It has long been established that cardiovascular risk increases with worsening chronic kidney disease, with the risk being extreme in patients with end-stage renal disease (ESRD) on dialysis [1]. Historically this association has been attributed to an increase in atheroembolic events. However, more recently it has become clear that the predominant cause of this increased cardiovascular mortality is not myocardial infarction and coronary events, but sudden cardiac death, accounting for up to 40% of all deaths and 78% of cardiovascular deaths in patients with ESRD [2, 3]. Importantly, this relationship also extends to the paediatric ESRD population [4].
WHAT IS MEANT BY SUDDEN CARDIAC DEATH?
The term sudden cardiac death (SCD) is applied to an unexpected death due to cardiac causes. These events are defined as those that are either preceded by a witnessed collapse, occur within 1 h of a sudden change in clinical condition or happen not >24 h since the deceased was known to be in their usual state of health [5]. However, exclusion of other potential causes, such as stroke, can be difficult. Furthermore, the time limits set by the definition mean that some potentially true SCDs are excluded. Although arrhythmia is proposed to be the major underlying factor in SCD, the direct evidence for this is surprisingly poor. In the general population, most SCD events are due to coronary artery disease, but only about half are thought to be due to myocardial infarction, the remainder are probably related to left ventricular scarring causing ventricular tachyarrhythmias, often ventricular tachycardia degenerating to ventricular fibrillation followed by asystole [5, 6]. In patients with heart failure, there may be diverse causes of SCD, including hyperkalaemia and pulmonary embolus [7], and little benefit from primary prevention by cardiac defibrillator (ICD) implantation in patients with non-ischaemic disease [8]. Efforts to refine patient selection for primary prevention by ICD implantation using techniques such as heart rate variability, signal averaged electrocardiograms (ECGs) and heart rate turbulence have been largely unsuccessful.
SCD AND CHRONIC KIDNEY DISEASE
Impaired renal function is associated with an increased risk of SCD. This association was first described in subanalyses of several randomized controlled trials. The Multicenter Automatic Defibrillator Implantation Trial II examined the efficacy of ICD in those with prior myocardial infarction and left ventricular ejection fraction <30%, finding that renal function was the most powerful baseline clinical parameter associated with SCD [9]. However, ICD implantation had no effect on all-cause mortality or SCD in those with an estimated glomerular filtration rate <35 mL/min/1.73 m2. This finding has also been repeated in observational studies [10], suggesting tachyarrhythmias might not be the major underlying cause of SCD in severe chronic kidney disease (CKD).
SCD IN END-STAGE RENAL DISEASE
Both administrative databases and prospective cohort studies attribute the majority of cardiovascular deaths in patients with end-stage renal disease (ESRD) to SCD [4]. However, the underlying causes are far from clear. Five recent studies using implantable loop recorders, enrolling 317 haemodialysis patients with a mean follow-up ranging from 14 to 21 months, have been reported [11]. Overall, there were 15 SCDs associated with bradyarrhythmias, 2 associated with tachyarrhythmias and 3 with unclear ECG morphology. Most deaths occurred during the long intradialytic period. Although it is too early to draw firm conclusions, these sorts of data suggest that, like chronic heart failure, the mechanisms of SCD in patients with CKD may be diverse, with perhaps only a minority of events due to ventricular tachyarrhythmias.
The notion that ventricular tachyarrhythmia is not the primary cause for SCD in ESRD is also supported by a recent trial that recruited 188 participants receiving regular haemodialysis with a left ventricular ejection fraction >35% who were randomized to either receive a prophylactic ICD or continued medical therapy and followed up for a median of 6.8 years [12]. The cumulative SCD incidence at 5 years was 9.7% in the ICD group and 7.8% in the control group, resulting in a non-significant hazard ratio of 1.32 (95% confidence interval 0.53–3.29; P = 0.55).
WHY IS THE RISK OF SCD INCREASED IN PATIENTS WITH CKD AND ESRD?
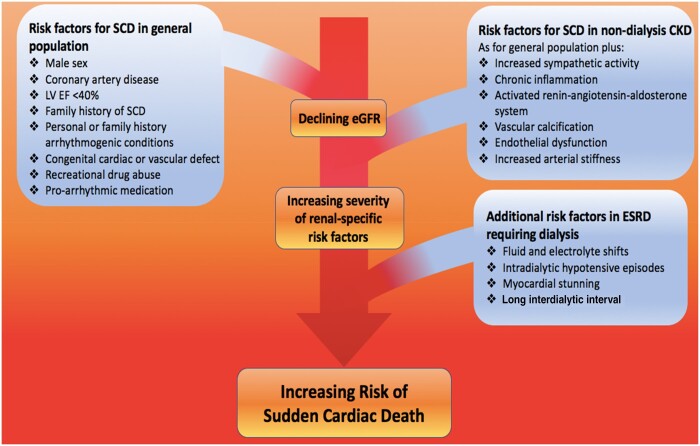
Risk factors for SCD in the general population and patients with CKD and ESRD are outlined in Figure 1. In general, the number and severity of risk factors increases with worsening renal function and is most extreme in patients with ESRD. In addition, there are unique features associated with starting a patient on haemodialysis as well as the intensity and intermittency of the individual treatments. Furthermore, both CKD and ESRD are associated with a unique cardiovascular phenotype associated with increasing endothelial dysfunction and arterial stiffness as well as the development of uraemic cardiomyopathy [1, 13]. Uraemic cardiomyopathy is characterized clinically by hypertrophy and diastolic and systolic left ventricular dysfunction and histologically by profound myocardial fibrosis [1, 13]. All of these features provide the substrate for a vulnerable myocardium and an increased risk of arrhythmias and SCD [4].
FIGURE 1.
Risk factors for sudden cardiac death in the general population, in those with chronic kidney disease, and those with end stage renal disease requiring dialysis.
POTENTIAL TREATMENTS TO PREVENT SCD IN PATIENTS WITH CKD OR ESRD
This is largely an evidence-free zone, with few clinical trials addressing this problem and extrapolation from studies obtained from other high-risk groups being potentially hazardous. However, because the links between CKD/ESRD and SCD are complex, aggressive treatment of common conditions including hypertension, diabetes and dyslipidaemia may be partially protective. β-blockers have been shown to reduce the risk of SCD in several at-risk populations, including patients with mild CKD and heart failure [14]. However, the potential exacerbation of intradialytic hypotension, conflicting data from observational studies and the emerging evidence of a preponderance of bradycardias as being the main arrhythmias in patients with ESRD suggests that β-blockers should not be routinely used to prevent SCD in the absence of adequately powered clinical trials. Similarly, while inhibitors of activation of the renin–angiotensin–aldosterone system, which reduce the occurrence of SCD in heart failure, have been shown to improve outcomes in high-risk populations with mild CKD, evidence for a role in advanced CKD/ESRD is lacking and the danger of hyperkalaemia is significant [4]. Currently there appears to be no role for the primary preventive use of ICDs in patients with severe CKD or ESRD, a finding consistent with other forms of non-ischaemic cardiomyopathy [10, 12].
Population studies highlight that following successful renal transplantation, mortality related to SCD and arrhythmia decreases from 40% to 16% [3]. However, the underlying mechanisms by which this happens are not understood and likely reflect, at least partially, many of the risk factors for SCD associated with ESRD. Research into this population might provide insights into potential mechanisms and therapies to reduce the risk of SCD in patients with ESRD.
CONCLUSIONS
Currently SCD accounts for a significant amount of the cardiovascular mortality observed in CKD and especially in ESRD patients. Unfortunately, problems with the definition of SCD, lack of knowledge of both the causes and underlying mechanisms, as well as a distinct lack of good-quality studies and trials prevent strong treatment recommendations being made [6]. High-quality research is urgently required to investigate the pathophysiology of SCD in patients with CKD/ESRD and to establish optimum management strategies.
FUNDING
L.C. P. and J.N.L. are supported by British Heart Foundation clinical research training fellowships (FS/18/29/33554, FS/19/16/34169).
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Ferro CJ, Mark PB, Kanbay M. et al. Lipid management in patients with chronic kidney disease. Nat Rev Nephrol 2018; 14: 727–749 [DOI] [PubMed] [Google Scholar]
- 2. Genovesi S, Boriani G, Covic A. et al. Sudden cardiac death in dialysis patients: different causes and management strategies. Nephrol Dial Transplant 2019; 10.1093/ndt/gfz182 [DOI] [PubMed] [Google Scholar]
- 3. Collins AJ, Foley RN, Chavers B. et al. United States Renal Data System 2011 Annual Data Report: atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis 2012; 59(1 Suppl 1): A1–A8, e1–e526 [DOI] [PubMed] [Google Scholar]
- 4. Whitman IR, Feldman HI, Deo R.. CKD and sudden cardiac death: epidemiology, mechanisms, and therapeutic approaches. J Am Soc Nephrol 2012; 23: 1929–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adabag AS, Luepker RV, Roger VL. et al. Sudden cardiac death: epidemiology and risk factors. Nat Rev Cardiol 2010; 7: 216–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Turakhia MP, Blankestijn PJ, Carrero JJ. et al. Chronic kidney disease and arrhythmias: conclusions from a kidney disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J 2018; 39: 2314–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luu M, Stevenson WG, Stevenson LW. et al. Diverse mechanisms of unexpected cardiac arrest in advanced heart failure. Circulation 1989; 80: 1675–1680 [DOI] [PubMed] [Google Scholar]
- 8. Kober L, Thune JJ, Nielsen JC. et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016; 375: 1221–1230 [DOI] [PubMed] [Google Scholar]
- 9. Goldenberg I, Moss AJ, McNitt S. et al. Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol 2006; 98: 485–490 [DOI] [PubMed] [Google Scholar]
- 10. Nakhoul GN, Schold JD, Arrigain S. et al. Implantable cardioverter-defibrillators in patients with CKD: a propensity-matched mortality analysis. Clin J Am Soc Nephrol 2015; 10: 1119–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalra PA, Green D, Poulikakos D.. Arrhythmia in hemodialysis patients and its relation to sudden death. Kidney Int 2018; 93: 781–783 [DOI] [PubMed] [Google Scholar]
- 12. Jukema JW, Timal RJ, Rotmans JI. et al. Prophylactic use of implantable cardioverter-defibrillators in the prevention of sudden cardiac death in dialysis patients. Circulation 2019; 139: 2628–2638 [DOI] [PubMed] [Google Scholar]
- 13. Edwards NC, Moody WE, Chue CD. et al. Defining the natural history of uremic cardiomyopathy in chronic kidney disease: the role of cardiovascular magnetic resonance. JACC Cardiovasc Imaging 2014; 7: 703–714 [DOI] [PubMed] [Google Scholar]
- 14. Badve SV, Roberts MA, Hawley CM. et al. Effects of beta-adrenergic antagonists in patients with chronic kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol 2011; 58: 1152–1161 [DOI] [PubMed] [Google Scholar]