Abstract
Background
Anti-CD20 B-cell depletion has not shown superior efficacy to standard immunosuppression in patients with systemic lupus erythematosus (SLE). Besides trial design, potential explanations are incomplete B-cell depletion in relation to substantial surges in B-cell-activating factor (BAFF). To improve B-cell targeting strategies, we conducted the first study in SLE patients aimed at investigating immunological effects and feasibility of combining rituximab (RTX; anti-CD20) and belimumab (BLM; anti-BAFF).
Methods
Reported is the long-term follow-up of a Phase 2 proof-of-concept study in 15 patients with SLE including 12 (80%) with lupus nephritis (LN).
Results
In 10/15 (67%) patients, a clinical response was observed by achievement of lupus low disease activity state, of which 8 (53%) continued treatment (BLM + ≤7.5 mg prednisolone) for the complete 2 years of follow-up. Five patients (33%) were referred to as ‘non-responders’ due to persistent LN, major flare or repetitive minor flares. Out of 12 LN patients, 9 (75%) showed a renal response including 8 (67%) complete renal responders. All anti-dsDNA+ patients converted to negative, and both anti-C1q and extractable nuclear antigen autoantibodies showed significant reductions. CD19+ B cells showed a median decrease from baseline of 97% at 24 weeks, with a persistent reduction of 84% up to 104 weeks. When comparing responders with non-responders, CD20+ B cells were depleted significantly less in non-responders and double-negative (DN) B cells repopulated significantly earlier.
Conclusions
Combined B-cell targeted therapy with RTX and BLM prevented full B-cell repopulation including DN B cells, with concomitant specific reduction of SLE-relevant autoantibodies. The observed immunological and clinical benefits in a therapy-refractory SLE population prompt further studies on RTX + BLM.
Keywords: autoantibodies, autoimmune glomerulonephritis, immune complex-mediated membranoproliferative, lupus nephritis, rituximab/belimumab, systemic lupus erythematosus
KEY LEARNING POINTS
What is already known about this subject?
Anti-CD20 B-cell depletion has not been superior to standard immunosuppressive regimens where incomplete B-cell depletion is associated with non-response as well as increased levels of B-cell-activating factor (BAFF) after B-cell depletion.
BAFF can be neutralized by the novel monoclonal antibody belimumab (BLM), which demonstrated superior efficacy in preclinical animal studies.
The combination therapy of anti-CD20 B-cell depletion by rituximab (RTX) with the addition of BAFF neutralization by BLM has only been published in a few case reports.
What this study adds?
The study is the first, proof-of-concept study designed to investigate the long-term feasibility of combining RTX + BLM in systemic lupus erythematosus (SLE) patients, which was well-tolerated without major safety issues throughout the 2-year follow-up and allowed the discontinuation of mycophenolate mofetil (MMF) associated with significant immune reconstitution.
RTX + BLM led to long-lasting and specific reduction of several anti-nuclear autoantibodies and prevented the full repopulation of B cells.
Response to treatment was associated with more profound depletion of CD20+ B cells and prolonged suppression of double-negative B cells.
What impact this may have on practice or policy?
RTX + BLM achieved targeting of pathological autoimmunity in SLE and showed a potential clinical benefit. Because no major safety issues were raised throughout the study, RTX + BLM can safely be studied in further clinical trials to establish its efficacy compared with standard treatment.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease in which loss of tolerance to nucleic acids and their binding proteins results in generation of autoantibodies (e.g. anti-DNA, anti-chromatin or anti-histone autoantibodies), leading to inflammation potentially involving almost every organ system, including the kidney [1]. Lupus nephritis (LN) is seen in 29–82% of patients [2] and remains difficult to treat, with short-term complete renal response (CRR) rates around 10–40% at 12 months [3] and occurrence of end-stage renal disease in 10% of LN patients [4]. Together with the fact that patients with refractory SLE receive high cumulative dosage of toxic immunosuppressive medication, exploration of new therapeutic options is important.
Since autoantibodies contribute to renal pathology in SLE, targeting autoreactive B cells has continued interest as a possible strategy for treating SLE patients. Targeting B cells with the anti-CD20 monoclonal antibody (mAb) rituximab (RTX) has been unsuccessful in randomized trials in both patients with extra-renal [5] and those with renal SLE [6]. Belimumab (BLM), an anti-B-cell-activating factor (BAFF) mAb, was approved for the treatment of active SLE. Approval of BLM included a special warning on its use with concomitant B-cell targeted therapy; however, RTX + BLM provides an opportunity to target the surge in circulating BAFF levels after B-cell depletion, thereby minimizing the survival of autoreactive B cells [7, 8].
The concept of combining anti-CD20 B-cell depletion with anti-BAFF cytokine inhibition is supported by mice studies showing the importance of the micro-environment and cellular competition in anti-CD20 mAb-mediated killing of B cells where cellular competition for survival factors (e.g. availability of BAFF) can underpin resistance to anti-CD20 therapy [9]. The importance of BAFF levels in anti-CD20 therapy is further illustrated in a study using an in vitro model of mature B cells, where BAFF was able to inhibit CD20-mediated apoptosis [10]. Additionally, in different lupus mouse models, a combination of anti-CD20 and anti-BAFF therapy led to improved disease control compared with each treatment separately or cyclophosphamide [11]. We have previously reported on the effects of combination treatment with CD20 and BAFF targeting in SLE patients [12]; however, the long-term effects on B-cell repopulation and B-cell composition have not been reported yet.
‘Synergetic B-cell immunomodulation in SLE’ (Synbiose) was designed as the first translational, single-arm, proof-of-concept study in SLE patients aimed at investigating the underpinning, immunological hypothesis of combining RTX + BLM in severe, refractory SLE patients. We previously reported the early effects of RTX + BLM, demonstrating a reduction in anti-nuclear antibodies (ANAs) and regression of excessive neutrophil extracellular trap formation [12]. We now report long-term effects of RTX + BLM on depletion of ANAs, B-cell repopulation and clinical response during 2 years of follow-up.
MATERIALS AND METHODS
Study design
The Synbiose study is a Phase 2, single-arm, open-label proof-of-concept study in which ‘severe, refractory SLE’ patients were included, defined as persisting or progressive disease despite conventional immunosuppressive treatment with an SLE disease activity index (SELENA-SLEDAI) score at time of inclusion of ≥12 points. Patients were treated with intravenous methylprednisolone pulse therapy at baseline, 1000 mg intravenous RTX at Weeks 0 + 2 and with intravenous 10 mg/kg BLM at Weeks 4 + 6 + 8 and then every 4 weeks until 104 weeks. Mycophenolate mofetil (MMF) was started but quickly tapered to avoid cumulative over-immunosuppression. Oral prednisolone was started at 1 mg/kg/day (maximum 60 mg/day) and tapered towards maintenance dose of ≤7.5 mg/day. The study was approved by the Dutch Leiden University Medical Center medical ethics committee and all patients provided written informed consent. The study was registered at ClinicalTrials.gov (NCT02284984).
A fully detailed methods section with description of the clinical parameters, methods and materials used for experiments and statistical analysis is available as Supplementary data, File S1.
RESULTS
Summarized patient characteristics
Baseline characteristics from all included patients have been reported previously [12]. Briefly, 16 patients (88% female) were included, with median age of 31 years (range 19–51). All patients had refractory disease, of which 12 (80%) had active LN at baseline. One patient experienced severe hypogammaglobulinaemia at Week 8 after completion of methylprednisolone and RTX; therefore, BLM treatment was not initiated. This patient was excluded from the long-term follow-up study. Fifteen patients reached the primary endpoint at Week 24. A flow diagram of the patients is included in Figure 1.
FIGURE 1.
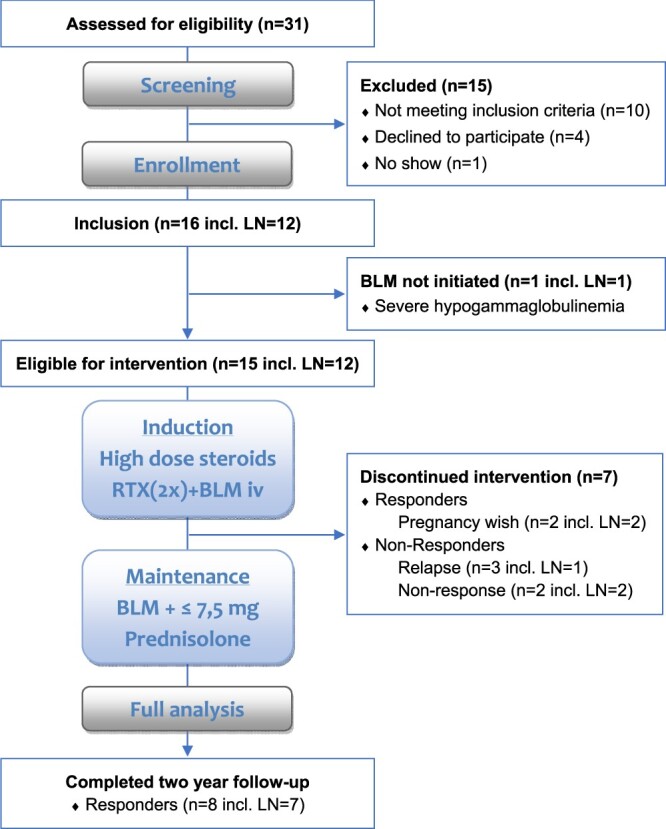
Flow diagram of patients.
Clinical response
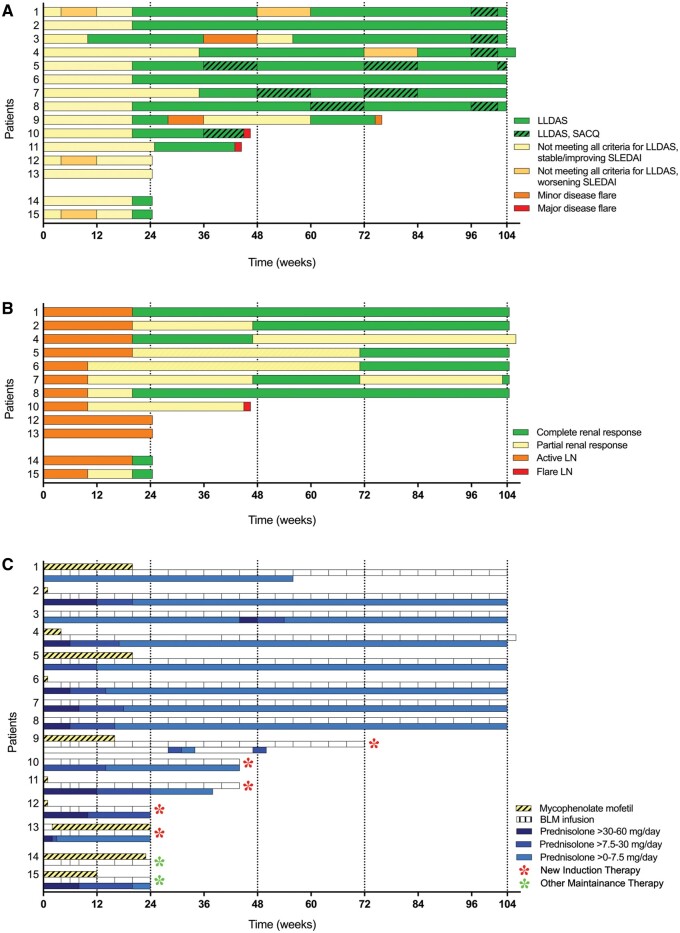
During the study period, 10 of 15 (67%) patients had a clinical response. At Week 104, this response was 8 of 13 (62%). Eight patients (53%) finished the complete follow-up of 104 weeks. Two patients with a clinical response stopped BLM treatment at Week 24, based on a pregnancy wish (Patients #14 and #15 in Figure 2). Clinical response is illustrated in Figure 2A defined by the time for patients to achieve and remain in lupus low disease activity state (LLDAS) and by attaining a renal response in patients with active LN at baseline (Figure 2B). In the eight responders available for analysis over the 2-year follow-up, the median time to the first achievement of LLDAS was 24 weeks (range 12–36) and the median time on LLDAS was 76 weeks (56–92). One patient had a minor flare with pericarditis and received 0.5 mg/kg prednisone and colchicine (Patient #3) followed by quick resolution of disease activity. At Week 104, seven of eight patients received maintenance therapy with glucocorticoids with median (range) dose of 7.5 mg/day (2.5–7.5), all patients continuously used hydroxychloroquine and BLM (Figure 2C).
FIGURE 2.
Overview of the clinical responses, renal responses and concomitant immunosuppression upon RTX + BLM treatment. (A) Achievement of LLDAS over time. (B) Achievement of a renal response in patients included with active LN (n = 12). CRR was achieved when proteinuria ≤0.7 g/day, normal serum albumin, stable kidney function and normal urinary sediment; partial response: >0.7–2.9g/24 h with a decrease in proteinuria of ≥50% from baseline, serum albumin >30 g/L and stable kidney function. When patients did not meet any of these criteria, they were considered to have persistent active LN. (C) Overview of concomitant treatment with BLM, MMF and prednisolone throughout the study’s follow-up. Patient numbers mentioned on the y-axis correspond between the three figures. SACQ, serologically active (positive antibody and or low complement) clinically quiescent.
In patients with active LN at baseline, 9 of 12 (75%) had a renal response during the trial period with CRR at Week 104 in 6 of 10 (60%), all had proteinuria <0.5 g/day. In renal responders that finished the study period (n = 7), proteinuria decreased from a median (range) of 4.6 g/day (1.3–11.2) to 0.3 (0.1–1) (P = 0.02 at Week 104), representing a median decrease of 96%. Despite rapid decline upon treatment, Patient #4 did not reach CRR due to persistent proteinuria >0.7 g/day, which clinically correlated with histologically proven chronic renal damage warranting the continuation in the study.
Five patients were classified as ‘non-responders’ and dropped out due to clinical relapse or non-response necessitating alternative induction treatment: two patients had a major flare: Patient #10 experienced a renal flare at Week 46 requiring cyclophosphamide treatment and Patient #11 experienced recurrence of transverse myelitis at Week 44, upon which induction treatment with RTX + steroids was given. Patients #12 and #13 had persistent features of active LN and were excluded at Week 24, as described in more detail previously [12]; one was treated with cyclophosphamide, while other was given an experimental induction treatment within another study. Patient #9 was excluded at Week 74 due to a recurrent minor flare (complement consumption, anti-dsDNA positivity and arthritis) and was switched to leflunomide with high-dose steroids (0.5 mg/kg/day). Baseline characteristics of the responders and non-responders are depicted in Table 1.
Table 1.
Baseline and historic disease characteristics of responders (n = 8) and non-responders (n = 5)
| Characteristic | Responders (n = 8) | Non- responders (n = 5) |
|---|---|---|
| Demographics | ||
| Age, median (range) | 31 (21–47) | 30 (19–51) |
| Female sex, n (%) | 6 (75) | 5 (100) |
| Race, n (%) | ||
| White/Caucasian | 2 (25) | 2 (40) |
| Black/African Origen | 6 (75) | 2 (40) |
| Asian/Oriental | 0 (0) | 1 (20) |
| Smoker, % | 2 (25) | 0 (0) |
| Baseline disease characteristics | ||
| SLEDAI, median (range) | 19 (12–26) | 18 (6–29) |
| Disease flare characteristics, n (%) | ||
| Renal flare | 7 (88) | 3 (60) |
| Transverse myelitis | 0 (0) | 1 (20) |
| Persistent disease activity despite treatment | 1 (13) | 1 (20) |
| LN disease characteristics | ||
| Histopathology, n (%) | ||
| Class II (±V) | 1 (14) | 0 (0) |
| Class III (±V) | 1 (14) | 2 (67) |
| Class IV (±V) | 4 (57) | 1 (33) |
| Class V | 1 (14) | 0 (0) |
| Proteinuria,a median (range), g/24 h | 4.6 (1.3–11.2) | 1.9 (1.0–8.4) |
| Treatment at disease flare | ||
| Glucocorticoidsb, n (%) | 8 (100) | 4 (80) |
| Dose, median (range), mg/day | 15 (5–60) | 15 (5–60) |
| MMF, n (%) | 5 (63) | 3 (60) |
| Dose, median (range), mg/day | 2000 (1500–4000) | 1500 (1000–3000) |
| Azathioprine, n (%) | 1 (13) | 1 (20) |
| Dose, median (range), mg/day | 200 | 100 |
| Hydroxychloroquinine, n (%) | 8 (100) | 1 (20) |
| Biomarkers | ||
| ANA positivity | 8 (100) | 5 (100) |
| Anti-dsDNA titre,c median (range), AU/mL | 268 (50–827) | 479 (33–1123) |
| Complement consumption,d % | 100 | 100 |
| C3,e median (range), g/L | 0.6 (0.3–0.8) | 0.6 (0.5–1.3) |
| C4,f median (range), mg/L | 96 (35–236) | 68 (21–260) |
| IgG, median (range), g/L | 11.5 (5–23.6) | 12.9 (4.9–16.6) |
| IgA, median (range), g/L | 3.0 (1.2–4.5) | 2.9 (1.6–6.3) |
| IgM, median (range), g/L | 0.7 (0.3–1.1) | 0.8 (0.4–1.1) |
| CD19+ B cells (×106 cells/L), median (range) | 90 (21–279) | 65 (37–300) |
| Historic disease characteristics | ||
| Disease duration, median (range), years | 7 (3–18) | 10 (2–24) |
| No. of previous relapses, median (range) | 3 (2–6) | 5 (1–5) |
| No. of renal relapses, median (range) | 2 (1–5) | 1 (0–3) |
| SLICC damage index, median (range) | 1 (0–3) | 1 (0–4) |
| Organ involvement, n (%) | ||
| Constitutional | 8 (100) | 5 (100) |
| Mucocutaneous | 7 (88) | 3 (60) |
| Neuropsychiatric | 1 (13) | 2 (40) |
| Musculoskeletal | 5 (63) | 4 (80) |
| Cardiorespiratory | 7 (88) | 4 (80) |
| Gastrointestinal | 0 (0) | 0 (0) |
| Ophtalmic | 0 (0) | 2 (40) |
| Renal | 8 (100) | 4 (80) |
| Haematology | 4 (50) | 4 (80) |
| Treatment history | ||
| Steroids, n (%) | 8 (100) | 5 (100) |
| MMF, n (%) | 8 (100) | 5 (100) |
| Cyclophosphamide, n (%) | 3 (38) | 3 (60) |
| Azathioprine, n (%) | 4 (50) | 3 (60) |
| Tacrolimus, n (%) | 1 (13) | 0 (0) |
| RTX, n (%) | 2 (25) | 1 (20) |
| Hydroxychloroquinine, n (%) | 8 (100) | 5 (100) |
Proteinuria did not differ significantly between both groups, P-value 0.67.
Patients were treated with the glucocorticoid equivalent prednisolone.
Normal anti-dsDNA IgG <10 IU/mL.
Complement consumption is defined as decreased classical pathway activation, decreased C3 or decreased C4.
Normal C3: 0.9–2 g/L.
Normal C4: 95–415 mg/L.
Long-term safety
Treatment-emergent adverse events (TEAEs) during the study period are summarized in Table 2. In all patients, adverse events (AEs) were reported with five serious AEs (SAEs) in four patients (27%) due to hospitalization for the suspicion of infection (n = 3) or laparoscopic cholecystectomy (n = 1) because of cholelithiasis. In all cases, suspected infections were gastrointestinal without detectable pathogen, requiring a one-night hospital admission without the need for antibiotic treatment. In nine patients (60%), a minor infection was observed, of which upper respiratory tract infections were most prevalent. A detailed description of all infectious AEs is provided in Supplementary data, File S2. Two patients suffered from mood disorders; one patient had glucocorticoid-induced mood disorder and psychosis after methylprednisolone infusions and another patient experienced depressive symptoms started at Week 95, leading to study treatment interruption to exclude progressive multifocal leukoencephalopathy (PML). Once PML and neuropsychiatric SLE were ruled out, a mild depressive disorder was diagnosed and BLM treatment reinstituted.
Table 2.
AEs during 104 weeks of study
| Treatment-emergent AEsa | n = 15 |
|---|---|
| All AEs | 15 (100) |
| SAEs (hospitalization) | 4 (26.7) |
| Major infection | 3 (20.0) |
| Cholelithiasis | 1 (6.7) |
| Minor infection | 8 (53.3) |
| Upper respiratory tract | 9 (60.0) |
| Lower respiratory tract | 3 (20.0) |
| Urinary tract | 4 (26.7) |
| Urogenital infection | 2 (13.3) |
| Sinusitis | 1 (6.7) |
| Influenza | 1 (6.7) |
| Herpes simplex1 | (6.7) |
| Skin 1 | (6.7) |
| HACA formation | 4 (26.7) |
| Symptomatic | 1 (6.7) |
| Asymptomatic | 3 (20.0) |
| Hypogammaglobulinaemia (<4.0 g/L)b | 2 (13.3) |
| Infusion-related reaction | 1 (6.7) |
| Myalgia | 7 (46.7) |
| Diarrhoea | 4 (26.7) |
| Headache | 2 (13.5) |
| Pyrexia | 2 (13.5) |
| Nausea | 2 (13.3) |
| Mood disorderc | 2 (13.3) |
| Fatigue | 2 (13.3) |
| Other | 10 (66.7) |
Depicted values are number of patients with percentage of patients that experienced one or more TEAE over the 104 weeks of study.
Study treatment was interrupted in one patient.
Study treatment was interrupted in one patient; in the other patient, symptoms were related to high-dose steroids.
HACA, human antichimeric antibody.
Long-term effects of RTX + BLM on B-cell immunology
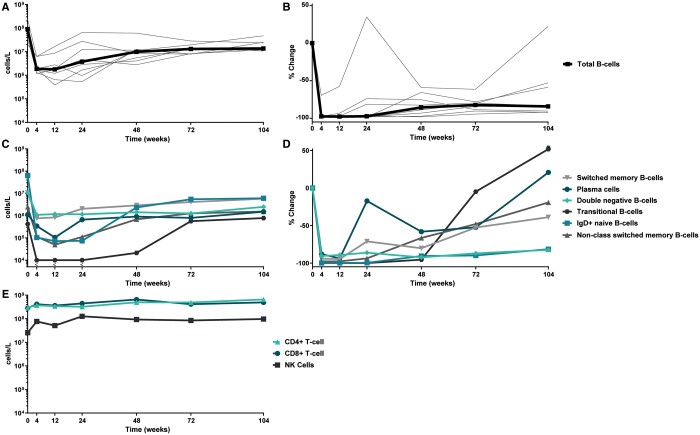
By employing high-sensitivity flow cytometry, we observed prolonged inhibition of B-cell repopulation: CD19+ B cells declined from a median of 100 × 106 cells/L (20.5; 248 × 106) at baseline to 3.75 × 106 cells/L (0.53; 64.7 × 106) (P = 0.005) at Week 24, representing a median decrease of 97% from baseline. At Week 104, the median number of CD19+ B cells was 13.6 × 106 cells/L (10.7; 47.3 × 106), representing a median decrease of 84% (−92; +22) from baseline (Figure 3A and B), illustrating that B cells did not repopulate to baseline values during continued BLM treatment. The low-level repopulation of B cells was dominated by an early recurrence of plasmablasts at Week 24 up to a median decrease of 17% [0.66 × 106 cells/L (<104; 18.3 × 106)] and to a lesser extent repopulation of switched memory B cells up to a median decrease of 71% [2.03 × 106 cells/L (<104; 41.9 × 106)] compared with baseline values. Only from 48 weeks onwards did the resurge of immature B cells occurr, with return of transitional B cells [+52%, 0.78 × 106 cells/L (0.19; 2.23 × 106)] and non-switched memory B cells [−19%, 1.53 × 106 cells/L (1.02; 3.19 × 106)] at Week 104. Interestingly, continuous BLM treatment prevented complete repopulation of naïve B cells [−81%, 6.07 × 106 cells/L (0.70; 25.8 × 106)] as well as double-negative (DN) B cells [−82%, 2.48 × 106 cells/L (0.46; 4.25 × 106)] at 104 weeks (Figure 3C and D).
FIGURE 3.
Longitudinal kinetics of circulating immune cells >2 years of follow-up after RTX + BLM treatment (n = 8 responders). (A and B) RTX + BLM prevents the complete repopulation of circulating B cells. Depicted are individual values of all responders with the median in bold representing change of CD19+ B cells in (A) absolute numbers and (B) the percentage of change from baseline. (C and D) Repopulation of B-cell subsets upon RTX + BLM. Depicted are the median change from baseline in (C) absolute numbers and (D) the percentage of change of the following B-cell subsets: plasmablasts (CD3−CD38brightCD27brightCD19+), non-switched memory B cells (CD3−CD19+CD27+IgD+), switched memory B cells (CD3−CD19+CD27+IgD−), naïve B cells (CD3−CD19+CD27−IgD+), DN B cells (CD3−CD19+CD27−IgD−) and transitional B cells (CD3−CD19+ CD38brightCD24bright). (E) Significant reconstitution of circulating CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+) and NK cells (CD16+CD56+). Depicted are the median changes from baseline in absolute numbers.
Long-term immune reconstituting effects
In the RTX + BLM treatment strategy, patients were able to taper steroids and stop MMF treatment before or at 24 weeks (Figure 2C). As a consequence, the observed low levels of T and NK cells at baseline significantly increased over time. Circulating CD4+ T cells increased from 234 × 106 cells/L (116; 530 × 106) at baseline to 658 × 106 cells/L (285; 1270 × 106) (P = 0.02 at Week 104), CD8+ T cells, from 276 × 106 cells/L (12.1; 418 × 106) at baseline to 493 × 106 cells/L (237; 1700 × 106) (P = 0.04) and in NK cells from 18 × 106 cells/L (0.4; 133 × 106) to 97 × 106 (38; 221 × 106) (P = 0.08; Figure 3E).
Long-term effects of RTX + BLM on humoral autoimmunity
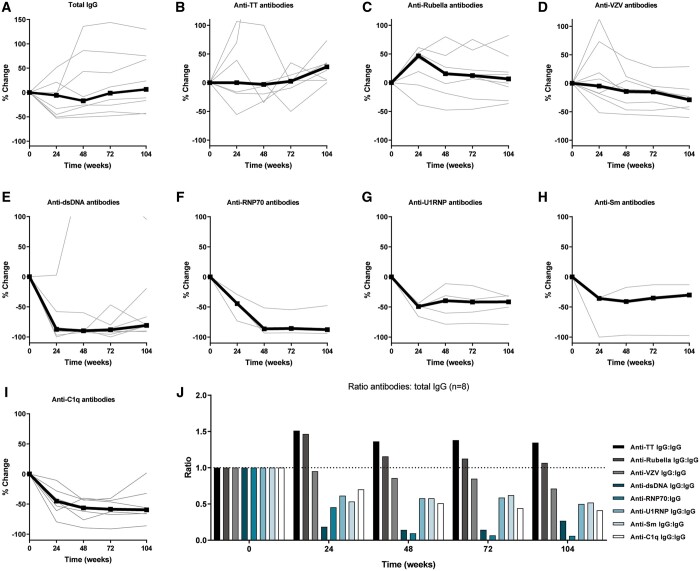
With respect to the effects of RTX + BLM on immunoglobulin levels, total immunoglobulin G (IgG) levels in comparison with baseline levels [median 11.3 g/L (5; 23.6)] initially decreased at 12 weeks [7.8 g/L (2.6; 14.4), P = 0.05] and stabilized from 24 weeks onwards [9.7 g/L (3.4; 16.4), Supplementary data, File S3]. At Week 104, IgG levels increased with 6.4% (−44; +30) compared with baseline levels (Figure 4A). IgA levels remained stable over the follow-up period while IgM levels gradually declined from 0.72 g/L (0.26; 1.06) at baseline to 0.27 g/L (0.2; 0.63) (P = 0.008) at Week 72 and increased to 0.37 g/L (0.2; 0.73) (P = 0.02) at Week 104 (Supplementary File S3). With regard to (auto)antigen-specific IgG, anti-tetanus and anti-rubella IgG remained stable during follow-up (Figure 4B and C), while anti-varicella zoster virus IgG (anti-VZV IgG) showed a significant decrease (Figure 4D) from 3435 mIU/mL (442; 4000) at baseline to 2436 (404; 3625) (P = 0.02) at Week 104. Of note, all measured anti-VZV IgG levels were within protective ranges.
FIGURE 4.
RTX + BLM resulted in prolonged, specific reduction of autoantibody levels >2 years follow-up (n = 8 responders). (A–D) Percentage change of physiological antibody levels are depicted, i.e. total IgG, anti-tetanus toxoid (TT), anti-rubella and anti-VZV antibodies. (E–G) Percentage change of SLE-relevant autoantibodies are depicted, i.e. anti-dsDNA (n = 8), anti-U1RNP (n = 4), anti-RNP70 (n = 3), anti-Sm antibodies (n = 3) and anti-C1q antibodies (n = 7). (J) To illustrate specific reductions in physiological antibody (anti-TT, anti-rubella and anti-VZV) and autoantibody levels (anti-dsDNA, anti-RNP70, anti-U1RNP, anti-Sm and anti-C1q), normalized ratio over total IgG was calculated and compared with baseline.
Anti-dsDNA levels of 268 AU/mL (50; 827) at baseline decreased at Week 24 to 29.6 (0; 104.5) (P = 0.02) equal to a median decrease of 87% (−100; +3) (Figure 4E). By Week 48 up to Week 104, all anti-dsDNA-positive patients converted to negative on immunofluorescence (CLIFT) with a median titre of 52 (23; 132) (P = 0.04) at Week 104, equal to a median decrease of 81% (−91; +95) from baseline. Similar reductions in anti-RNP70, anti-U1RNP, anti-Sm and anti-C1q autoantibodies levels were observed as illustrated in Figure 4F–I. Briefly, at 104 weeks, anti-RNP70 antibody levels were reduced with a median of 88% (−94; −48) (P = 0.25), anti-U1RNP with 41% (−79; −31) (P = 0.13), anti-Sm with 30% (−97; −13) and anti-C1q antibodies with 60% (−86; +2) (P = 0.03). The relative reductions of autoantibody compared with alloantibody levels over total IgG is illustrated in Figure 4J, demonstrating that RTX + BLM preferentially targeted humoral autoimmunity.
With respect to complement levels, normalization of C3 levels was seen at 104 weeks in seven of eight patients with median C3 levels of 1.0 g/L (0.8; 1.3) compared with baseline C3 levels of 0.6 g/L (0.3; 0.8) (P = 0.008). Also, C4 levels increased from 54 (35; 80) to 147 mg/L (74; 279) (P = 0.25; Supplementary data, File S3).
Associations of immunological effects with clinical response to RTX + BLM
We investigated immunological parameters that could potentially discriminate long-term responders (n = 8) from non-responders (n = 5) depicted in Supplementary data, Files S3 and S4. We observed that, not unexpectedly, after 4, 12 and 24 weeks, a significantly larger increase in C3 levels was seen in responders versus non-responders (respectively, 27% versus 0%, P = 0.03, 42% versus 8%, P = 0.01 and 79% versus 8%, P = 0.008). With high sensitivity flow cytometry, we observed two noteworthy findings: first, the total number of CD20+ B cells at Week 24 was significantly lower in responders [1.83 × 106 (0.10; 17.2 × 106)] compared with the non-responders [15.8 × 106 (3.01; 22.1 × 106), P = 0.045]. Secondly, repopulation of DN B cells occurred earlier in the non-responder group, at Week 24 (12; 24) [nadir levels of 0.48 × 106 cells/L (0.17; 1.02 × 106)], while in responders repopulation of DN B cells occurred at Week 72 (48; 104) (P = 0.0008) [nadir levels of 0.32 × 106 (0.11; 2.34 × 106)]. Finally, a trend for higher baseline BAFF levels was found in non-responders versus responders [respectively, 0.97 ng/mL (0.48–1.4) versus 0.44 ng/mL (0.26; 0.91), P = 0.06], while the decrease at 24 weeks was similar between responders [0.11 ng/mL (0.09–0.19)] and non-responders [0.15 ng/mL (0.08–0.35), P = 0.12].
DISCUSSION
In this proof-of-concept study, the long-term immunological and clinical effects of RTX + BLM in patients with severe, refractory SLE (including LN) are described. Long-lasting, specific reduction of anti-dsDNA, anti-C1q and even extractable nuclear antigen autoantibodies were observed and full B-cell repopulation was prevented throughout the 2-year follow-up. Clinical response persisted in two-thirds of the patients during follow-up with maintenance treatment consisting of BLM and low-dose prednisolone and allowed discontinuation of MMF associated with significant immune reconstitution. Profound depletion of CD20+ B cells, prolonged suppression of DN B cells and higher serum BAFF levels potentially discriminated responders from non-responders, and should be validated in larger clinical trials.
The study encompassed refractory SLE patients in which we were unable to continue immunomonitoring in non-responders who required different conventional induction therapies or in responders with a pregnancy wish. Within this limitation, we investigated potential predictors of non-response to RTX + BLM predominantly in the first 6 months. It is known that the B-cell-depleting potential of RTX has an inter-person variation and that the association of clinical outcome with the depth of B-cell depletion has been made [13, 14]. We found that less profound depletion of CD20+ B cells was associated with a poor response, in line with findings of a post hoc analysis of the LUNAR trial [15] where rapidness and duration of complete peripheral B-cell depletion were associated with complete response. Our observations in B-cell subsets are also in line with a recent study investigating B-cell subsets with Cytof in SLE patients during BLM therapy [16], where long-term depletion of CD20+ B cells and naïve B cells was seen. The loss of naïve B cells during BLM therapy has been shown before [16–18]. We found that only transitional B cells and plasma cells repopulated to baseline levels and that naïve B-cell repopulation present at 48 weeks remained suppressed for the duration of the follow-up period. Even more interesting are the DN B cells that showed a less profound depletion but did not repopulate throughout the 104 weeks. Additionally, we observed that early repopulation of DN B-cell associated with poor response. DN B cells in SLE are shown to be a major source of autoantibody-secreting cells (ASCs) [19], and the number of DN B cells is associated with disease activity and the presence of LN [20]. Moreover, further characterization of the DN B-cell population elucidated that these cells were hardly found in healthy or disease controls and were highly responsive to Toll-like receptor-7 stimulation inducing their differentiation to ASCs [20]. Unfortunately, we were limited in the depth of phenotyping DN B cells in this study, partly because this subpopulation had not been described at the time of study design and initiation. Notwithstanding, taken together with our observation that memory B cells and plasmablasts fully repopulated after RTX + BLM while long-lasting reductions of autoantibodies persisted, this suggested that a prolonged suppression of autoreactive DN B cells can be beneficial to SLE patients. Therefore, DN B cells are highly interesting biomarker to further study in the context of RTX + BLM treatment for SLE and LN patients.
Throughout the 2-year follow-up, no major safety issues were raised. The frequency of TEAEs was registered in 100% of patients containing 27% SAEs and 60% infections and was comparable to the LUNAR (99, 27 and 85%, respectively) and BLISS studies (93, 42 and 75%, respectively). Also, preliminary results of the CALIBRATE study (NCT02260934), in which 43 LN patients were randomized to receive RTX, cyclophosphamide and prednisone with or without additional BLM treatment, showed a non-significant difference on Grade 3 or higher infectious AEs (9% with BLM versus 23% without BLM, P = 0.25), confirming that RTX + BLM is well-tolerated. In addition, the CALIBRATE study reported 52% renal responders in the BLM group versus 41% in the placebo group. This non-significant difference could possibly be explained by the use of cyclophosphamide for induction treatment, in contrast to mycophenolate in the present study and the relative high dose of prednisolone maintenance (10 mg/day) continued throughout 2 years. It is of interest that preliminary reports from the CALIBRATE study showed impaired B-cell repopulation during BLM treatment as well as specific decrease in the naïve B-cell compartment upon BLM.
Our study observed 60% CRR rate at 104 weeks using the pre-defined CRR criteria containing proteinuria levels of ≤0.7 g/24 h, in comparison with ≤0.5 g/24 h used by landmark LN trials (LUNAR [6], ACCESS [21], ALLURE [22] and ALMS [23]). Reanalysation of the results showed that patients with a CRR at 104 weeks all have proteinuria levels <0.5 g/24 h. Based on LLDAS, clinical response to RTX + BLM was observed in 62% of patients in the present study. We demonstrated that responders to RTX + BLM had lasting LLDAS, which is associated with reduced damage accrual [24] and better quality of life [25], and can be used as an endpoint for clinical trials [26]. In this small trial, clinical benefit was achieved with RTX + BLM and persisted despite tapering of steroids to a dosage ≤7.5 mg and discontinuation of MMF. Although unconventional, tapering of MMF was added in the study design because at that time combined B-cell targeting with RTX + BLM had not been given to patients structurally (besides case reports [27–31]) and was intended to avoid over-immunosuppression which was fundamental of the previously mentioned label warning of BLM. Indeed, MMF tapering allowed for significant reconstitution of circulating CD4+ T cells and is a unique achievement for LN patients. Altogether, these data are reassuring for further studies to study clinical efficacy of RTX + BLM for active SLE including LN in a randomized setting.
It is noteworthy to establish that the primary null-hypothesis to study the combination of RTX + BLM, i.e. to induce long-term B-cell depletion and indirectly (autoreactive) plasmablast depletion, was wrong. The null-hypothesis was based on dual B-cell therapy in murine studies [11] but the contrary was observed: plasmablasts repopulated fastest among the studied B-cell subsets. Importantly, this was not associated with (recurrence of) disease activity or with autoantibodies. It was remarkable that RTX + BLM preferentially targeted humoral autoimmunity without affecting protective ranges of anti-viral antibody levels. It can be speculated that autoreactive B cells have an increased BAFF dependence due to the continuous presence of antigens compared with alloreactive B cells. This might also explain the significant drop, although not below protective levels, of antibodies against the VZV, which remains inactive in the body for many years.
The most important limitations of this study are the small number of treated patients and the single-arm design. The latter impairs the ability to place the observed effects into perspective to standard treatment regimens and it could be argued that the observed effects are solely due to RTX treatment combined with concomitant immunosuppressants. However, profound B-cell depletion by RTX has shown to be highly variable in SLE patients with a median time to repopulation around 32 weeks [32], and only 0–11% of patients with sustained low B-cell counts for 1–2 years without retreatment [13, 32–34]. In a comparable cohort of seven severe, refractory SLE patients retreated with RTX, a median duration of clinical response of 13 months and B-cell depletion of 6 months were reported [35], together suggesting a synergistic role of BLM in RTX-treated SLE patients.
In conclusion, this study was the first to pioneer the combination of RTX + BLM in patients with severe, refractory SLE aiming to establish its feasibility and better understand its immunological effects.
RTX + BLM treatment appears to be a promising strategy to target pathological autoimmunity mechanisms in SLE with suggestions towards beneficial clinical effects. We are therefore reassured that RTX + BLM can be safely studied in further clinical trials to assess its added value in the treatment of SLE patients with and without renal involvement.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
The work of L.S.v.D. is supported by FOREUM (SLE project). Support for the design of the patient informational material was received from the National Association for LUPUS, APS, Scleroderma and MCTD. Results were presented prior to publication at the EULAR [36].
FUNDING
This work is funded by the Dutch Kidney Foundation (17OKG04) and Clinical Fellowship from the Netherlands Organization for Scientific Research (90713460), and GlaxoSmithKline provided belimumab and an unrestricted grant for the clinical study described in this manuscript.
AUTHORS’ CONTRIBUTIONS
T.K and E.J.A. contributed equally as first authors. T.K. and Y.K.O.T. contributed to the design of the study, acquisition of data, analysis and interpretation of data and manuscript preparation. E.J.A. contributed to the acquisition of data, analysis and interpretation of data and manuscript preparation. L.S.v.D. contributed to the acquisition of data, interpretation of data and manuscript preparation. P.L.A.v.D., O.W.B. and A.R. contributed significantly to the recruitment and follow-up of patients, and acquisition of data. S.W.A.K., J.A.B. and H.U.S. contributed to the acquisition of data. T.J.W.H., T.J.R. and C.v.K. contributed to the analysis and interpretation of data and manuscript preparation. All authors discussed and agreed on the content of the manuscript before submission.
CONFLICT OF INTEREST STATEMENT
Y.K.O.T. received consultancy fees from GlaxoSmithKline and Aurinia Pharmaceuticals.
REFERENCES
- 1. Lech M, Anders HJ.. The pathogenesis of lupus nephritis. J Am Soc Nephrol 2013; 24: 1357–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Almaani S, Meara A, Rovin BH.. Update on lupus nephritis. Clin J Am Soc Nephrol 2017; 12: 825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parikh SV, Rovin BH.. Current and emerging therapies for lupus nephritis. J Am Soc Nephrol 2016; 27: 2929–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hanly JG, O’Keeffe AG, Su L. et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford) 2016; 55: 252–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Merrill JT, Neuwelt CM, Wallace DJ. et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 2010; 62: 222–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rovin BH, Furie R, Latinis K. et al.; LUNAR Investigator Group. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the lupus nephritis assessment with rituximab study. Arthritis Rheum 2012; 64: 1215–1226 [DOI] [PubMed] [Google Scholar]
- 7. Ehrenstein MR, Wing C.. The BAFFling effects of rituximab in lupus: danger ahead? Nat Rev Rheumatol 2016; 12: 367–372 [DOI] [PubMed] [Google Scholar]
- 8. Cambridge G, Stohl W, Leandro MJ. et al. Circulating levels of B lymphocyte stimulator in patients with rheumatoid arthritis following rituximab treatment: relationships with B cell depletion, circulating antibodies, and clinical relapse. Arthritis Rheum 2006; 54: 723–732 [DOI] [PubMed] [Google Scholar]
- 9. Gong Q, Ou Q, Ye S. et al. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol 2005; 174: 817–826 [DOI] [PubMed] [Google Scholar]
- 10. Saito Y, Miyagawa Y, Onda K. et al. B-cell-activating factor inhibits CD20-mediated and B-cell receptor-mediated apoptosis in human B cells. Immunology 2008; 125: 570–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin W, Seshasayee D, Lee WP. et al. Dual B cell immunotherapy is superior to individual anti-CD20 depletion or BAFF blockade in murine models of spontaneous or accelerated lupus. Arthritis Rheumatol 2015; 67: 215–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kraaij T, Kamerling SWA, de Rooij ENM. et al. The NET-effect of combining rituximab with belimumab in severe systemic lupus erythematosus. J Autoimmun 2018; 91: 45–54 [DOI] [PubMed] [Google Scholar]
- 13. Vital EM, Dass S, Buch MH. et al. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum 2011; 63: 3038–3047 [DOI] [PubMed] [Google Scholar]
- 14. Md Yusof MY, Shaw D, El-Sherbiny YM. et al. Predicting and managing primary and secondary non-response to rituximab using B-cell biomarkers in systemic lupus erythematosus. Ann Rheum Dis 2017; 76: 1829–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gomez Mendez LM, Cascino MD, Garg J. et al. Peripheral blood B cell depletion after rituximab and complete response in lupus nephritis. Clin J Am Soc Nephrol 2018; 13: 1502–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ramskold D, Parodis I, Lakshmikanth T. et al. B cell alterations during BAFF inhibition with belimumab in SLE. EBioMedicine 2019; 40: 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacobi AM, Huang W, Wang T. et al. Effect of long-term belimumab treatment on B cells in systemic lupus erythematosus: extension of a phase II, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum 2010; 62: 201–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stohl W, Hiepe F, Latinis KM. et al.; on behalf of the BLISS-52 and BLISS-76 Study Groups. Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis Rheum 2012; 64: 2328–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tipton CM, Fucile CF, Darce J. et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol 2015; 16: 755–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jenks SA, Cashman KS, Zumaquero E. et al. Distinct effector B cells induced by unregulated toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity 2018; 49: 725–739.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Group AT. Treatment of lupus nephritis with abatacept: the abatacept and cyclophosphamide combination efficacy and safety study. Arthritis Rheumatol 2014; 66: 3096–3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Furie R, Dooley MA, Wofsy D. et al. OP0253 A phase iii randomised, double-blind, placebo-controlled study to evaluate the efficacy and safety of abatacept or placebo on standard of care in patients with active class iii or iv lupus nephritis. Ann Rheum Dis 2018; 77 (Suppl 2): 176–177 [Google Scholar]
- 23. Appel GB, Contreras G, Dooley MA. et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol 2009; 20: 1103–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsang A, Bultink IE, Heslinga M. et al. Both prolonged remission and lupus low disease activity state are associated with reduced damage accrual in systemic lupus erythematosus. Rheumatology (Oxford) 2017; 56: 121–128 [DOI] [PubMed] [Google Scholar]
- 25. Golder V, Kandane-Rathnayake R, Hoi AY. et al.; for the Asia-Pacific Lupus Collaboration. Association of the lupus low disease activity state (LLDAS) with health-related quality of life in a multinational prospective study. Arthritis Res Ther 2017; 19: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morand EF, Trasieva T, Berglind A. et al. Lupus low disease activity state (LLDAS) attainment discriminates responders in a systemic lupus erythematosus trial: post-hoc analysis of the Phase IIb MUSE trial of anifrolumab. Ann Rheum Dis 2018; 77: 706–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gonzalez-Echavarri C, Ugarte A, Ruiz-Irastorza G.. Rituximab-refractory lupus nephritis successfully treated with belimumab. Clin Exp Rheumatol 2016; 34: 355–356 [PubMed] [Google Scholar]
- 28. Kraaij T, Huizinga TW, Rabelink TJ. et al. Belimumab after rituximab as maintenance therapy in lupus nephritis. Rheumatology (Oxford) 2014; 53: 2122–2124 [DOI] [PubMed] [Google Scholar]
- 29. Simonetta F, Allali D, Roux-Lombard P. et al. Successful treatment of refractory lupus nephritis by the sequential use of rituximab and belimumab. Joint Bone Spine 2017; 84: 235–236 [DOI] [PubMed] [Google Scholar]
- 30. Psarelis S, Nikiphorou E, Boumpas DT.. Successful use of sequential B-cell depletion therapy in lupus. Lupus 2018; 27: 345–346 [DOI] [PubMed] [Google Scholar]
- 31. Gualtierotti R, Borghi MO, Gerosa M. et al. Successful sequential therapy with rituximab and belimumab in patients with active systemic lupus erythematosus: a case series. Clin Exp Rheumatol 2018; 36: 643–647 [PubMed] [Google Scholar]
- 32. Lazarus MN, Turner-Stokes T, Chavele KM. et al. B-cell numbers and phenotype at clinical relapse following rituximab therapy differ in SLE patients according to anti-dsDNA antibody levels. Rheumatology (Oxford) 2012; 51: 1208–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dias SS, Rodriguez-Garcia V, Nguyen H. et al. Longer duration of B cell depletion is associated with better outcome. Rheumatology (Oxford) 2015; 54: 1876–1881 [DOI] [PubMed] [Google Scholar]
- 34. Anolik JH, Campbell D, Felgar RE. et al. The relationship of FcgammaRIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Arthritis Rheum 2003; 48: 455–459 [DOI] [PubMed] [Google Scholar]
- 35. Ng KP, Leandro MJ, Edwards JC. et al. Repeated B cell depletion in treatment of refractory systemic lupus erythematosus. Ann Rheum Dis 2005; 65: 942–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kraaij T, Arends EJ, Dam L. et al. OP0042 Long-term effects of synergetic B cell immunomodulation with rituximab and belimumab combination treatment in severe, refractory SLE: two year results. Ann Rheum Dis 2019; 78 (Suppl 2): 91.2–9230337425 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.