCC following the results of the REFLECT trial, a randomized phase III noninferiority trial by Kudo et al[225], which showed that lenvatinib was not inferior to sorafenib in overall survival in untreated advanced HCC[225]. Further multicenter findings have confirmed the efficacy of lenvatinib with or without previous TKI therapies[226,227]. Lenvatinib’s nephrotoxic profile is similar to that sorafenib, including proteinuria, renal failure, TMA, and FSGS[225,228-231]. The enrollment criteria in the original REFLECT trial included adequate renal function, which was defined as creatinine clearance (CCR) > 30 mL/min as calculated using the Cockcroft–Gault formula[225]. In the FDA label, no dose adjustment is recommended for patients with mild (CCR 60-89 mL/min) or moderate (CCR 30-59 mL/min) renal impairment. Lenvatinib concentrations may increase in patients with differentiated thyroid cancer (DTC) or renal cell carcinoma (RCC) and severe (CCR 15-29 mL/min) renal impairment. It is recommended to reduce the dose for patients with DTC or RCC who also have severe renal impairment. However, there exists no recommended dose for lenvatinib in patients with HCC and severe renal impairment. Lenvatinib has not been studied in patients with ESRD[232].
Regorafenib was approved as the second-line therapy for advanced HCC following the results of the RESORCE trial. This randomized, double-blind, placebo-controlled phase III trial demonstrated the effectiveness of regorafenib in patients progressing after sorafenib treatment. The study confirmed the potential of second-line agents and ushered in the era of second-line therapy[233]. Further multicenter studies have verified the efficacy and safety indicated in the RESORCE trial[234,235]. The nephrotoxic effects include proteinuria and renal failure[236,237]. In regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT), an international, multicenter, randomized, placebo-controlled, and phase III trial reported diarrhea in 34% of patients, with 7% experiencing grade 3 or 4 diarrhea, leading to fluid and electrolyte depletion. The sequelae of fluid and electrolyte depletion may result in dehydration, renal failure, and potential cardiovascular compromise[238]. According to a pharmacokinetic modeling and simulation study, the pharmacokinetics of regorafenib are unlikely to be impacted by any stage of renal impairment[239]. The FDA label suggests that no dose adjustment is recommended for patients with renal impairment. The pharmacokinetics of regorafenib have not been studied in patients on dialysis and there exists no recommended dose for this patient population[240].
Cabozantinib is another TKI that blocks the receptors involved in oncogenesis and angiogenesis, including VEGFR 1, 2, and 3; hepatocyte growth factor receptor (MET); AXL; and the angiopoietin receptors TIE-2, RET, c-Kit, and FLT-3 in vitro and in vivo. Cabozantinib was also indicated to be a second-line treatment in the progression of HCC with acquired resistance to sorafenib[241]. In the CELESTIAL trial, cabozantinib achieved significantly superior overall survival compared with the placebo group and was thus approved by the FDA[242]. The nephrotoxic profile of cabozantinib is similar to those of sorafenib and lenvatinib, including renal failure, proteinuria, and TMA[243-245]. However, in the CELESTIAL trial, grade 5 adverse events considered to be related to the drug were reported in six patients in the cabozantinib group (one event each of hepatic failure, bronchoesophageal fistula, portal-vein thrombosis, upper gastrointestinal hemorrhage, pulmonary embolism, and HRS)[242]. The enrollment criteria for the CELESTIAL trial included serum creatinine ≤ 1.5 times the upper normal limit or calculated CCR ≥ 40 mL/min using the Cockcroft–Gault formula occurring in conjunction with either urine protein/creatinine ratio ≤ 1 mg/mg or 24-h urine protein < 1 g[242]. Two clinical pharmacology studies were conducted to characterize the single-dose pharmacokinetics of cabozantinib in individuals with renal and hepatic impairment, respectively[243]. Although mild-to-moderate renal impairment (eGFR ≥ 30 mL/min/1.73 m2) did not result in a clinically relevant difference in the pharmacokinetics of cabozantinib, the author concluded that cabozantinib should be used cautiously in individuals with mild or moderate renal impairment. According to the FDA label, no dose adjustment is recommended in patients with mild or moderate renal impairment[246]. No experience of cabozantinib in patients with severe renal impairment or requiring dialysis has been documented.
Ramucirumab is a fully human recombinant immunoglobulin G (IgG) 1 monoclonal antibody targeting the VEGF2 receptor. A randomized, multicenter, double-blind, placebo-controlled, and phase III trial (REACH) was conducted to examine the safety and efficacy of ramucirumab as a second-line agent for HCC[247]. In the REACH trial, although the second-line treatment with ramucirumab did not significantly improve survival over placebo in patients with advanced HCC, a subgroup analysis revealed better survival in patients with AFP ≥ 400 ng/mL[248,249]. This was later verified in the REACH-2 trial, which was the first positive phase III trial conducted in a biomarker-selected patient population with HCC[250]. Therefore, ramucirumab was approved by the FDA as a second-line treatment for advanced HCC. The renal toxicity profile of ramucirumab includes proteinuria and nephrotic syndrome[251,252]. Renal failure and TMA have also been reported[250,253]. Notably, several predictors of ramucirumab-induced proteinuria have been identified, including systemic blood pressure, the number of cycles, and calcium channel blocker use[248,254]. Notably, in the REACH-2 trial, three deaths in the ramucirumab group were judged to be related to study treatment: one each from AKI, HRS, and renal failure[250]. The FDA label reports no clinically meaningful effect on the pharmacokinetics of ramucirumab in patients with renal impairment (CCR calculated using Cockcroft–Gault, 15-89 mL/min), and thus, no dose adjustment is suggested[255]. The pharmacokinetics of ramucirumab in patients with ESRD are unknown.
Bevacizumab is a humanized anti-VEGF monoclonal antibody that was previously approved by the FDA as a first-line treatment for metastatic colorectal cancer in combination with chemotherapy. In a global, open-label, and phase III trial conducted in 2020, patients with unresectable HCC who had not previously received systemic treatment were randomly assigned at a 2:1 ratio to receive either atezolizumab (discussed later in the text) plus bevacizumab or sorafenib until unacceptable toxic effects or a loss of clinical benefit occurred[256]. The primary end points were overall survival and progression-free survival in the intention-to-treat population, as assessed at an independent review facility according to the Response Evaluation Criteria in Solid Tumors, version 1.1. The study revealed that in patients with unresectable HCC, atezolizumab combined with bevacizumab resulted in superior overall and progression-free survival outcomes than sorafenib did, which led to the combined therapy of atezolimumab plus bevacizumab being approved as the first-line treatment for unresectable HCC by the FDA. Similar to other anti-VEGF or VEGFR blocking agents, the renal toxicity profile of bevacizumab encompasses renal failure[257,258], proteinuria, and nephrotic syndrome[259,260]. Microvascular diseases such as TMA or hemolytic uremic syndrome are not particularly uncommon[261-263]. In addition, sporadic cases of interstitial nephritis have been documented, as verified by renal biopsy findings, improvement after steroid treatment, and cessation of the offending agents[264,265]. A case of minimal change disease was also reported[266]. Per the manufacturer’s instructions, no studies have investigated the pharmacokinetics of bevacizumab in patients with CKD because the kidneys are not major organs for bevacizumab metabolism or excretion[267]. Only one report has been published about the pharmacokinetics of bevacizumab in a dialysis-dependent patient with metastatic renal cancer, who received 5 mg/kg every 2 wk[268]. The drug was not dialyzable, and its pharmacokinetic parameters were similar to the reference values of patients with normal renal function. The author concluded that the drug can be administered any time before or after hemodialysis. The FDA label does not provide information on dose adjustment in patients with renal dysfunction[267].
Immunotherapy: Immunotherapy has been proven to be effective and safe in treating various solid tumors, prolonging overall survival, and offering a tolerable toxicity profile[269]. Immunotherapy negates tumor-expressed extracellular ligands that suppress intrinsic immune response and can be achieved through three main approaches[270]. One approach is to target the inhibitory proteins that prevent T cells from recognizing and eliminating cancer cells and allow regulatory cells to avoid autoimmune destruction by downregulating T-cell activation[269]. Examples of these molecules are cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), programmed cell death protein-1 (PD-1) in addition to its ligand PD-L1, and T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3)[271,272]. Checkpoint inhibitors are antibodies that activate T-cell mediated antitumor responses by selectively blocking the checkpoint receptors PD-1, PD-L1, and CTLA-4[271]. Conversely, therapeutic cancer vaccines that use a tumor-associated antigen (TAA) originating either from whole-cell tumor lysates and recombinant tumor peptides or recombinant viruses encoding for TAAs bring new prospects in treating cancers. TAAs are transferred and presented by major histocompatibility complex class I molecules in atrial premature complexes to effectively induce the activation of cytotoxic T-lymphocytes[273,274]. Another strategy in immune-regulated antitumor response is that of adoptive cell transfer. Immune cells are extracted from patients’ peripheral blood and undergo genetic engineering to express chimeric antigen receptors. These cell membrane proteins bind to specific cancer antigens and stimulate the immune destruction of tumor cells[275].
In HCC, two categories of immune checkpoint inhibitors have been thoroughly examined in clinical trials, namely PD-1/PD-L1 and CTLA-4. Other promising markers are being investigated in animal models, and new agents are being tested in clinical trials[272]. Currently, the FDA has approved checkpoint inhibitors for advanced HCC, including atezolizumab, pembrolizumab, nivolumab, and ipilimumab. The following paragraphs discuss the mechanism of action, common adverse effects, and points of caution for people with renal dysfunction in the use of these agents. The anticipating checkpoint molecule TIM-3 blockade and the related clinical trials are mentioned as well.
Atezolizumab is an engineered IgG1 monoclonal antibody targeting PD-L1. Patients with unresectable HCC who had not previously received systemic treatment were randomly assigned at a 2:1 ratio to receive either atezolizumab plus bevacizumab or sorafenib until unacceptable toxic effects or a loss of clinical benefit occurred[256]. Atezolizumab combined with bevacizumab resulted in superior overall and progression-free survival outcomes than did sorafenib, which led to the FDA approving the combined therapy of atezolizumab plus bevacizumab as the first-line treatment for unresectable HCC (see the preceding paragraph on bevacizumab). In a study focusing on the use of atezolizumab in patients with renal insufficiency, the efficacy and safety of atezolizumab in these special subpopulations from an expanded access program was reported[276]. Objective responses occurred in 0/6 (0%), 4/19 (21%), 1/27 (3.7%), and 12/62 (19%) of evaluable patients with CCR < 30, 30-45, 45-60, and ≥ 60 mL/min, respectively, and stable disease course was observed in three patients with CCR < 30 mL/min. The author concluded that these findings verified the clinical benefit of atezolizumab in patients with compromised renal function. In one case report, a male patient with metastatic urothelial cell carcinoma and ESRD on dialysis was safely treated with atezolizumab[277]. The main kidney-related side effect caused by atezolizumab is acute tubulointerstitial nephritis, as reported in biopsy-proven cases[278,279]. Based on the FDA label, mild or moderate renal impairment (eGFR 30-89 mL/min/1.73 m2) has no clinically significant effect on systemic exposure to atezolizumab; however, the effects of severe renal impairment (eGFR < 30 mL/min/1.73 m2) or severe hepatic impairment on the pharmacokinetics of atezolizumab is unknown[280].
Pembrolizumab is an anti-PD-1 monoclonal antibody. The antitumor effects of pembrolizumab were examined in a phase II trial in patients who were previously treated with advanced HCC (KEYNOTE-224)[281]. Subsequently, a randomized, double-blind, and phase III study (KEYNOTE-240) was conducted to further verify the efficacy and safety of pembrolizumab in this population[282]. The study indicated a favorable risk-to-benefit ratio for pembrolizumab in this population, but the overall and progression-free survival did not reach statistical significance per the specified criteria. Based on the aforementioned trials, the FDA granted accelerated approval to pembrolizumab for patients with HCC who have been previously treated with sorafenib. Several adverse renal effects have been noted during the use of pembrolizumab, including acute tubular injury, acute interstitial nephritis, and minimal change disease; moreover, kidney biopsy was recommended for suspected pembrolizumab-related cases of AKI[283-285]. According to the FDA label, regarding the risk of immune-mediated nephritis, changes in renal function should be monitored during use. The FDA also advised withholding pembrolizumab and administering corticosteroids for grade 2 nephritis or higher, and they also advised permanently discontinuing the drug for severe (Grade 3) or life-threatening (Grade 4) nephritis. Renal impairment (eGFR ≥ 15 mL/min/1.73 m2) has no clinically significant effect on the clearance of pembrolizumab. Insufficient information exists regarding whether clinically important differences exist in the clearance of pembrolizumab in patients with eGFR < 15 mL/min/1.73 m2[286].
Nivolumab is another anti-PD-1 monoclonal antibody. An open-label, noncomparative, phase 1/2, and dose escalation and expansion trial (CheckMate-040) was conducted to assess the safety and efficacy of nivolumab in patients with advanced HCC with or without chronic viral hepatitis[287]. The FDA later granted accelerated approval to nivolumab for patients with HCC who have previously been treated with sorafenib. A randomized, multicenter phase III study (CheckMate-459) of nivolumab vs sorafenib in patients with advanced HCC is currently ongoing to examine the use of nivolumab as a first-line treatment[288]. The renal toxicity profile of nivolumab is similar to that of pembrolizumab, including AKI, acute tubular injury, and immune complex-mediated glomerulonephritis[284,289,290]. The FDA label contains special warnings, prescribes precaution for immune-mediated nephritis and renal dysfunction during use, and advises that patients should be monitored for changes in renal function. The drug should be withdrawn in cases of moderate or severe serum creatinine elevation and permanently discontinued in cases of life-threatening serum creatinine elevation. The effect of renal impairment on the clearance of nivolumab was evaluated through a population pharmacokinetics analysis in patients with mild (eGFR: 60-89 mL/min/1.73 m2), moderate (eGFR: 30-59 mL/min/1.73 m2), or severe (eGFR: 15-29 mL/min/1.73 m2) renal impairment. No clinically important differences in the clearance of nivolumab were found between patients with renal impairment and those with normal renal function. The FDA suggests no dose adjustment in patients with renal impairment[291].
Ipilimumab is a CTLA-4 immune checkpoint inhibitor. The anti-HCC effect of ipilimumab was demonstrated in the nivolumab plus ipilimumab cohort in CheckMate-040, a multicenter, open-label, and phase 1/2 study (described in the preceding paragraph on nivolumab)[292]. The FDA granted accelerated approval to the combination of nivolumab and ipilimumab for patients with HCC who have previously been treated with sorafenib. As with those for other immunotherapy agents, evidence of the adverse renal effects of ipilimumab has indicated their presence in the forms of acute interstitial nephritis and AKI, constituting a cause for alarm[293]. However, the onset of kidney injury as indicated by CTLA-4 antagonist-related renal injury occurs earlier (2–3 mo) than that indicated by PD-1 inhibitors (3–10 mo)[284,294]. Furthermore, a case of ipilimumab-induced lupus nephritis was also reported[295]. Notably, ipilimumab has also been associated with electrolyte disturbances. Ipilimumab-induced hyponatremia caused by pituitary hypophysitis has been documented in case reports[296,297]. The FDA label suggests that patients should be monitored for changes in renal function. Furthermore, the drug should be withdrawn in cases of moderate or severe serum creatinine elevation and permanently discontinued in cases of life-threatening serum creatinine elevation. The effect of renal impairment on the clearance of ipilimumab was evaluated in patients with mild (eGFR: 60-89 mL/min/1.73 m2), moderate (eGFR: 30-59 mL/min/1.73 m2), or severe (eGFR: 15-29 mL/min/1.73 m2) renal impairment compared with patients with normal renal function (eGFR: ≥ 90 mL/min/1.73 m2) in a population pharmacokinetics analysis. No clinically important differences in the clearance of ipilimumab were found between patients with renal impairment and patients with normal renal function. The FDA recommended no dose adjustment for patients with renal impairment[298].
In addition to antibodies against CTLA-4 and PD-1/PD-L1, checkpoint inhibitor targeting TIM-3 is another potential and promising candidate of immunotherapy for cancer treatment[272,299]. TIM-3, a type I surface glycoproteins encoded by the gene on chromosome 5q33.2, was first discovered in 2001 and identified as an immune checkpoint that specifically expressed on interferon-γ-secreting CD4(+) T helper 1 and CD8(+) T cytotoxic cells in both mice and humans[300,301]. TIM-3 acts as a negative regulator of T cell function by triggering cell death upon interaction with its ligand, galectin-9. TIM-3 overexpression has been implicated in the suppression of T-cell responses and T-cell dysfunction; a state referred to as T-cell exhaustion[302]. TIM-3 also has other ligands and is expressed on other cell types like dendritic cells[303], monocytes[304], and mast cells[305]. In chronic HBV infection, TIM-3 expression is elevated in T helper cells, cytotoxic T lymphocytes, dendritic cells, macrophages, and natural killer cells, accompanied by impaired function of these immunocytes[306]. The TIM-3/galectin-9 signaling pathway was found to mediate T-cell senescence in HBV-associated HCC[307]. In addition to the immunomodulation effect, the expression of TIM-3 on tumor cells has been found to regulate the function of tumor cells directly[308]. A mechanistic study showed that TIM-3 expressed by malignant hepatocytes served as a tumor cell-intrinsic receptor to promote tumor growth via triggering NF-κB/IL-6/STAT3 axis[309]. Therefore, TIM-3 is a drug target for treating both chronic viral infection and HCC.
Several clinical trials about the use of anti-TIM-3 monoclonal antibodies in different types of cancer have been registered on ClinicalTrials.gov. MBG453, an anti-TIM-3 monoclonal antibody, was tested for the safety and efficacy of a single agent or in combination with PDR001 (anti-PD-1 antibody) in adult patients with advanced malignancies in a phase I-Ib/II open-label multicenter study (NCT02608268). TSR-022 is another anti-TIM-3 monoclonal antibody and its safety and efficacy are assessed alone in patients with advanced solid tumors (NCT02817633) or in combination with TSR-042 (anti-PD-1 antibody) (NCT03307785). Notably, a phase II trial studying the effect of TSR-022 with TSR-042 in the treatment of patients with locally advanced or metastatic liver cancer is recruiting and results are pending in October 2023 (NCT03680508). There are also various TIM-3 inhibitors studied in the phase I trials, including Sym023 (NCT03489343), BMS986258 (NCT3446040), and RO7121661 (NCT03708328).
In the mice model of nephrotoxic serum nephritis, TIM-3 was found up-regulated in kidneys and exerted a protective role. Administration of the anti-TIM-3 antibody aggravated nephritis as shown by significantly increased albuminuria, respective histological changes, and expression of the renal injury molecule lipocalin-2[310]. Paradoxically, in the other mice model of diabetic nephropathy, TIM-3 was found to worsen the disease via the NF-κB/TNF-α pathway, and its performance in macrophage worsened podocyte injury both in vivo and in vitro studies[311]. Given that limited data is available, its exact role in the development of renal diseases remains unclear. Currently, the renal side effects of anti-TIM-3 antibodies in humans are still unknown. The renal safety profile from the clinical trials is still awaited.
Antivirals use during HCC treatment
Concomitant antiviral therapy is common during HCC treatment, especially the use of anti-HBV nucleoside or nucleotide analogues (NUCs). NUCs therapy could suppress HBV viral replication, achieve biochemical remission, and ameliorate liver inflammation[312-315]. In addition, NUCs therapy could reduce the incidence of liver decompensation, particularly in HCC patients undergoing LT on immunosuppressant and TACE which are prone to cause HBV reactivation or flare[316-319]. Though there is emerging evidence shows NUCs could decrease HCC incidence and recurrence[315,320-325], the extent to which NUCs therapy may reduce the risk for HCC has been debated[326-328]. Recent mainstay therapies for HBV include NUCs with high potency and high genetic barriers, such as entecavir (ETV), tenofovir disoproxil fumarate (TDF), and tenofovir alafenamide (TAF). However, when using in patients with renal dysfunction, there are special considerations that need to be watched.
ETV belongs to a nucleoside analogue with high potency. However, it has lower efficacy if lamivudine resistance presents previously[329]. Relatively safe renal safety profiles were reported both in rats and humans[330-333]. Since ETV is eliminated primarily from kidneys, renal dose adjustment is needed in patients with CCR less than 50 mL/min to avoid over-exposure to this drug[334,335].
TDF, a nucleotide analogue, is a prodrug of tenofovir that is absorbed from the intestine and cleaved to release tenofovir, which is then phosphorylated inside hepatocytes to form active tenofovir diphosphate targeting viral reverse transcriptase[336]. The adverse effects of long-term use include elevated creatinine, Fanconi syndrome, and osteoporosis[337,338]. TDF is also eliminated by the kidneys in the majority and is not suggested in patients with CCR less than 50 mL/min by some society guidelines because of its nephrotoxicity to proximal renal tubules[339]. Furthermore, when using in the scenario of HCC treatment, renal functions of patients receiving repeated computed tomography exams should be closely followed in case of deterioration[340].
TAF is the other novel prodrug of tenofovir. In an in vitro study, TAF resulted in high levels of the pharmacologically active metabolite tenofovir diphosphate than TDF[341]. A recent study showed comparable viral suppression and serologic response between TAF and TDF in non-cirrhotic HBV patients[342]. Besides, TAF has no proximal renal transporter-dependent cytotoxicity, which may lead to an improved renal safety profile[343]. Nevertheless, there was insufficient data in patients whose CCR below 15mL/min not receiving chronic hemodialysis and thus TAF is not recommended in this patient group[344].
CONCLUSION
The HCC patient population highly overlaps with those for CKD and ESRD. This article summarized the possible causes of the high comorbidity of HCC and renal dysfunction (Figure 1), including the possible mechanisms of CKD causing HCC, the pathophysiology of HCC affecting renal function, and the common risk factors shared by both HCC and CKD (Table 1). Both CKD and ESRD have been reported to negatively affect the prognosis of HCC. The article then adopted the well-known BCLC guidelines as a template (Figure 2) to discuss the indications for each stage of HCC treatment, the treatment-related adverse renal effects, and the concerns that are specific to patients with pre-existing renal dysfunction in the application of aggressive treatments such as liver resection, SLKT, RFA, and TACE, and in the use of the latest systemic target and immunotherapy approaches among the CKD and ESRD population. This article provides a comprehensive review of HCC patients with renal dysfunction from disease onset to treatment; the findings are expected to aid clinicians and scholars.
ACKNOWLEDGEMENTS
The authors wish to thank Miss Ingrid Kuo and the Center for Big Data Analytics and Statistics at Chang Gung Memorial Hospital for creating the illustrations used herein.
Footnotes
Conflict-of-interest statement: The authors declare no competing financial interests.
Manuscript source: Invited manuscript
Peer-review started: January 28, 2021
First decision: March 6, 2021
Article in press: June 16, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhu J S-Editor: Fan JR L-Editor: A P-Editor: Xing YX
Contributor Information
Hsuan Yeh, Department of Nephrology, Chang Gung Memorial Hospital and Chang Gung University, Taipei 105, Taiwan.
Chung-Cheng Chiang, Department of Gastroenterology and Hepatology, Chang Gung Memorial Hospital, Taoyuan 333, Taiwan.
Tzung-Hai Yen, Department of Nephrology, Chang Gung Memorial Hospital and Chang Gung University, Taipei 105, Taiwan. m19570@adm.cgmh.org.tw.
References
- 1.Baecker A, Liu X, La Vecchia C, Zhang ZF. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur J Cancer Prev. 2018;27:205–212. doi: 10.1097/CEJ.0000000000000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mak LY, Cruz-Ramón V, Chinchilla-López P, Torres HA, LoConte NK, Rice JP, Foxhall LE, Sturgis EM, Merrill JK, Bailey HH, Méndez-Sánchez N, Yuen MF, Hwang JP. Global Epidemiology, Prevention, and Management of Hepatocellular Carcinoma. Am Soc Clin Oncol Educ Book. 2018;38:262–279. doi: 10.1200/EDBK_200939. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 4.El Nahas M. The global challenge of chronic kidney disease. Kidney Int. 2005;68:2918–2929. doi: 10.1111/j.1523-1755.2005.00774.x. [DOI] [PubMed] [Google Scholar]
- 5.Stewart JH, Vajdic CM, van Leeuwen MT, Amin J, Webster AC, Chapman JR, McDonald SP, Grulich AE, McCredie MR. The pattern of excess cancer in dialysis and transplantation. Nephrol Dial Transplant. 2009;24:3225–3231. doi: 10.1093/ndt/gfp331. [DOI] [PubMed] [Google Scholar]
- 6.Stengel B. Chronic kidney disease and cancer: a troubling connection. J Nephrol. 2010;23:253–262. [PMC free article] [PubMed] [Google Scholar]
- 7.Zacks SL, Fried MW. Hepatitis B and C and renal failure. Infect Dis Clin North Am. 2001;15:877–899. doi: 10.1016/s0891-5520(05)70177-x. [DOI] [PubMed] [Google Scholar]
- 8.Chen YC, Su YC, Li CY, Wu CP, Lee MS. A nationwide cohort study suggests chronic hepatitis B virus infection increases the risk of end-stage renal disease among patients in Taiwan. Kidney Int. 2015;87:1030–1038. doi: 10.1038/ki.2014.363. [DOI] [PubMed] [Google Scholar]
- 9.Lin HF, Li YH, Wang CH, Chou CL, Kuo DJ, Fang TC. Increased risk of cancer in chronic dialysis patients: a population-based cohort study in Taiwan. Nephrol Dial Transplant. 2012;27:1585–1590. doi: 10.1093/ndt/gfr464. [DOI] [PubMed] [Google Scholar]
- 10.Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, Wolfe RA, Jones E, Disney AP, Briggs D, McCredie M, Boyle P. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet. 1999;354:93–99. doi: 10.1016/s0140-6736(99)06154-1. [DOI] [PubMed] [Google Scholar]
- 11.Chan-Yeung M. A clinician's approach to determine the diagnosis, prognosis, and therapy of occupational asthma. Med Clin North Am. 1990;74:811–822. doi: 10.1016/s0025-7125(16)30554-5. [DOI] [PubMed] [Google Scholar]
- 12.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 13.Chapman JR, Webster AC, Wong G. Cancer in the transplant recipient. Cold Spring Harb Perspect Med. 2013;3 doi: 10.1101/cshperspect.a015677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vamvakas S, Bahner U, Heidland A. Cancer in end-stage renal disease: potential factors involved -editorial- Am J Nephrol. 1998;18:89–95. doi: 10.1159/000013314. [DOI] [PubMed] [Google Scholar]
- 15.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 16.Sun CY, Chang SC, Wu MS. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS One. 2012;7:e34026. doi: 10.1371/journal.pone.0034026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han H, Chen Y, Zhu Z, Su X, Ni J, Du R, Zhang R, Jin W. p-Cresyl sulfate promotes the formation of atherosclerotic lesions and induces plaque instability by targeting vascular smooth muscle cells. Front Med. 2016;10:320–329. doi: 10.1007/s11684-016-0463-x. [DOI] [PubMed] [Google Scholar]
- 18.Opdebeeck B, Maudsley S, Azmi A, De Maré A, De Leger W, Meijers B, Verhulst A, Evenepoel P, D'Haese PC, Neven E. Indoxyl Sulfate and p-Cresyl Sulfate Promote Vascular Calcification and Associate with Glucose Intolerance. J Am Soc Nephrol. 2019;30:751–766. doi: 10.1681/ASN.2018060609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nitta T, Kim JS, Mohuczy D, Behrns KE. Murine cirrhosis induces hepatocyte epithelial mesenchymal transition and alterations in survival signaling pathways. Hepatology. 2008;48:909–919. doi: 10.1002/hep.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dooley S, Hamzavi J, Ciuclan L, Godoy P, Ilkavets I, Ehnert S, Ueberham E, Gebhardt R, Kanzler S, Geier A, Breitkopf K, Weng H, Mertens PR. Hepatocyte-specific Smad7 expression attenuates TGF-beta-mediated fibrogenesis and protects against liver damage. Gastroenterology. 2008;135:642–659. doi: 10.1053/j.gastro.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 21.Popov Y, Schuppan D. Epithelial-to-mesenchymal transition in liver fibrosis: dead or alive? Gastroenterology. 2010;139:722–725. doi: 10.1053/j.gastro.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Wells RG. The epithelial-to-mesenchymal transition in liver fibrosis: here today, gone tomorrow? Hepatology. 2010;51:737–740. doi: 10.1002/hep.23529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee WR, Kim KH, An HJ, Kim JY, Lee SJ, Han SM, Pak SC, Park KK. Apamin inhibits hepatic fibrosis through suppression of transforming growth factor β1-induced hepatocyte epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2014;450:195–201. doi: 10.1016/j.bbrc.2014.05.089. [DOI] [PubMed] [Google Scholar]
- 24.Kong D, Zhang F, Shao J, Wu L, Zhang X, Chen L, Lu Y, Zheng S. Curcumin inhibits cobalt chloride-induced epithelial-to-mesenchymal transition associated with interference with TGF-β/Smad signaling in hepatocytes. Lab Invest. 2015;95:1234–1245. doi: 10.1038/labinvest.2015.107. [DOI] [PubMed] [Google Scholar]
- 25.Ramakrishna G, Rastogi A, Trehanpati N, Sen B, Khosla R, Sarin SK. From cirrhosis to hepatocellular carcinoma: new molecular insights on inflammation and cellular senescence. Liver Cancer. 2013;2:367–383. doi: 10.1159/000343852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Tsai IT, Wang CP, Yu TH, Lu YC, Lin CW, Lu LF, Wu CC, Chung FM, Lee YJ, Hung WC, Hsu CC. Circulating visfatin level is associated with hepatocellular carcinoma in chronic hepatitis B or C virus infection. Cytokine. 2017;90:54–59. doi: 10.1016/j.cyto.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Hwang JC, Weng SF, Weng RH. High incidence of hepatocellular carcinoma in ESRD patients: caused by high hepatitis rate or 'uremia'? Jpn J Clin Oncol. 2012;42:780–786. doi: 10.1093/jjco/hys100. [DOI] [PubMed] [Google Scholar]
- 29.Cavaillon JM, Poignet JL, Fitting C, Delons S. Serum interleukin-6 in long-term hemodialyzed patients. Nephron. 1992;60:307–313. doi: 10.1159/000186770. [DOI] [PubMed] [Google Scholar]
- 30.Herbelin A, Nguyen AT, Zingraff J, Ureña P, Descamps-Latscha B. Influence of uremia and hemodialysis on circulating interleukin-1 and tumor necrosis factor alpha. Kidney Int. 1990;37:116–125. doi: 10.1038/ki.1990.16. [DOI] [PubMed] [Google Scholar]
- 31.Descamps-Latscha B, Herbelin A, Nguyen AT, Roux-Lombard P, Zingraff J, Moynot A, Verger C, Dahmane D, de Groote D, Jungers P. Balance between IL-1 beta, TNF-alpha, and their specific inhibitors in chronic renal failure and maintenance dialysis. Relationships with activation markers of T cells, B cells, and monocytes. J Immunol. 1995;154:882–892. [PubMed] [Google Scholar]
- 32.Rapa SF, Di Iorio BR, Campiglia P, Heidland A, Marzocco S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int J Mol Sci. 2019;21 doi: 10.3390/ijms21010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rapicetta M, Ferrari C, Levrero M. Viral determinants and host immune responses in the pathogenesis of HBV infection. J Med Virol. 2002;67:454–457. doi: 10.1002/jmv.10096. [DOI] [PubMed] [Google Scholar]
- 34.Freeman AJ, Marinos G, Ffrench RA, Lloyd AR. Immunopathogenesis of hepatitis C virus infection. Immunol Cell Biol. 2001;79:515–536. doi: 10.1046/j.1440-1711.2001.01036.x. [DOI] [PubMed] [Google Scholar]
- 35.Fernández-Fresnedo G, Ramos MA, González-Pardo MC, de Francisco AL, López-Hoyos M, Arias M. B lymphopenia in uremia is related to an accelerated in vitro apoptosis and dysregulation of Bcl-2. Nephrol Dial Transplant. 2000;15:502–510. doi: 10.1093/ndt/15.4.502. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt S, Westhoff TH, Krauser P, Ignatius R, Jankowski J, Jankowski V, Zidek W, van der Giet M. The uraemic toxin phenylacetic acid impairs macrophage function. Nephrol Dial Transplant. 2008;23:3485–3493. doi: 10.1093/ndt/gfn266. [DOI] [PubMed] [Google Scholar]
- 37.Meier P, Dayer E, Blanc E, Wauters JP. Early T cell activation correlates with expression of apoptosis markers in patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:204–212. doi: 10.1681/ASN.V131204. [DOI] [PubMed] [Google Scholar]
- 38.Taborelli M, Toffolutti F, Del Zotto S, Clagnan E, Furian L, Piselli P, Citterio F, Zanier L, Boscutti G, Serraino D Italian Transplant & Cancer Cohort Study. Increased cancer risk in patients undergoing dialysis: a population-based cohort study in North-Eastern Italy. BMC Nephrol. 2019;20:107. doi: 10.1186/s12882-019-1283-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong G, Turner RM, Chapman JR, Howell M, Lim WH, Webster AC, Craig JC. Time on dialysis and cancer risk after kidney transplantation. Transplantation. 2013;95:114–121. doi: 10.1097/TP.0b013e31827743b4. [DOI] [PubMed] [Google Scholar]
- 40.Duong MC, Nguyen VTT, Otsu S, McLaws ML. Prevalence of hepatitis B and C virus infections in hemodialysis patients in Vietnam: A systematic review and meta-analysis. JGH Open. 2020;4:29–38. doi: 10.1002/jgh3.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fabrizi F, Poordad FF, Martin P. Hepatitis C infection and the patient with end-stage renal disease. Hepatology. 2002;36:3–10. doi: 10.1053/jhep.2002.34613. [DOI] [PubMed] [Google Scholar]
- 42.Fabrizi F, Lunghi G, Martin P. Hepatitis B virus infection in hemodialysis: recent discoveries. J Nephrol. 2002;15:463–468. [PubMed] [Google Scholar]
- 43.Vial T, Descotes J. Immunosuppressive drugs and cancer. Toxicology. 2003;185:229–240. doi: 10.1016/s0300-483x(02)00612-1. [DOI] [PubMed] [Google Scholar]
- 44.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905–913. doi: 10.1111/j.1600-6143.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 45.Yeh SH, Chen PJ. Gender disparity of hepatocellular carcinoma: the roles of sex hormones. Oncology. 2010;78 Suppl 1:172–179. doi: 10.1159/000315247. [DOI] [PubMed] [Google Scholar]
- 46.Chiu CM, Yeh SH, Chen PJ, Kuo TJ, Chang CJ, Yang WJ, Chen DS. Hepatitis B virus X protein enhances androgen receptor-responsive gene expression depending on androgen level. Proc Natl Acad Sci U S A. 2007;104:2571–2578. doi: 10.1073/pnas.0609498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu MW, Cheng SW, Lin MW, Yang SY, Liaw YF, Chang HC, Hsiao TJ, Lin SM, Lee SD, Chen PJ, Liu CJ, Chen CJ. Androgen-receptor gene CAG repeats, plasma testosterone levels, and risk of hepatitis B-related hepatocellular carcinoma. J Natl Cancer Inst. 2000;92:2023–2028. doi: 10.1093/jnci/92.24.2023. [DOI] [PubMed] [Google Scholar]
- 48.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 49.Prieto J. Inflammation, HCC and sex: IL-6 in the centre of the triangle. J Hepatol. 2008;48:380–381. doi: 10.1016/j.jhep.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 50.Weng PH, Hung KY, Huang HL, Chen JH, Sung PK, Huang KC. Cancer-specific mortality in chronic kidney disease: longitudinal follow-up of a large cohort. Clin J Am Soc Nephrol. 2011;6:1121–1128. doi: 10.2215/CJN.09011010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nallu A, Sharma S, Ramezani A, Muralidharan J, Raj D. Gut microbiome in chronic kidney disease: challenges and opportunities. Transl Res. 2017;179:24–37. doi: 10.1016/j.trsl.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cigarran Guldris S, González Parra E, Cases Amenós A. Gut microbiota in chronic kidney disease. Nefrologia. 2017;37:9–19. doi: 10.1016/j.nefro.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Zitvogel L, Galluzzi L, Viaud S, Vétizou M, Daillère R, Merad M, Kroemer G. Cancer and the gut microbiota: an unexpected link. Sci Transl Med. 2015;7:271ps1. doi: 10.1126/scitranslmed.3010473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.composition of alcoholic beverages, additives and contaminants. IARC Monogr Eval Carcinog Risks Hum. 1988;44:71–99. [PMC free article] [PubMed] [Google Scholar]
- 55.Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong W, Tang L, Lin Y, He YQ, Zou SS, Wang C, Zhang HL, Cao GW, Wu MC, Wang HY. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010;52:1322–1333. doi: 10.1002/hep.23845. [DOI] [PubMed] [Google Scholar]
- 56.Yamada S, Takashina Y, Watanabe M, Nagamine R, Saito Y, Kamada N, Saito H. Bile acid metabolism regulated by the gut microbiota promotes non-alcoholic steatohepatitis-associated hepatocellular carcinoma in mice. Oncotarget. 2018;9:9925–9939. doi: 10.18632/oncotarget.24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, Sanguinetti M, Morelli D, Paroni Sterbini F, Petito V, Reddel S, Calvani R, Camisaschi C, Picca A, Tuccitto A, Gasbarrini A, Pompili M, Mazzaferro V. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology. 2019;69:107–120. doi: 10.1002/hep.30036. [DOI] [PubMed] [Google Scholar]
- 58.Schwabe RF, Greten TF. Gut microbiome in HCC - Mechanisms, diagnosis and therapy. J Hepatol. 2020;72:230–238. doi: 10.1016/j.jhep.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 59.Na SY, Sung JY, Chang JH, Kim S, Lee HH, Park YH, Chung W, Oh KH, Jung JY. Chronic kidney disease in cancer patients: an independent predictor of cancer-specific mortality. Am J Nephrol. 2011;33:121–130. doi: 10.1159/000323740. [DOI] [PubMed] [Google Scholar]
- 60.Erly B, Carey WD, Kapoor B, McKinney JM, Tam M, Wang W. Hepatorenal Syndrome: A Review of Pathophysiology and Current Treatment Options. Semin Intervent Radiol. 2015;32:445–454. doi: 10.1055/s-0035-1564794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Licata A, Maida M, Bonaccorso A, Macaluso FS, Cappello M, Craxì A, Almasio PL. Clinical course and prognostic factors of hepatorenal syndrome: A retrospective single-center cohort study. World J Hepatol. 2013;5:685–691. doi: 10.4254/wjh.v5.i12.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 63.Cooney TG, Bauer DC, Knauer CM. Portal hypertension associated with hepatocellular carcinoma with cirrhosis. Am J Gastroenterol. 1980;74:436–438. [PubMed] [Google Scholar]
- 64.Aron M, Nair M, Hemal AK. Renal metastasis from primary hepatocellular carcinoma. A case report and review of the literature. Urol Int. 2004;73:89–91. doi: 10.1159/000078812. [DOI] [PubMed] [Google Scholar]
- 65.D'Antonio A, Caleo A, Caleo O, Addesso M, Boscaino A. Hepatocellular carcinoma metastatic to the kidney mimicking renal oncocytoma. Hepatobiliary Pancreat Dis Int. 2010;9:550–552. [PubMed] [Google Scholar]
- 66.Jang TY, Yeh ML. Hepatocellular carcinoma metastatic to kidney mimicking renal cell carcinoma. Kaohsiung J Med Sci. 2017;33:161–162. doi: 10.1016/j.kjms.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 67.Thomas DM, Nasim MM, Gullick WJ, Alison MR. Immunoreactivity of transforming growth factor alpha in the normal adult gastrointestinal tract. Gut. 1992;33:628–631. doi: 10.1136/gut.33.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pelosof LC, Gerber DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc. 2010;85:838–854. doi: 10.4065/mcp.2010.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hyun HS, Park PG, Kim JC, Hong KT, Kang HJ, Park KD, Shin HY, Kang HG, Ha IS, Cheong HI. A Case of Severe Hypercalcemia Causing Acute Kidney Injury: An Unusual Presentation of Acute Lymphoblastic Leukemia. Child Kidney Dis. 2017;21:21–25. doi: 10.3339/jkspn.2017.21.1.21. [DOI] [Google Scholar]
- 70.Moysés-Neto M, Guimarães FM, Ayoub FH, Vieira-Neto OM, Costa JA, Dantas M. Acute renal failure and hypercalcemia. Ren Fail. 2006;28:153–159. doi: 10.1080/08860220500531005. [DOI] [PubMed] [Google Scholar]
- 71.Yen TC, Hwang SJ, Wang CC, Lee SD, Yeh SH. Hypercalcemia and parathyroid hormone-related protein in hepatocellular carcinoma. Liver. 1993;13:311–315. doi: 10.1111/j.1600-0676.1993.tb00651.x. [DOI] [PubMed] [Google Scholar]
- 72.Leone N, Debernardi-Venon W, Marzano A, Massari M, Rizzetto M. Hypercalcaemia secondary to hepatocellular carcinoma. Ital J Gastroenterol Hepatol. 1999;31:604–606. [PubMed] [Google Scholar]
- 73.Kwon HJ, Kim JW, Kim H, Choi Y, Ahn S. Combined Hepatocellular Carcinoma and Neuroendocrine Carcinoma with Ectopic Secretion of Parathyroid Hormone: A Case Report and Review of the Literature. J Pathol Transl Med. 2018;52:232–237. doi: 10.4132/jptm.2018.05.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jimi S, Uchiyama M, Takaki A, Suzumiya J, Hara S. Mechanisms of cell death induced by cadmium and arsenic. Ann N Y Acad Sci. 2004;1011:325–331. doi: 10.1007/978-3-662-41088-2_32. [DOI] [PubMed] [Google Scholar]
- 75.Johri N, Jacquillet G, Unwin R. Heavy metal poisoning: the effects of cadmium on the kidney. Biometals. 2010;23:783–792. doi: 10.1007/s10534-010-9328-y. [DOI] [PubMed] [Google Scholar]
- 76.Cheng YY, Huang NC, Chang YT, Sung JM, Shen KH, Tsai CC, Guo HR. Associations between arsenic in drinking water and the progression of chronic kidney disease: A nationwide study in Taiwan. J Hazard Mater. 2017;321:432–439. doi: 10.1016/j.jhazmat.2016.09.032. [DOI] [PubMed] [Google Scholar]
- 77.Satarug S. Long-term exposure to cadmium in food and cigarette smoke, liver effects and hepatocellular carcinoma. Curr Drug Metab. 2012;13:257–271. doi: 10.2174/138920012799320446. [DOI] [PubMed] [Google Scholar]
- 78.Shen J, Wanibuchi H, Salim EI, Wei M, Kinoshita A, Yoshida K, Endo G, Fukushima S. Liver tumorigenicity of trimethylarsine oxide in male Fischer 344 rats--association with oxidative DNA damage and enhanced cell proliferation. Carcinogenesis. 2003;24:1827–1835. doi: 10.1093/carcin/bgg143. [DOI] [PubMed] [Google Scholar]
- 79.Yamamoto S, Konishi Y, Matsuda T, Murai T, Shibata MA, Matsui-Yuasa I, Otani S, Kuroda K, Endo G, Fukushima S. Cancer induction by an organic arsenic compound, dimethylarsinic acid (cacodylic acid), in F344/DuCrj rats after pretreatment with five carcinogens. Cancer Res. 1995;55:1271–1276. [PubMed] [Google Scholar]
- 80.Chiu HF, Ho SC, Wang LY, Wu TN, Yang CY. Does arsenic exposure increase the risk for liver cancer? J Toxicol Environ Health A. 2004;67:1491–1500. doi: 10.1080/15287390490486806. [DOI] [PubMed] [Google Scholar]
- 81.Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I, Duggan HM, Wood R, Kosnett MJ, Smith MT. Cancer risks from arsenic in drinking water. Environ Health Perspect. 1992;97:259–267. doi: 10.1289/ehp.9297259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu J, Waalkes MP. Liver is a target of arsenic carcinogenesis. Toxicol Sci. 2008;105:24–32. doi: 10.1093/toxsci/kfn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sabolić I, Breljak D, Skarica M, Herak-Kramberger CM. Role of metallothionein in cadmium traffic and toxicity in kidneys and other mammalian organs. Biometals. 2010;23:897–926. doi: 10.1007/s10534-010-9351-z. [DOI] [PubMed] [Google Scholar]
- 84.Souza V, Escobar Md Mdel C, Gómez-Quiroz L, Bucio L, Hernández E, Cossio EC, Gutiérrez-Ruiz MC. Acute cadmium exposure enhances AP-1 DNA binding and induces cytokines expression and heat shock protein 70 in HepG2 cells. Toxicology. 2004;197:213–228. doi: 10.1016/j.tox.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 85.Hong F, Si C, Gao P, Cederbaum AI, Xiong H, Lu Y. The role of CYP2A5 in liver injury and fibrosis: chemical-specific difference. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:33–43. doi: 10.1007/s00210-015-1172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baker JR, Edwards RJ, Lasker JM, Moore MR, Satarug S. Renal and hepatic accumulation of cadmium and lead in the expression of CYP4F2 and CYP2E1. Toxicol Lett. 2005;159:182–191. doi: 10.1016/j.toxlet.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 87.Baker JR, Satarug S, Reilly PE, Edwards RJ, Ariyoshi N, Kamataki T, Moore MR, Williams DJ. Relationships between non-occupational cadmium exposure and expression of nine cytochrome P450 forms in human liver and kidney cortex samples. Biochem Pharmacol. 2001;62:713–721. doi: 10.1016/s0006-2952(01)00716-x. [DOI] [PubMed] [Google Scholar]
- 88.Carcinoma in Relation to Occupational and Environmental Heavy metals Exposure. Eur J Community Med. 2015;33:33–46. doi: 10.21608/ejcm.2015.714. [DOI] [Google Scholar]
- 89.Li H, Xing L, Zhang M, Wang J, Zheng N. The Toxic Effects of Aflatoxin B1 and Aflatoxin M1 on Kidney through Regulating L-Proline and Downstream Apoptosis. Biomed Res Int. 2018;2018:9074861. doi: 10.1155/2018/9074861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kew MC. Aflatoxins as a cause of hepatocellular carcinoma. J Gastrointestin Liver Dis. 2013;22:305–310. [PubMed] [Google Scholar]
- 91.Wu HC, Santella R. The role of aflatoxins in hepatocellular carcinoma. Hepat Mon. 2012;12:e7238. doi: 10.5812/hepatmon.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reginster F, Jadoul M, van Ypersele de Strihou C. Chinese herbs nephropathy presentation, natural history and fate after transplantation. Nephrol Dial Transplant. 1997;12:81–86. doi: 10.1093/ndt/12.1.81. [DOI] [PubMed] [Google Scholar]
- 93.Lord GM, Tagore R, Cook T, Gower P, Pusey CD. Nephropathy caused by Chinese herbs in the UK. Lancet. 1999;354:481–482. doi: 10.1016/S0140-6736(99)03380-2. [DOI] [PubMed] [Google Scholar]
- 94.Lord GM, Cook T, Arlt VM, Schmeiser HH, Williams G, Pusey CD. Urothelial malignant disease and Chinese herbal nephropathy. Lancet. 2001;358:1515–1516. doi: 10.1016/s0140-6736(01)06576-x. [DOI] [PubMed] [Google Scholar]
- 95.Mei N, Arlt VM, Phillips DH, Heflich RH, Chen T. DNA adduct formation and mutation induction by aristolochic acid in rat kidney and liver. Mutat Res. 2006;602:83–91. doi: 10.1016/j.mrfmmm.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ng AWT, Poon SL, Huang MN, Lim JQ, Boot A, Yu W, Suzuki Y, Thangaraju S, Ng CCY, Tan P, Pang ST, Huang HY, Yu MC, Lee PH, Hsieh SY, Chang AY, Teh BT, Rozen SG. Aristolochic acids and their derivatives are widely implicated in liver cancers in Taiwan and throughout Asia. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aan6446. [DOI] [PubMed] [Google Scholar]
- 97.Nault JC, Letouzé E. Mutational Processes in Hepatocellular Carcinoma: The Story of Aristolochic Acid. Semin Liver Dis. 2019;39:334–340. doi: 10.1055/s-0039-1685516. [DOI] [PubMed] [Google Scholar]
- 98.Shi J, Zhu L, Liu S, Xie WF. A meta-analysis of case-control studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma in China. Br J Cancer. 2005;92:607–612. doi: 10.1038/sj.bjc.6602333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arbuthnot P, Kew M. Hepatitis B virus and hepatocellular carcinoma. Int J Exp Pathol. 2001;82:77–100. doi: 10.1111/j.1365-2613.2001.iep0082-0077-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 101.Chisari FV, Klopchin K, Moriyama T, Pasquinelli C, Dunsford HA, Sell S, Pinkert CA, Brinster RL, Palmiter RD. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell. 1989;59:1145–1156. doi: 10.1016/0092-8674(89)90770-8. [DOI] [PubMed] [Google Scholar]
- 102.Ringelhan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philos Trans R Soc Lond B Biol Sci. 2017;372 doi: 10.1098/rstb.2016.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hong YS, Ryu S, Chang Y, Caínzos-Achirica M, Kwon MJ, Zhao D, Shafi T, Lazo M, Pastor-Barriuso R, Shin H, Cho J, Guallar E. Hepatitis B virus infection and development of chronic kidney disease: a cohort study. BMC Nephrol. 2018;19:353. doi: 10.1186/s12882-018-1154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bhimma R, Coovadia HM. Hepatitis B virus-associated nephropathy. Am J Nephrol. 2004;24:198–211. doi: 10.1159/000077065. [DOI] [PubMed] [Google Scholar]
- 105.Deng CL, Song XW, Liang HJ, Feng C, Sheng YJ, Wang MY. Chronic hepatitis B serum promotes apoptotic damage in human renal tubular cells. World J Gastroenterol. 2006;12:1752–1756. doi: 10.3748/wjg.v12.i11.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Si J, Yu C, Guo Y, Bian Z, Qin C, Yang L, Chen Y, Yin L, Li H, Lan J, Chen J, Chen Z, Lv J, Li L China Kadoorie Biobank Collaborative Group. Chronic hepatitis B virus infection and risk of chronic kidney disease: a population-based prospective cohort study of 0.5 million Chinese adults. BMC Med. 2018;16:93. doi: 10.1186/s12916-018-1084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen YC, Lin HY, Li CY, Lee MS, Su YC. A nationwide cohort study suggests that hepatitis C virus infection is associated with increased risk of chronic kidney disease. Kidney Int. 2014;85:1200–1207. doi: 10.1038/ki.2013.455. [DOI] [PubMed] [Google Scholar]
- 108.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 110.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 111.Moore MA, Park CB, Tsuda H. Implications of the hyperinsulinaemia-diabetes-cancer link for preventive efforts. Eur J Cancer Prev. 1998;7:89–107. [PubMed] [Google Scholar]
- 112.Goyal R, Faizy AF, Siddiqui SS, Singhai M. Evaluation of TNF-α and IL-6 Levels in Obese and Non-obese Diabetics: Pre- and Postinsulin Effects. N Am J Med Sci. 2012;4:180–184. doi: 10.4103/1947-2714.94944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 115.Takuma Y, Nouso K. Nonalcoholic steatohepatitis-associated hepatocellular carcinoma: our case series and literature review. World J Gastroenterol. 2010;16:1436–1441. doi: 10.3748/wjg.v16.i12.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dhamija E, Paul SB, Kedia S. Non-alcoholic fatty liver disease associated with hepatocellular carcinoma: An increasing concern. Indian J Med Res. 2019;149:9–17. doi: 10.4103/ijmr.IJMR_1456_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nagaoki Y, Hyogo H, Aikata H, Tanaka M, Naeshiro N, Nakahara T, Honda Y, Miyaki D, Kawaoka T, Takaki S, Hiramatsu A, Waki K, Imamura M, Kawakami Y, Takahashi S, Chayama K. Recent trend of clinical features in patients with hepatocellular carcinoma. Hepatol Res. 2012;42:368–375. doi: 10.1111/j.1872-034X.2011.00929.x. [DOI] [PubMed] [Google Scholar]
- 118.Diehl AM, Li ZP, Lin HZ, Yang SQ. Cytokines and the pathogenesis of non-alcoholic steatohepatitis. Gut. 2005;54:303–306. doi: 10.1136/gut.2003.024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang Y, Ausman LM, Greenberg AS, Russell RM, Wang XD. Nonalcoholic steatohepatitis induced by a high-fat diet promotes diethylnitrosamine-initiated early hepatocarcinogenesis in rats. Int J Cancer. 2009;124:540–546. doi: 10.1002/ijc.23995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kamada Y, Matsumoto H, Tamura S, Fukushima J, Kiso S, Fukui K, Igura T, Maeda N, Kihara S, Funahashi T, Matsuzawa Y, Shimomura I, Hayashi N. Hypoadiponectinemia accelerates hepatic tumor formation in a nonalcoholic steatohepatitis mouse model. J Hepatol. 2007;47:556–564. doi: 10.1016/j.jhep.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 121.Byrne CD, Targher G. NAFLD as a driver of chronic kidney disease. J Hepatol. 2020;72:785–801. doi: 10.1016/j.jhep.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 122.Park H, Dawwas GK, Liu X, Nguyen MH. Nonalcoholic fatty liver disease increases risk of incident advanced chronic kidney disease: a propensity-matched cohort study. J Intern Med. 2019;286:711–722. doi: 10.1111/joim.12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Targher G, Mantovani A, Alisi A, Mosca A, Panera N, Byrne CD, Nobili V. Relationship Between PNPLA3 rs738409 Polymorphism and Decreased Kidney Function in Children With NAFLD. Hepatology. 2019;70:142–153. doi: 10.1002/hep.30625. [DOI] [PubMed] [Google Scholar]
- 124.Leung PS, Carlsson PO. Tissue renin-angiotensin system: its expression, localization, regulation and potential role in the pancreas. J Mol Endocrinol. 2001;26:155–164. doi: 10.1677/jme.0.0260155. [DOI] [PubMed] [Google Scholar]
- 125.Siragy HM, Carey RM. Role of the intrarenal renin-angiotensin-aldosterone system in chronic kidney disease. Am J Nephrol. 2010;31:541–550. doi: 10.1159/000313363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brewster UC, Perazella MA. The renin-angiotensin-aldosterone system and the kidney: effects on kidney disease. Am J Med. 2004;116:263–272. doi: 10.1016/j.amjmed.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 127.Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116:288–296. doi: 10.1172/JCI27699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Anderson S, Rennke HG, Brenner BM. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest. 1986;77:1993–2000. doi: 10.1172/JCI112528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 130.Simões E Silva AC, Miranda AS, Rocha NP, Teixeira AL. Renin angiotensin system in liver diseases: Friend or foe? World J Gastroenterol. 2017;23:3396–3406. doi: 10.3748/wjg.v23.i19.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bataller R, Ginès P, Nicolás JM, Görbig MN, Garcia-Ramallo E, Gasull X, Bosch J, Arroyo V, Rodés J. Angiotensin II induces contraction and proliferation of human hepatic stellate cells. Gastroenterology. 2000;118:1149–1156. doi: 10.1016/s0016-5085(00)70368-4. [DOI] [PubMed] [Google Scholar]
- 132.Tuncer I, Ozbek H, Ugras S, Bayram I. Anti-fibrogenic effects of captopril and candesartan cilexetil on the hepatic fibrosis development in rat. The effect of AT1-R blocker on the hepatic fibrosis. Exp Toxicol Pathol. 2003;55:159–166. doi: 10.1078/0940-2993-00309. [DOI] [PubMed] [Google Scholar]
- 133.Wei YH, Jun L, Qiang CJ. Effect of losartan, an angiotensin II antagonist, on hepatic fibrosis induced by CCl4 in rats. Dig Dis Sci. 2004;49:1589–1594. doi: 10.1023/b:ddas.0000043369.88701.5b. [DOI] [PubMed] [Google Scholar]
- 134.Yoshiji H, Noguchi R, Kuriyama S, Yoshii J, Ikenaka Y, Yanase K, Namisaki T, Kitade M, Yamazaki M, Uemura M, Fukui H. Suppression of renin-angiotensin system attenuates hepatocarcinogenesis via angiogenesis inhibition in rats. Anticancer Res. 2005;25:3335–3340. [PubMed] [Google Scholar]
- 135.Lee CH, Hsieh SY, Chang CC, Wang IK, Huang WH, Weng CH, Hsu CW, Yen TH. Hepatocellular carcinoma in hemodialysis patients. Oncotarget. 2017;8:73154–73161. doi: 10.18632/oncotarget.17127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee YH, Hsu CY, Hsia CY, Huang YH, Su CW, Lin HC, Lee RC, Chiou YY, Huo TI. Hepatocellular carcinoma in uremic patients: is there evidence for an increased risk of mortality? J Gastroenterol Hepatol. 2013;28:348–356. doi: 10.1111/jgh.12062. [DOI] [PubMed] [Google Scholar]
- 137.Sarno G, Montalti R, Giglio MC, Rompianesi G, Tomassini F, Scarpellini E, De Simone G, De Palma GD, Troisi RI. Hepatocellular carcinoma in patients with chronic renal disease: Challenges of interventional treatment. Surg Oncol. 2021;36:42–50. doi: 10.1016/j.suronc.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 138.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 139.Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 140.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 141.Thelen A, Benckert C, Tautenhahn HM, Hau HM, Bartels M, Linnemann J, Bertolini J, Moche M, Wittekind C, Jonas S. Liver resection for hepatocellular carcinoma in patients without cirrhosis. Br J Surg. 2013;100:130–137. doi: 10.1002/bjs.8962. [DOI] [PubMed] [Google Scholar]
- 142.Smoot RL, Nagorney DM, Chandan VS, Que FG, Schleck CD, Harmsen WS, Kendrick ML. Resection of hepatocellular carcinoma in patients without cirrhosis. Br J Surg. 2011;98:697–703. doi: 10.1002/bjs.7401. [DOI] [PubMed] [Google Scholar]
- 143.Ercolani G, Grazi GL, Ravaioli M, Del Gaudio M, Gardini A, Cescon M, Varotti G, Cetta F, Cavallari A. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003;237:536–543. doi: 10.1097/01.SLA.0000059988.22416.F2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Santambrogio R, Kluger MD, Costa M, Belli A, Barabino M, Laurent A, Opocher E, Azoulay D, Cherqui D. Hepatic resection for hepatocellular carcinoma in patients with Child-Pugh's A cirrhosis: is clinical evidence of portal hypertension a contraindication? HPB (Oxford) 2013;15:78–84. doi: 10.1111/j.1477-2574.2012.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 146.European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 147.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Allaire M, Goumard C, Lim C, Le Cleach A, Wagner M, Scatton O. New frontiers in liver resection for hepatocellular carcinoma. JHEP Rep. 2020;2:100134. doi: 10.1016/j.jhepr.2020.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cloyd JM, Ma Y, Morton JM, Kurella Tamura M, Poultsides GA, Visser BC. Does chronic kidney disease affect outcomes after major abdominal surgery? J Gastrointest Surg. 2014;18:605–612. doi: 10.1007/s11605-013-2390-3. [DOI] [PubMed] [Google Scholar]
- 150.Toshima T, Shirabe K, Yoshiya S, Muto J, Ikegami T, Yoshizumi T, Maehara Y. Outcome of hepatectomy for hepatocellular carcinoma in patients with renal dysfunction. HPB (Oxford) 2012;14:317–324. doi: 10.1111/j.1477-2574.2012.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shirata C, Hasegawa K, Kokudo T, Yamashita S, Yamamoto S, Arita J, Akamatsu N, Kaneko J, Sakamoto Y, Kokudo N. Liver Resection for Hepatocellular Carcinoma in Patients with Renal Dysfunction. World J Surg. 2018;42:4054–4062. doi: 10.1007/s00268-018-4698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cheng SB, Wu CC, Shu KH, Ho WL, Chen JT, Yeh DC, Liu TJ, P'eng FK. Liver resection for hepatocellular carcinoma in patients with end-stage renal failure. J Surg Oncol. 2001;78:241–6; discussion 246. doi: 10.1002/jso.1160. [DOI] [PubMed] [Google Scholar]
- 153.Yeh CN, Lee WC, Chen MF. Hepatic resection for hepatocellular carcinoma in end-stage renal disease patients: two decades of experience at Chang Gung Memorial Hospital. World J Gastroenterol. 2005;11:2067–2071. doi: 10.3748/wjg.v11.i14.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yeh CC, Lin JT, Jeng LB, Charalampos I, Chen TT, Lee TY, Wu MS, Kuo KN, Liu YY, Wu CY. Hepatic resection for hepatocellular carcinoma patients on hemodialysis for uremia: a nationwide cohort study. World J Surg. 2013;37:2402–2409. doi: 10.1007/s00268-013-2137-z. [DOI] [PubMed] [Google Scholar]
- 155.Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17 Suppl 2:S44–S57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- 156.Moreno R, Berenguer M. Post-liver transplantation medical complications. Ann Hepatol. 2006;5:77–85. [PubMed] [Google Scholar]
- 157.Liu LU, Schiano TD. Long-term care of the liver transplant recipient. Clin Liver Dis. 2007;11:397–416. doi: 10.1016/j.cld.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 158.McCauley J, Van Thiel DH, Starzl TE, Puschett JB. Acute and chronic renal failure in liver transplantation. Nephron. 1990;55:121–128. doi: 10.1159/000185938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Karapanagiotou A, Kydona C, Dimitriadis C, Sgourou K, Giasnetsova T, Fouzas I, Imvrios G, Gritsi-Gerogianni N. Acute kidney injury after orthotopic liver transplantation. Transplant Proc. 2012;44:2727–2729. doi: 10.1016/j.transproceed.2012.09.096. [DOI] [PubMed] [Google Scholar]
- 160.Bilbao I, Charco R, Balsells J, Lazaro JL, Hidalgo E, Llopart L, Murio E, Margarit C. Risk factors for acute renal failure requiring dialysis after liver transplantation. Clin Transplant. 1998;12:123–129. [PubMed] [Google Scholar]
- 161.Klaus F, Keitel da Silva C, Meinerz G, Carvalho LM, Goldani JC, Cantisani G, Zanotelli ML, Duro Garcia V, Keitel E. Acute kidney injury after liver transplantation: incidence and mortality. Transplant Proc. 2014;46:1819–1821. doi: 10.1016/j.transproceed.2014.05.053. [DOI] [PubMed] [Google Scholar]
- 162.Chen X, Ding X, Shen B, Teng J, Zou J, Wang T, Zhou J, Chen N, Zhang B. Incidence and outcomes of acute kidney injury in patients with hepatocellular carcinoma after liver transplantation. J Cancer Res Clin Oncol. 2017;143:1337–1346. doi: 10.1007/s00432-017-2376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fraley DS, Burr R, Bernardini J, Angus D, Kramer DJ, Johnson JP. Impact of acute renal failure on mortality in end-stage liver disease with or without transplantation. Kidney Int. 1998;54:518–524. doi: 10.1046/j.1523-1755.1998.00004.x. [DOI] [PubMed] [Google Scholar]
- 164.Zhu M, Li Y, Xia Q, Wang S, Qiu Y, Che M, Dai H, Qian J, Ni Z, Axelsson J, Yan Y. Strong impact of acute kidney injury on survival after liver transplantation. Transplant Proc. 2010;42:3634–3638. doi: 10.1016/j.transproceed.2010.08.059. [DOI] [PubMed] [Google Scholar]
- 165.Barri YM, Sanchez EQ, Jennings LW, Melton LB, Hays S, Levy MF, Klintmalm GB. Acute kidney injury following liver transplantation: definition and outcome. Liver Transpl. 2009;15:475–483. doi: 10.1002/lt.21682. [DOI] [PubMed] [Google Scholar]
- 166.Weber ML, Ibrahim HN, Lake JR. Renal dysfunction in liver transplant recipients: evaluation of the critical issues. Liver Transpl. 2012;18:1290–1301. doi: 10.1002/lt.23522. [DOI] [PubMed] [Google Scholar]
- 167.Herrero JI, Cuervas-Mons V, Gómez-Bravo MÁ, Fabregat J, Otero A, Bilbao I, Salcedo MM, González-Diéguez ML, Fernández JR, Serrano MT, Jiménez M, Rodrigo JM, Narváez I, Sánchez G. Prevalence and progression of chronic kidney disease after liver transplant: a prospective, real-life, observational, two-year multicenter study. Rev Esp Enferm Dig. 2018;110:538–543. doi: 10.17235/reed.2018.5431/2017. [DOI] [PubMed] [Google Scholar]
- 168.Kalisvaart M, Schlegel A, Trivedi PJ, Roberts K, Mirza DF, Perera T, Isaac JI, Ferguson J, de Jonge J, Muiesan P. Chronic Kidney Disease After Liver Transplantation: Impact of Extended Criteria Grafts. Liver Transpl. 2019;25:922–933. doi: 10.1002/lt.25468. [DOI] [PubMed] [Google Scholar]
- 169.Mason J. The effect of cyclosporin on renal function. J Autoimmun. 1992;5 Suppl A:349–354. doi: 10.1016/0896-8411(92)90053-s. [DOI] [PubMed] [Google Scholar]
- 170.O'Riordan A, Dutt N, Cairns H, Rela M, O'Grady JG, Heaton N, Hendry BM. Renal biopsy in liver transplant recipients. Nephrol Dial Transplant. 2009;24:2276–2282. doi: 10.1093/ndt/gfp112. [DOI] [PubMed] [Google Scholar]
- 171.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 172.Herrero JI, Quiroga J, Sangro B, Girala M, Gómez-Manero N, Pardo F, Alvárez-Cienfuegos J, Prieto J. Conversion of liver transplant recipients on cyclosporine with renal impairment to mycophenolate mofetil. Liver Transpl Surg. 1999;5:414–420. doi: 10.1002/lt.500050513. [DOI] [PubMed] [Google Scholar]
- 173.Fisher NC, Nightingale PG, Gunson BK, Lipkin GW, Neuberger JM. Chronic renal failure following liver transplantation: a retrospective analysis. Transplantation. 1998;66:59–66. doi: 10.1097/00007890-199807150-00010. [DOI] [PubMed] [Google Scholar]
- 174.Brown RS Jr, Lombardero M, Lake JR. Outcome of patients with renal insufficiency undergoing liver or liver-kidney transplantation. Transplantation. 1996;62:1788–1793. doi: 10.1097/00007890-199612270-00018. [DOI] [PubMed] [Google Scholar]
- 175.Lafayette RA, Paré G, Schmid CH, King AJ, Rohrer RJ, Nasraway SA. Pretransplant renal dysfunction predicts poorer outcome in liver transplantation. Clin Nephrol. 1997;48:159–164. [PubMed] [Google Scholar]
- 176.David AI, Coelho MP, Paes AT, Leite AK, Della Guardia B, de Almeida MD, Meira SP, de Rezende MB, Afonso RC, Ferraz-Neto BH. Liver transplant outcome: a comparison between high and low MELD score recipients. Einstein (Sao Paulo) 2012;10:57–61. doi: 10.1590/s1679-45082012000100012. [DOI] [PubMed] [Google Scholar]
- 177.Aguilera V, Ferrer I, Berenguer M, Rivera J, Rubín Á, Moya Á, Pareja E, Sánchez J, Prieto M, Mir J. Comparison of results of combined liver-kidney transplantation vs. isolated liver transplantation. Ann Hepatol. 2013;12:274–281. [PubMed] [Google Scholar]
- 178.Kliem V, Ringe B, Frei U, Pichlmayr R. Single-center experience of combined liver and kidney transplantation. Clin Transplant. 1995;9:39–44. [PubMed] [Google Scholar]
- 179.Tanriover B, MacConmara MP, Parekh J, Arce C, Zhang S, Gao A, Mufti A, Levea SL, Sandikci B, Ayvaci MU, Ariyamuthu VK, Hwang C, Mohan S, Mete M, Vazquez MA, Marrero JA. Simultaneous liver kidney transplantation in liver transplant candidates with renal dysfunction: Importance of creatinine levels, dialysis, and organ quality in survival. Kidney Int Rep. 2016;1:221–229. doi: 10.1016/j.ekir.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Margreiter R, Steurer W, Spechtenhauser B, Königsrainer A. Kidney transplantation together with another solid organ from the same donor--a single-center progress report. Clin Nephrol. 2000;53:suppl 38–suppl 43. [PubMed] [Google Scholar]
- 181.Zhu XF, He XS, Chen GH, Chen LZ, Wang CX, Huang JF. Combined liver and kidney transplantation in Guangzhou, China. Hepatobiliary Pancreat Dis Int. 2007;6:585–589. [PubMed] [Google Scholar]
- 182.Pardo F, Pons JA, Castells L, Colmenero J, Gómez MÁ, Lladó L, Pérez B, Prieto M, Briceño J. VI consensus document by the Spanish Liver Transplantation Society. Gastroenterol Hepatol. 2018;41:406–421. doi: 10.1016/j.gastrohep.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 183.Singal AK, Salameh H, Kuo YF, Wiesner RH. Evolving frequency and outcomes of simultaneous liver kidney transplants based on liver disease etiology. Transplantation. 2014;98:216–221. doi: 10.1097/TP.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 184.Rich N, Tanriover B, Singal AG, Marrero JA. Outcomes of Simultaneous Liver Kidney Transplantation in Patients With Hepatocellular Carcinoma. Transplantation. 2017;101:e12–e19. doi: 10.1097/TP.0000000000001515. [DOI] [PubMed] [Google Scholar]
- 185.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 186.Takaki H, Yamakado K, Nakatsuka A, Yamada T, Shiraki K, Takei Y, Takeda K. Frequency of and risk factors for complications after liver radiofrequency ablation under CT fluoroscopic guidance in 1500 sessions: single-center experience. AJR Am J Roentgenol. 2013;200:658–664. doi: 10.2214/AJR.12.8691. [DOI] [PubMed] [Google Scholar]
- 187.Li H, Li B, Wei Y, Liu F. Hemolysis as a complication of radiofrequency ablation for hepatocellular carcinoma must be paid more attention. Dig Dis Sci. 2011;56:3391–3392. doi: 10.1007/s10620-011-1737-4. [DOI] [PubMed] [Google Scholar]
- 188.Keltner JR, Donegan E, Hynson JM, Shapiro WA. Acute renal failure after radiofrequency liver ablation of metastatic carcinoid tumor. Anesth Analg. 2001;93:587–589. doi: 10.1097/00000539-200109000-00011. [DOI] [PubMed] [Google Scholar]
- 189.Tsui SL, Lee AK, Lui SK, Poon RT, Fan ST. Acute intraoperative hemolysis and hemoglobinuria during radiofrequency ablation of hepatocellular carcinoma. Hepatogastroenterology. 2003;50:526–529. [PubMed] [Google Scholar]
- 190.Metzner J, Evans JL, Domino KB. Life-threatening hyperkalemia during radiofrequency ablation of hepatocellular carcinoma. J Clin Anesth. 2010;22:473–476. doi: 10.1016/j.jclinane.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 191.Jiao LR, Hansen PD, Havlik R, Mitry RR, Pignatelli M, Habib N. Clinical short-term results of radiofrequency ablation in primary and secondary liver tumors. Am J Surg. 1999;177:303–306. doi: 10.1016/s0002-9610(99)00043-4. [DOI] [PubMed] [Google Scholar]
- 192.Hargreaves GM, Adam R, Bismuth H. Results after nonsurgical local treatment of primary liver malignancies. Langenbecks Arch Surg. 2000;385:185–193. doi: 10.1007/s004230050263. [DOI] [PubMed] [Google Scholar]
- 193.Fukumori K, Shakado S, Makihata T, Takemoto R, Fukuizumi K, Miyahara T, Yasumori K, Muranaka T, Watanabe J, Saitsu H, Sakai H, Sata M. [A case of chronic renal failure caused hyperkalemia following percutaneous radiofrequency ablation therapy for hepatocellular carcinoma] Nihon Shokakibyo Gakkai Zasshi. 2003;100:702–706. [PubMed] [Google Scholar]
- 194.Hiraoka A, Kumada T, Michitaka K, Toyoda H, Tada T, Takaguchi K, Tsuji K, Itobayashi E, Takizawa D, Hirooka M, Koizumi Y, Ochi H, Joko K, Kisaka Y, Shimizu Y, Tajiri K, Tani J, Taniguchi T, Toshimori A, Fujioka S Real-Life Practice Experts For Hepatocellular Carcinoma (HCC) (RELPEC) Study Group and The HCC 48 Group (HCC experts from 48 clinics) Clinical features of hemodialysis patients treated for hepatocellular carcinoma: Comparison between resection and radiofrequency ablation. Mol Clin Oncol. 2017;6:455–461. doi: 10.3892/mco.2017.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kondo Y, Yoshida H, Tomizawa Y, Tateishi R, Shiina S, Tagawa K, Omata M. Percutaneous radiofrequency ablation of hepatocellular carcinoma in 14 patients undergoing regular hemodialysis for end-stage renal disease. AJR Am J Roentgenol. 2009;193:964–969. doi: 10.2214/AJR.08.2236. [DOI] [PubMed] [Google Scholar]
- 196.Sato M, Tateishi R, Yasunaga H, Matsui H, Horiguchi H, Fushimi K, Koike K. Mortality and hemorrhagic complications associated with radiofrequency ablation for treatment of hepatocellular carcinoma in patients on hemodialysis for end-stage renal disease: A nationwide survey. J Gastroenterol Hepatol. 2017;32:1873–1878. doi: 10.1111/jgh.13780. [DOI] [PubMed] [Google Scholar]
- 197.Verhoeven BH, Haagsma EB, Appeltans BM, Slooff MJ, de Jong KP. Hyperkalaemia after radiofrequency ablation of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2002;14:1023–1024. doi: 10.1097/00042737-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 198.Waghray A, Murali AR, Menon KN. Hepatocellular carcinoma: From diagnosis to treatment. World J Hepatol. 2015;7:1020–1029. doi: 10.4254/wjh.v7.i8.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lee BC, Liu KL, Lin CL, Kao CH. Risk of acute kidney injury after transarterial chemoembolisation in hepatocellular carcinoma patients: A nationwide population-based cohort study. Eur Radiol. 2017;27:4482–4489. doi: 10.1007/s00330-017-4893-x. [DOI] [PubMed] [Google Scholar]
- 200.Hsu CY, Huang YH, Su CW, Lin HC, Chiang JH, Lee PC, Lee FY, Huo TI, Lee SD. Renal failure in patients with hepatocellular carcinoma and ascites undergoing transarterial chemoembolization. Liver Int. 2010;30:77–84. doi: 10.1111/j.1478-3231.2009.02128.x. [DOI] [PubMed] [Google Scholar]
- 201.Zhou C, Wang R, Ding Y, Du L, Hou C, Lu D, Hao L, Lv W. Prognostic factors for acute kidney injury following transarterial chemoembolization in patients with hepatocellular carcinoma. Int J Clin Exp Pathol. 2014;7:2579–2586. [PMC free article] [PubMed] [Google Scholar]
- 202.Huo TI, Wu JC, Lee PC, Chang FY, Lee SD. Incidence and risk factors for acute renal failure in patients with hepatocellular carcinoma undergoing transarterial chemoembolization: a prospective study. Liver Int. 2004;24:210–215. doi: 10.1111/j.1478-3231.2004.00911.x. [DOI] [PubMed] [Google Scholar]
- 203.Aspelin P, Aubry P, Fransson SG, Strasser R, Willenbrock R, Berg KJ Nephrotoxicity in High-Risk Patients Study of Iso-Osmolar and Low-Osmolar Non-Ionic Contrast Media Study Investigators. Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. 2003;348:491–499. doi: 10.1056/NEJMoa021833. [DOI] [PubMed] [Google Scholar]
- 204.Hong CX, Lv LW, Hua LZ, Bo S, Sen CX, Xin NY, Wei YJ, Rui XJ, Qiang DX, Zhou ZJ. Epidemiology and Management of Acute Kidney Injury in Hepatocellular Carcinoma Patients Undergoing Transcatheter Arterial Chemoembolization. Curr Protein Pept Sci. 2017;18:1218–1223. doi: 10.2174/1389203717666160909124305. [DOI] [PubMed] [Google Scholar]
- 205.Hao JF, Zhang LW, Bai JX, Li YJ, Liu JN, Zhang XL, Han JM, Li X, Jiang H, Cao N. Incidence, risk factors, and prognosis of acute kidney injury following transarterial chemoembolization in patients with hepatocellular carcinoma: a prospective cohort study. Indian J Cancer. 2015;51 Suppl 2:e3–e8. doi: 10.4103/0019-509X.151984. [DOI] [PubMed] [Google Scholar]
- 206.Hsu CY, Huang YH, Su CW, Chiang JH, Lin HC, Lee PC, Lee FY, Huo TI, Lee SD. Transarterial chemoembolization in patients with hepatocellular carcinoma and renal insufficiency. J Clin Gastroenterol. 2010;44:e171–e177. doi: 10.1097/MCG.0b013e3181c88235. [DOI] [PubMed] [Google Scholar]
- 207.Lin WC, Chang CW, Wang TE, Chen MJ, Wang HY. Challenges of transarterial therapy for hepatocellular carcinoma in patients with chronic kidney disease. Medicine (Baltimore) 2019;98:e17007. doi: 10.1097/MD.0000000000017007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Watanabe M, Shibuya A, Minamino T, Murano J, Matsunaga K, Fujii K, Ogasawara G, Irie T, Woodhams R, Koizumi W. Benefits and problems of transarterial therapy in patients with hepatocellular carcinoma and chronic kidney disease. J Vasc Interv Radiol. 2014;25:1947–55; quiz 1955. doi: 10.1016/j.jvir.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 209.Sato M, Tateishi R, Yasunaga H, Matsui H, Fushimi K, Ikeda H, Yatomi Y, Koike K. In-hospital mortality associated with transcatheter arterial embolization for treatment of hepatocellular carcinoma in patients on hemodialysis for end stage renal disease: a matched-pair cohort study using a nationwide database. BJR Open. 2019;1:20190004. doi: 10.1259/bjro.20190004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12:408–424. doi: 10.1038/nrclinonc.2015.103. [DOI] [PubMed] [Google Scholar]
- 211.Roberts LR, Gores GJ. Hepatocellular carcinoma: molecular pathways and new therapeutic targets. Semin Liver Dis. 2005;25:212–225. doi: 10.1055/s-2005-871200. [DOI] [PubMed] [Google Scholar]
- 212.Chen S, Cao Q, Wen W, Wang H. Targeted therapy for hepatocellular carcinoma: Challenges and opportunities. Cancer Lett. 2019;460:1–9. doi: 10.1016/j.canlet.2019.114428. [DOI] [PubMed] [Google Scholar]
- 213.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 214.Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 215.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Maynard S, Epstein FH, Karumanchi SA. Preeclampsia and angiogenic imbalance. Annu Rev Med. 2008;59:61–78. doi: 10.1146/annurev.med.59.110106.214058. [DOI] [PubMed] [Google Scholar]
- 217.Patel TV, Morgan JA, Demetri GD, George S, Maki RG, Quigley M, Humphreys BD. A preeclampsia-like syndrome characterized by reversible hypertension and proteinuria induced by the multitargeted kinase inhibitors sunitinib and sorafenib. J Natl Cancer Inst. 2008;100:282–284. doi: 10.1093/jnci/djm311. [DOI] [PubMed] [Google Scholar]
- 218.Okuno Y, Kume H, Hosoda C, Homma Y. Development of nephrotic syndrome after administration of sorafenib in a case of metastatic renal cell carcinoma. Case Rep Med. 2011;2011:710216. doi: 10.1155/2011/710216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hanna RM, Selamet U, Hasnain H, El-Masry M, Saab S, Wallace WD, Yanny B, Wilson J. Development of Focal Segmental Glomerulosclerosis and Thrombotic Microangiopathy in a Liver Transplant Patient on Sorafenib for Hepatocellular Carcinoma: A Case Report. Transplant Proc. 2018;50:4033–4037. doi: 10.1016/j.transproceed.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 220.Hilger RA, Richly H, Grubert M, Kredtke S, Thyssen D, Eberhardt W, Hense J, Schuler M, Scheulen ME. Pharmacokinetics of sorafenib in patients with renal impairment undergoing hemodialysis. Int J Clin Pharmacol Ther. 2009;47:61–64. doi: 10.5414/cpp47061. [DOI] [PubMed] [Google Scholar]
- 221.Ishii T, Hatano E, Taura K, Mizuno T, Kawai T, Fukudo M, Katsura T, Uemoto S. Sorafenib in a hepatocellular carcinoma patient with end-stage renal failure: A pharmacokinetic study. Hepatol Res. 2014;44:685–688. doi: 10.1111/hepr.12156. [DOI] [PubMed] [Google Scholar]
- 222.Bayer HealthCare Pharmaceuticals Inc. NEXAVAR (sorafenib) [package insert]. U.S. Food and Drug Administration website. [cited 12 March 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021923s024lblrpl.pdf .
- 223.Masini C, Sabbatini R, Porta C, Procopio G, Di Lorenzo G, Onofri A, Buti S, Iacovelli R, Invernizzi R, Moscetti L, Aste MG, Pagano M, Grosso F, Lucia Manenti A, Ortega C, Cosmai L, Del Giovane C, Conte PF. Use of tyrosine kinase inhibitors in patients with metastatic kidney cancer receiving haemodialysis: a retrospective Italian survey. BJU Int. 2012;110:692–698. doi: 10.1111/j.1464-410X.2012.10946.x. [DOI] [PubMed] [Google Scholar]
- 224.Huang A, Yang XR, Chung WY, Dennison AR, Zhou J. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5:146. doi: 10.1038/s41392-020-00264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 226.Hiraoka A, Kumada T, Kariyama K, Takaguchi K, Itobayashi E, Shimada N, Tajiri K, Tsuji K, Ishikawa T, Ochi H, Hirooka M, Tsutsui A, Shibata H, Tada T, Toyoda H, Nouso K, Joko K, Hiasa Y, Michitaka K Real-life Practice Experts for HCC (RELPEC) Study Group and the HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan) Therapeutic potential of lenvatinib for unresectable hepatocellular carcinoma in clinical practice: Multicenter analysis. Hepatol Res. 2019;49:111–117. doi: 10.1111/hepr.13243. [DOI] [PubMed] [Google Scholar]
- 227.Takeda H, Nishijima N, Nasu A, Komekado H, Kita R, Kimura T, Kudo M, Osaki Y. Long-term antitumor effect of lenvatinib on unresectable hepatocellular carcinoma with portal vein invasion. Hepatol Res. 2019;49:594–599. doi: 10.1111/hepr.13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hiraoka A, Kumada T, Kariyama K, Takaguchi K, Atsukawa M, Itobayashi E, Tsuji K, Tajiri K, Hirooka M, Shimada N, Shibata H, Ishikawa T, Ochi H, Tada T, Toyoda H, Nouso K, Tsutsui A, Itokawa N, Imai M, Joko K, Hiasa Y, Michitaka K Real-life Practice Experts for HCC (RELPEC) Study Group; HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan) Clinical features of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions: Multicenter analysis. Cancer Med. 2019;8:137–146. doi: 10.1002/cam4.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Iwasaki H, Yamazaki H, Takasaki H, Suganuma N, Sakai R, Nakayama H, Toda S, Masudo K. Renal dysfunction in patients with radioactive iodine-refractory thyroid cancer treated with tyrosine kinase inhibitors: A retrospective study. Medicine (Baltimore) 2019;98:e17588. doi: 10.1097/MD.0000000000017588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hyogo Y, Kiyota N, Otsuki N, Goto S, Imamura Y, Chayahara N, Toyoda M, Nibu KI, Hyodo T, Hara S, Masuoka H, Kasahara T, Ito Y, Miya A, Hirokawa M, Miyauchi A, Minami H. Thrombotic Microangiopathy with Severe Proteinuria Induced by Lenvatinib for Radioactive Iodine-Refractory Papillary Thyroid Carcinoma. Case Rep Oncol. 2018;11:735–741. doi: 10.1159/000494080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Furuto Y, Hashimoto H, Namikawa A, Outi H, Takahashi H, Horiuti H, Honda K, Shibuya Y. Focal segmental glomerulosclerosis lesion associated with inhibition of tyrosine kinases by lenvatinib: a case report. BMC Nephrol. 2018;19:273. doi: 10.1186/s12882-018-1074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Eisai Inc. LENVIMA (lenvatinib) [package insert]. U.S. Food and Drug Administration website. [cited 12 March 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/206947s018lbl.pdf .
- 233.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 234.Yoo C, Park JW, Kim YJ, Kim DY, Yu SJ, Lim TS, Lee SJ, Ryoo BY, Lim HY. Multicenter retrospective analysis of the safety and efficacy of regorafenib after progression on sorafenib in Korean patients with hepatocellular carcinoma. Invest New Drugs. 2019;37:567–572. doi: 10.1007/s10637-018-0707-5. [DOI] [PubMed] [Google Scholar]
- 235.Finn RS, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Gerolami R, Caparello C, Cabrera R, Chang C, Sun W, LeBerre MA, Baumhauer A, Meinhardt G, Bruix J. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353–358. doi: 10.1016/j.jhep.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 236.Eisen T, Joensuu H, Nathan PD, Harper PG, Wojtukiewicz MZ, Nicholson S, Bahl A, Tomczak P, Pyrhonen S, Fife K, Bono P, Boxall J, Wagner A, Jeffers M, Lin T, Quinn DI. Regorafenib for patients with previously untreated metastatic or unresectable renal-cell carcinoma: a single-group phase 2 trial. Lancet Oncol. 2012;13:1055–1062. doi: 10.1016/S1470-2045(12)70364-9. [DOI] [PubMed] [Google Scholar]
- 237.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 238.Krishnamoorthy SK, Relias V, Sebastian S, Jayaraman V, Saif MW. Management of regorafenib-related toxicities: a review. Therap Adv Gastroenterol. 2015;8:285–297. doi: 10.1177/1756283X15580743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Sturm A. Cleton MBBPJGKSMGF-THSRFHZJTI. Evaluation of exposure of regorafenib and its metabolites in cancer patients with renal impairment by modelling, simulation, and clinical study. Ann Oncol. 2016;27:vi526–vi544. doi: 10.1093/annonc/mdw392.37. [DOI] [Google Scholar]
- 240.Bayer HealthCare Pharmaceuticals Inc. STIVARGA (regorafenib) [package insert]. U.S. Food and Drug Administration website. [cited 12 March 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/203085s013lbl.pdf .
- 241.Cochin V, Gross-Goupil M, Ravaud A, Godbert Y, Le Moulec S. [Cabozantinib: Mechanism of action, efficacy and indications] Bull Cancer. 2017;104:393–401. doi: 10.1016/j.bulcan.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 242.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Nguyen L, Holland J, Ramies D, Mamelok R, Benrimoh N, Ciric S, Marbury T, Preston RA, Heuman DM, Gavis E, Lacy S. Effect of Renal and Hepatic Impairment on the Pharmacokinetics of Cabozantinib. J Clin Pharmacol. 2016;56:1130–1140. doi: 10.1002/jcph.714. [DOI] [PubMed] [Google Scholar]
- 244.Cappagli V, Moriconi D, Bonadio AG, Giannese D, La Manna G, Egidi MF, Comai G, Vischini G, Bottici V, Elisei R, Viola D. Proteinuria is a late-onset adverse event in patients treated with cabozantinib. J Endocrinol Invest. 2021;44:95–103. doi: 10.1007/s40618-020-01272-y. [DOI] [PubMed] [Google Scholar]
- 245.Paschke L, Lincke T, Mühlberg KS, Jabs WJ, Lindner TH, Paschke R. Anti VEGF-TKI Treatment and New Renal Adverse Events Not Reported in Phase III Trials. Eur Thyroid J. 2018;7:308–312. doi: 10.1159/000491387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Exelixis Inc. COMETRIQ (cabozantinib) [package insert]. U.S. Food and Drug Administration website. [cited 12 March 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/203756s009lbl.pdf .
- 247.Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, Blanc JF, Chung HC, Baron AD, Pfiffer TE, Okusaka T, Kubackova K, Trojan J, Sastre J, Chau I, Chang SC, Abada PB, Yang L, Schwartz JD, Kudo M REACH Trial Investigators. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859–870. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- 248.Park JO, Ryoo BY, Yen CJ, Kudo M, Yang L, Abada PB, Cheng R, Orlando M, Zhu AX, Okusaka T. Second-line ramucirumab therapy for advanced hepatocellular carcinoma (REACH): an East Asian and non-East Asian subgroup analysis. Oncotarget. 2016;7:75482–75491. doi: 10.18632/oncotarget.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zhu AX, Baron AD, Malfertheiner P, Kudo M, Kawazoe S, Pezet D, Weissinger F, Brandi G, Barone CA, Okusaka T, Wada Y, Park JO, Ryoo BY, Cho JY, Chung HC, Li CP, Yen CJ, Lee KD, Chang SC, Yang L, Abada PB, Chau I. Ramucirumab as Second-Line Treatment in Patients With Advanced Hepatocellular Carcinoma: Analysis of REACH Trial Results by Child-Pugh Score. JAMA Oncol. 2017;3:235–243. doi: 10.1001/jamaoncol.2016.4115. [DOI] [PubMed] [Google Scholar]
- 250.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 251.Fujii T, Kawasoe K, Tonooka A, Ohta A, Nitta K. Nephrotic syndrome associated with ramucirumab therapy: A single-center case series and literature review. Medicine (Baltimore) 2019;98:e16236. doi: 10.1097/MD.0000000000016236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Abdel-Rahman O, ElHalawani H. Proteinuria in Patients with Solid Tumors Treated with Ramucirumab: A Systematic Review and Meta-Analysis. Chemotherapy. 2014;60:325–333. doi: 10.1159/000437253. [DOI] [PubMed] [Google Scholar]
- 253.Yamada R, Okawa T, Matsuo K, Suzuki M, Mori N, Mori K. Renal-limited thrombotic microangiopathy after switching from bevacizumab to ramucirumab: a case report. BMC Nephrol. 2019;20:14. doi: 10.1186/s12882-018-1194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Hirai T, Shuji Y, Takiyama M, Hanada K, Itoh T. Renin-angiotensin system inhibitors for countering proteinuria induced by angiogenesis inhibitors: a retrospective observational analysis. Cancer Chemother Pharmacol. 2019;84:195–202. doi: 10.1007/s00280-019-03876-5. [DOI] [PubMed] [Google Scholar]
- 255.Eli Lilly and Company. CYRAMZA (ramucirumab) [package insert]. U.S. Food and Drug Administration website. [cited 12 March 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125477s037lbl.pdf .
- 256.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 257.Sassier M, Dugué AE, Clarisse B, Lesueur P, Avrillon V, Bizieux-Thaminy A, Auliac JB, Kaluzinski L, Tillon J, Robinet G, Le Caer H, Monnet I, Madroszyk A, Boza G, Falchero L, Fournel P, Egenod T, Toffart AC, Leiber N, Do P, Gervais R. Renal insufficiency is the leading cause of double maintenance (bevacizumab and pemetrexed) discontinuation for toxicity to advanced non-small cell lung cancer in real world setting. Lung Cancer. 2015;89:161–166. doi: 10.1016/j.lungcan.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 258.Zhao J, Li H, Wang M. Acute renal failure in a patient receiving anti-VEGF therapy for advanced non-small cell lung cancer. J Thorac Oncol. 2009;4:1185–1187. doi: 10.1097/JTO.0b013e3181b2362f. [DOI] [PubMed] [Google Scholar]
- 259.Wu S, Kim C, Baer L, Zhu X. Bevacizumab increases risk for severe proteinuria in cancer patients. J Am Soc Nephrol. 2010;21:1381–1389. doi: 10.1681/ASN.2010020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Zhao T, Wang X, Xu T, Xu X, Liu Z. Bevacizumab significantly increases the risks of hypertension and proteinuria in cancer patients: A systematic review and comprehensive meta-analysis. Oncotarget. 2017;8:51492–51506. doi: 10.18632/oncotarget.18190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Usui J, Glezerman IG, Salvatore SP, Chandran CB, Flombaum CD, Seshan SV. Clinicopathological spectrum of kidney diseases in cancer patients treated with vascular endothelial growth factor inhibitors: a report of 5 cases and review of literature. Hum Pathol. 2014;45:1918–1927. doi: 10.1016/j.humpath.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 262.Uy AL, Simper NB, Champeaux AL, Perkins RM. Progressive bevacizumab-associated renal thrombotic microangiopathy. NDT Plus. 2009;2:36–39. doi: 10.1093/ndtplus/sfn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Vakiti A, Singh D, Pilla R, Alhaj-Moustafa M, Fitzpatrick KW. Bevacizumab-induced atypical hemolytic uremic syndrome and treatment with eculizumab. J Oncol Pharm Pract. 2019;25:1011–1015. doi: 10.1177/1078155218774895. [DOI] [PubMed] [Google Scholar]
- 264.Lomax AJ, Hill PA, Ashley DM. Case report of interstitial nephritis induced by bevacizumab therapy for glioblastoma multiforme. J Oncol Pharm Pract. 2013;19:365–368. doi: 10.1177/1078155212466421. [DOI] [PubMed] [Google Scholar]
- 265.Barakat RK, Singh N, Lal R, Verani RR, Finkel KW, Foringer JR. Interstitial nephritis secondary to bevacizumab treatment in metastatic leiomyosarcoma. Ann Pharmacother. 2007;41:707–710. doi: 10.1345/aph.1H635. [DOI] [PubMed] [Google Scholar]
- 266.Hanna RM, Lopez E, Wilson J, Barathan S, Cohen AH. Minimal change disease onset observed after bevacizumab administration. Clin Kidney J. 2016;9:239–244. doi: 10.1093/ckj/sfv139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Genentech Inc. AVASTIN (bevacizumab) [package insert]. U.S. Food and Drug Administration website. [cited 12 March 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125085s337lbl.pdf .
- 268.Garnier-Viougeat N, Rixe O, Paintaud G, Ternant D, Degenne D, Mouawad R, Deray G, Izzedine H. Pharmacokinetics of bevacizumab in haemodialysis. Nephrol Dial Transplant. 2007;22:975. doi: 10.1093/ndt/gfl664. [DOI] [PubMed] [Google Scholar]
- 269.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kole C, Charalampakis N, Tsakatikas S, Vailas M, Moris D, Gkotsis E, Kykalos S, Karamouzis MV, Schizas D. Immunotherapy for Hepatocellular Carcinoma: A 2021 Update. Cancers (Basel) 2020;12 doi: 10.3390/cancers12102859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Rosenberg SA. Decade in review-cancer immunotherapy: entering the mainstream of cancer treatment. Nat Rev Clin Oncol. 2014;11:630–632. doi: 10.1038/nrclinonc.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Greten TF, Sangro B. Targets for immunotherapy of liver cancer. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Cerezo D, Peña MJ, Mijares M, Martínez G, Blanca I, De Sanctis JB. Peptide vaccines for cancer therapy. Recent Pat Inflamm Allergy Drug Discov. 2015;9:38–45. doi: 10.2174/1872213x09666150131141953. [DOI] [PubMed] [Google Scholar]
- 274.Parmiani G, Russo V, Maccalli C, Parolini D, Rizzo N, Maio M. Peptide-based vaccines for cancer therapy. Hum Vaccin Immunother. 2014;10:3175–3178. doi: 10.4161/hv.29418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Akce M, Zaidi MY, Waller EK, El-Rayes BF, Lesinski GB. The Potential of CAR T Cell Therapy in Pancreatic Cancer. Front Immunol. 2018;9:2166. doi: 10.3389/fimmu.2018.02166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Hoffman-Censits J, Pal S, Kaiser C, Ding B, Bellmunt J. Atezolizumab in patients with renal insufficiency and mixed variant histology: analyses from an expanded access program in platinum-treated locally advanced or metastatic urothelial carcinoma. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Parisi A, Cortellini A, Cannita K, Bersanelli M, Ficorella C. Safe Administration of anti-PD-L1 Atezolizumab in a Patient with Metastatic Urothelial Cell Carcinoma and End-Stage Renal Disease on Dialysis. Case Rep Oncol Med. 2019;2019:3452762. doi: 10.1155/2019/3452762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, Fong L, Joseph RW, Pal SK, Reeves JA, Sznol M, Hainsworth J, Rathmell WK, Stadler WM, Hutson T, Gore ME, Ravaud A, Bracarda S, Suárez C, Danielli R, Gruenwald V, Choueiri TK, Nickles D, Jhunjhunwala S, Piault-Louis E, Thobhani A, Qiu J, Chen DS, Hegde PS, Schiff C, Fine GD, Powles T. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24:749–757. doi: 10.1038/s41591-018-0053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.McDermott DF, Sosman JA, Sznol M, Massard C, Gordon MS, Hamid O, Powderly JD, Infante JR, Fassò M, Wang YV, Zou W, Hegde PS, Fine GD, Powles T. Atezolizumab, an Anti-Programmed Death-Ligand 1 Antibody, in Metastatic Renal Cell Carcinoma: Long-Term Safety, Clinical Activity, and Immune Correlates From a Phase Ia Study. J Clin Oncol. 2016;34:833–842. doi: 10.1200/JCO.2015.63.7421. [DOI] [PubMed] [Google Scholar]
- 280.Genentech Inc. TECENTRIQ (atezolizumab) [package insert]. U.S. Food and Drug Administration website. [cited 12 March 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761034s020lbl.pdf .
- 281.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 282.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 283.Izzedine H, Mathian A, Champiat S, Picard C, Mateus C, Routier E, Varga A, Malka D, Leary A, Michels J, Michot JM, Marabelle A, Lambotte O, Amoura Z, Soria JC, Kaaki S, Quellard N, Goujon JM, Brocheriou I. Renal toxicities associated with pembrolizumab. Clin Kidney J. 2019;12:81–88. doi: 10.1093/ckj/sfy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Wanchoo R, Karam S, Uppal NN, Barta VS, Deray G, Devoe C, Launay-Vacher V, Jhaveri KD Cancer and Kidney International Network Workgroup on Immune Checkpoint Inhibitors. Adverse Renal Effects of Immune Checkpoint Inhibitors: A Narrative Review. Am J Nephrol. 2017;45:160–169. doi: 10.1159/000455014. [DOI] [PubMed] [Google Scholar]
- 285.Mamlouk O, Selamet U, Machado S, Abdelrahim M, Glass WF, Tchakarov A, Gaber L, Lahoti A, Workeneh B, Chen S, Lin J, Abdel-Wahab N, Tayar J, Lu H, Suarez-Almazor M, Tannir N, Yee C, Diab A, Abudayyeh A. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. J Immunother Cancer. 2019;7:2. doi: 10.1186/s40425-018-0478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Merck Sharp & Dohme Corp. KEYTRUDA (pembrolizumab) [package insert]. U.S. Food and Drug Administration website. [cited 12 March 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125514s088lbl.pdf .
- 287.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Sangro B, Park J-W, Cruz CMD, Anderson J, Lang L, Neely J, Shaw JW, Cheng AL. A randomized, multicenter, phase 3 study of nivolumab vs sorafenib as first-line treatment in patients (pts) with advanced hepatocellular carcinoma (HCC): CheckMate-459. J Clin Oncol. 2016;34:TPS4147–TPS4147. doi: 10.1200/JCO.2016.34.15_suppl.TPS4147. [DOI] [Google Scholar]
- 289.Jung K, Zeng X, Bilusic M. Nivolumab-associated acute glomerulonephritis: a case report and literature review. BMC Nephrol. 2016;17:188. doi: 10.1186/s12882-016-0408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Uchida N, Tsuji S, Fujita K, Koizumi M, Moriyoshi K, Mio T. Nivolumab-induced severe acute kidney injury with a long latent phase in a patient with non-small-cell lung cancer: A case report. Clin Case Rep. 2018;6:2185–2188. doi: 10.1002/ccr3.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Bristol-Myers Squibb Company. OPDIVO (nivolumab) [package insert]. U.S. Food and Drug Administration website. [cited 12 March 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125527s000lbl.pdf .
- 292.Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6:e204564. doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, Le DT, Lipson EJ, Glezerman IG, Wolchok J, Cornell LD, Feldman P, Stokes MB, Zapata SA, Hodi FS, Ott PA, Yamashita M, Leaf DE. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016;90:638–647. doi: 10.1016/j.kint.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Iannello A, Thompson TW, Ardolino M, Marcus A, Raulet DH. Immunosurveillance and immunotherapy of tumors by innate immune cells. Curr Opin Immunol. 2016;38:52–58. doi: 10.1016/j.coi.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Fadel F, El Karoui K, Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med. 2009;361:211–212. doi: 10.1056/NEJMc0904283. [DOI] [PubMed] [Google Scholar]
- 296.Barnard ZR, Walcott BP, Kahle KT, Nahed BV, Coumans JV. Hyponatremia associated with Ipilimumab-induced hypophysitis. Med Oncol. 2012;29:374–377. doi: 10.1007/s12032-010-9794-7. [DOI] [PubMed] [Google Scholar]
- 297.Chodakiewitz Y, Brown S, Boxerman JL, Brody JM, Rogg JM. Ipilimumab treatment associated pituitary hypophysitis: clinical presentation and imaging diagnosis. Clin Neurol Neurosurg. 2014;125:125–130. doi: 10.1016/j.clineuro.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 298.Bristol-Myers Squibb Company. YERVOY (ipilimumab) [package insert]. U.S. Food and Drug Administration website. [cited 12 March 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125377s119lbl.pdf .
- 299.Wang Z, Yin N, Zhang Z, Zhang Y, Zhang G, Chen W. Upregulation of T-cell Immunoglobulin and Mucin-Domain Containing-3 (Tim-3) in Monocytes/Macrophages Associates with Gastric Cancer Progression. Immunol Invest. 2017;46:134–148. doi: 10.1080/08820139.2016.1229790. [DOI] [PubMed] [Google Scholar]
- 300.Ganjalikhani Hakemi M, Jafarinia M, Azizi M, Rezaeepoor M, Isayev O, Bazhin AV. The Role of TIM-3 in Hepatocellular Carcinoma: A Promising Target for Immunotherapy? Front Oncol. 2020;10:601661. doi: 10.3389/fonc.2020.601661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Zhu C, Anderson AC, Kuchroo VK. TIM-3 and its regulatory role in immune responses. Curr Top Microbiol Immunol. 2011;350:1–15. doi: 10.1007/82_2010_84. [DOI] [PubMed] [Google Scholar]
- 302.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M, Bruce JN, Kane LP, Kuchroo VK, Hafler DA. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 304.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 305.Wiener Z, Kohalmi B, Pocza P, Jeager J, Tolgyesi G, Toth S, Gorbe E, Papp Z, Falus A. TIM-3 is expressed in melanoma cells and is upregulated in TGF-beta stimulated mast cells. J Invest Dermatol. 2007;127:906–914. doi: 10.1038/sj.jid.5700616. [DOI] [PubMed] [Google Scholar]
- 306.Pfeffer CR. Assessment of suicidal children and adolescents. Psychiatr Clin North Am. 1989;12:861–872. [PubMed] [Google Scholar]
- 307.Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, Liu J, Shi L, Liu C, Wang G, Zou W. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342–1351. doi: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- 308.Liu F, Liu Y, Chen Z. Tim-3 expression and its role in hepatocellular carcinoma. J Hematol Oncol. 2018;11:126. doi: 10.1186/s13045-018-0667-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Zhang H, Song Y, Yang H, Liu Z, Gao L, Liang X, Ma C. Tumor cell-intrinsic Tim-3 promotes liver cancer via NF-κB/IL-6/STAT3 axis. Oncogene. 2018;37:2456–2468. doi: 10.1038/s41388-018-0140-4. [DOI] [PubMed] [Google Scholar]
- 310.Schroll A, Eller K, Huber JM, Theurl IM, Wolf AM, Weiss G, Rosenkranz AR. Tim3 is upregulated and protective in nephrotoxic serum nephritis. Am J Pathol. 2010;176:1716–1724. doi: 10.2353/ajpath.2010.090859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Yang H, Xie T, Li D, Du X, Wang T, Li C, Song X, Xu L, Yi F, Liang X, Gao L, Yang X, Ma C. Tim-3 aggravates podocyte injury in diabetic nephropathy by promoting macrophage activation via the NF-κB/TNF-α pathway. Mol Metab. 2019;23:24–36. doi: 10.1016/j.molmet.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Berg T, Zoulim F, Moeller B, Trinh H, Marcellin P, Chan S, Kitrinos KM, Dinh P, Flaherty JF Jr, McHutchison JG, Manns M. Long-term efficacy and safety of emtricitabine plus tenofovir DF vs. tenofovir DF monotherapy in adefovir-experienced chronic hepatitis B patients. J Hepatol. 2014;60:715–722. doi: 10.1016/j.jhep.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 313.Buti M, Tsai N, Petersen J, Flisiak R, Gurel S, Krastev Z, Aguilar Schall R, Flaherty JF, Martins EB, Charuworn P, Kitrinos KM, Subramanian GM, Gane E, Marcellin P. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig Dis Sci. 2015;60:1457–1464. doi: 10.1007/s10620-014-3486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Ahn J, Lee HM, Lim JK, Pan CQ, Nguyen MH, Ray Kim W, Mannalithara A, Trinh H, Chu D, Tran T, Min A, Do S, Te H, Reddy KR, Lok AS. Entecavir safety and effectiveness in a national cohort of treatment-naïve chronic hepatitis B patients in the US - the ENUMERATE study. Aliment Pharmacol Ther. 2016;43:134–144. doi: 10.1111/apt.13440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Watanabe T, Tokumoto Y, Joko K, Michitaka K, Mashiba T, Hiraoka A, Ochi H, Koizumi Y, Tada F, Hirooka M, Yoshida O, Imai Y, Abe M, Hiasa Y. Effects of long-term entecavir treatment on the incidence of hepatocellular carcinoma in chronic hepatitis B patients. Hepatol Int. 2016;10:320–327. doi: 10.1007/s12072-015-9647-8. [DOI] [PubMed] [Google Scholar]
- 316.Loomba R, Liang TJ. Hepatitis B Reactivation Associated With Immune Suppressive and Biological Modifier Therapies: Current Concepts, Management Strategies, and Future Directions. Gastroenterology. 2017;152:1297–1309. doi: 10.1053/j.gastro.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Lok AS, Ward JW, Perrillo RP, McMahon BJ, Liang TJ. Reactivation of hepatitis B during immunosuppressive therapy: potentially fatal yet preventable. Ann Intern Med. 2012;156:743–745. doi: 10.7326/0003-4819-156-10-201205150-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Nagington J. Reactivation of hepatitis b after transplantation operations. Lancet. 1977;1:558–560. doi: 10.1016/s0140-6736(77)91995-x. [DOI] [PubMed] [Google Scholar]
- 319.Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT American Gastroenterological Association Institute. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:215–9; quiz e16. doi: 10.1053/j.gastro.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 320.Choi J, Jo C, Lim YS. Tenofovir Versus Entecavir on Recurrence of Hepatitis B Virus-Related Hepatocellular Carcinoma After Surgical Resection. Hepatology. 2021;73:661–673. doi: 10.1002/hep.31289. [DOI] [PubMed] [Google Scholar]
- 321.Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y, Ikeda K, Kumada H. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107. doi: 10.1002/hep.26180. [DOI] [PubMed] [Google Scholar]
- 322.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J Cirrhosis Asian Lamivudine Multicentre Study Group. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 323.Tseng CH, Hsu YC, Chen TH, Ji F, Chen IS, Tsai YN, Hai H, Thuy LTT, Hosaka T, Sezaki H, Borghi JA, Cheung R, Enomoto M, Nguyen MH. Hepatocellular carcinoma incidence with tenofovir versus entecavir in chronic hepatitis B: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:1039–1052. doi: 10.1016/S2468-1253(20)30249-1. [DOI] [PubMed] [Google Scholar]
- 324.Yip TC, Wong VW, Chan HL, Tse YK, Lui GC, Wong GL. Tenofovir Is Associated With Lower Risk of Hepatocellular Carcinoma Than Entecavir in Patients With Chronic HBV Infection in China. Gastroenterology 2020; 158: 215-225. :e6. doi: 10.1053/j.gastro.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 325.Papatheodoridis GV, Idilman R, Dalekos GN, Buti M, Chi H, van Boemmel F, Calleja JL, Sypsa V, Goulis J, Manolakopoulos S, Loglio A, Siakavellas S, Keskın O, Gatselis N, Hansen BE, Lehretz M, de la Revilla J, Savvidou S, Kourikou A, Vlachogiannakos I, Galanis K, Yurdaydin C, Berg T, Colombo M, Esteban R, Janssen HLA, Lampertico P. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B. Hepatology. 2017;66:1444–1453. doi: 10.1002/hep.29320. [DOI] [PubMed] [Google Scholar]
- 326.Pellicelli AM, Vignally P, Messina V, Izzi A, Mazzoni E, Barlattani A, Bacca D, Romano M, Mecenate F, Stroffolini T, Furlan C, Picardi A, Gentilucci UV, Gulminetti R, Bonaventura ME, Villani R, D'Ambrosio C, Paffetti A, Mastropietro C, Marignani M, Fondacaro L, Cerasari G, Andreoli A, Barbarini G. Long term nucleotide and nucleoside analogs treatment in chronic hepatitis B HBeAg negative genotype D patients and risk for hepatocellular carcinoma. Ann Hepatol. 2014;13:376–385. [PubMed] [Google Scholar]
- 327.Kim SS, Hwang JC, Lim SG, Ahn SJ, Cheong JY, Cho SW. Effect of virological response to entecavir on the development of hepatocellular carcinoma in hepatitis B viral cirrhotic patients: comparison between compensated and decompensated cirrhosis. Am J Gastroenterol. 2014;109:1223–1233. doi: 10.1038/ajg.2014.145. [DOI] [PubMed] [Google Scholar]
- 328.Cho JY, Paik YH, Sohn W, Cho HC, Gwak GY, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC. Patients with chronic hepatitis B treated with oral antiviral therapy retain a higher risk for HCC compared with patients with inactive stage disease. Gut. 2014;63:1943–1950. doi: 10.1136/gutjnl-2013-306409. [DOI] [PubMed] [Google Scholar]
- 329.Tenney DJ, Rose RE, Baldick CJ, Levine SM, Pokornowski KA, Walsh AW, Fang J, Yu CF, Zhang S, Mazzucco CE, Eggers B, Hsu M, Plym MJ, Poundstone P, Yang J, Colonno RJ. Two-year assessment of entecavir resistance in Lamivudine-refractory hepatitis B virus patients reveals different clinical outcomes depending on the resistance substitutions present. Antimicrob Agents Chemother. 2007;51:902–911. doi: 10.1128/AAC.00833-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Tsai MC, Chen CH, Tseng PL, Hung CH, Chiu KW, Wang JH, Lu SN, Lee CM, Chang KC, Yen YH, Lin MT, Chou YP, Hu TH. Comparison of renal safety and efficacy of telbivudine, entecavir and tenofovir treatment in chronic hepatitis B patients: real world experience. Clin Microbiol Infect 2016; 22: 95.e1-95. :e7. doi: 10.1016/j.cmi.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 331.Bryant ML, Bridges EG, Placidi L, Faraj A, Loi AG, Pierra C, Dukhan D, Gosselin G, Imbach JL, Hernandez B, Juodawlkis A, Tennant B, Korba B, Cote P, Marion P, Cretton-Scott E, Schinazi RF, Sommadossi JP. Antiviral L-nucleosides specific for hepatitis B virus infection. Antimicrob Agents Chemother. 2001;45:229–235. doi: 10.1128/AAC.45.1.229-235.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Genovesi EV, Lamb L, Medina I, Taylor D, Seifer M, Innaimo S, Colonno RJ, Standring DN, Clark JM. Efficacy of the carbocyclic 2'-deoxyguanosine nucleoside BMS-200475 in the woodchuck model of hepatitis B virus infection. Antimicrob Agents Chemother. 1998;42:3209–3217. doi: 10.1128/AAC.42.12.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Suzuki K, Suda G, Yamamoto Y, Furuya K, Baba M, Kimura M, Maehara O, Shimazaki T, Yamamoto K, Shigesawa T, Nakamura A, Ohara M, Kawagishi N, Nakai M, Sho T, Natsuizaka M, Morikawa K, Ogawa K, Sakamoto N NORTE Study Group. Entecavir treatment of hepatitis B virus-infected patients with severe renal impairment and those on hemodialysis. Hepatol Res. 2019;49:1294–1304. doi: 10.1111/hepr.13399. [DOI] [PubMed] [Google Scholar]
- 334.Matthews SJ. Entecavir for the treatment of chronic hepatitis B virus infection. Clin Ther. 2006;28:184–203. doi: 10.1016/j.clinthera.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 335.Bristol-Myers Squibb Company. BARACLUDE (entecavir) [package insert]. U.S. Food and Drug Administration website. [cited 12 March 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021797s023, 021798s024lbl.pdf.
- 336.Gilead Sciences, Inc. VIREAD (tenofovir disoproxil fumarate) [package insert]. U.S. Food and Drug Administration website. [cited 12 March 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021356s058,022577s014lbl.pdf .
- 337.Ustianowski A, Arends JE. Tenofovir: What We Have Learnt After 7.5 Million Person-Years of Use. Infect Dis Ther. 2015;4:145–157. doi: 10.1007/s40121-015-0070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Morlat P, Vivot A, Vandenhende MA, Dauchy FA, Asselineau J, Déti E, Gerard Y, Lazaro E, Duffau P, Neau D, Bonnet F, Chêne G Groupe D’epidémiologie Clinique du Sida en Aquitaine (Gecsa) Role of traditional risk factors and antiretroviral drugs in the incidence of chronic kidney disease, ANRS CO3 Aquitaine cohort, France, 2004-2012. PLoS One. 2013;8:e66223. doi: 10.1371/journal.pone.0066223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Lucas GM, Ross MJ, Stock PG, Shlipak MG, Wyatt CM, Gupta SK, Atta MG, Wools-Kaloustian KK, Pham PA, Bruggeman LA, Lennox JL, Ray PE, Kalayjian RC HIV Medicine Association of the Infectious Diseases Society of America. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e96–138. doi: 10.1093/cid/ciu617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Jeon MY, Lee JS, Lee HW, Kim BK, Park JY, Kim DY, Han KH, Ahn SH, Kim SU. Entecavir and tenofovir on renal function in patients with hepatitis B virus-related hepatocellular carcinoma. J Viral Hepat. 2020;27:932–940. doi: 10.1111/jvh.13313. [DOI] [PubMed] [Google Scholar]
- 341.Murakami E, Wang T, Park Y, Hao J, Lepist EI, Babusis D, Ray AS. Implications of efficient hepatic delivery by tenofovir alafenamide (GS-7340) for hepatitis B virus therapy. Antimicrob Agents Chemother. 2015;59:3563–3569. doi: 10.1128/AAC.00128-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Agarwal K, Brunetto M, Seto WK, Lim YS, Fung S, Marcellin P, Ahn SH, Izumi N, Chuang WL, Bae H, Sharma M, Janssen HLA, Pan CQ, Çelen MK, Furusyo N, Shalimar D, Yoon KT, Trinh H, Flaherty JF, Gaggar A, Lau AH, Cathcart AL, Lin L, Bhardwaj N, Suri V, Mani Subramanian G, Gane EJ, Buti M, Chan HLY GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J Hepatol. 2018;68:672–681. doi: 10.1016/j.jhep.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 343.Bam RA, Yant SR, Cihlar T. Tenofovir alafenamide is not a substrate for renal organic anion transporters (OATs) and does not exhibit OAT-dependent cytotoxicity. Antivir Ther. 2014;19:687–692. doi: 10.3851/IMP2770. [DOI] [PubMed] [Google Scholar]
- 344.Gilead Sciences, Inc. VEMLIDY (tenofovir alafenamide) [package insert]. U.S. Food and Drug Administration website. [cited 12 March 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/Label/2021/208464s012. Lbl.pdf.