Abstract
Objective
To synthesize the literature on the proportion of health care providers who access and use prescription monitoring program data in their practice, as well as associated barriers to the use of such data.
Design
We performed a systematic review using a standard systematic review method with meta-analysis and qualitative meta-summary. We included full-published peer-reviewed reports of study data, as well as theses and dissertations.
Methods
We identified relevant quantitative and qualitative studies. We synthesized outcomes related to prescription monitoring program data use (i.e., ever used, frequency of use). We pooled the proportion of health care providers who had ever used prescription monitoring program data by using random effects models, and we used meta-summary methodology to identify prescription monitoring program use barriers.
Results
Fifty-three studies were included in our review, all from the United States. Of these, 46 reported on prescription monitoring program use and 32 reported on barriers. The pooled proportion of health care providers who had ever used prescription monitoring program data was 0.57 (95% confidence interval: 0.48–0.66). Common barriers to prescription monitoring program data use included time constraints and administrative burdens, low perceived value of prescription monitoring program data, and problems with prescription monitoring program system usability.
Conclusions
Our study found that health care providers underutilize prescription monitoring program data and that many barriers exist to prescription monitoring program data use.
Keywords: Prescription Monitoring Programs, Prescription Drug Monitoring Programs, Opioids, Utilization, Systematic Review
Introduction
North America and Western Europe are amid an opioid crisis. In the United States, opioids were found to account for two thirds of all drug overdose deaths, and in Canada, the rate of opioid-related deaths was found to be 7.8 per 100,000 [1, 2]. In Europe, the role of opioids in mortality is also apparent, with opioids being found in 81% of all fatal overdoses [3].
Although considerable attention has been directed to the use of illicit opioids, the crisis is strongly linked to the emergence of prescription opioids as the primary treatment modality to address acute and chronic pain over the past few decades [4–8]. As many patients with pain turned to opioids for treatment, prescription opioids, such as morphine, hydromorphone, and oxycodone, were revealed to have a high risk for abuse and potential for dependence, which has been since reflected in their classification as Schedule II controlled substances [9]. Many strategies developed to combat this crisis have, therefore, also targeted prescription opioids, with a goal to promote appropriate use of these medications while reducing dependence and potential for harm.
One such strategy is the implementation of prescription monitoring programs (PMPs). PMPs house prescribing and dispensing data for controlled substances from health care providers such as physicians and pharmacists. Opioids are among these controlled substances, making PMPs an important source of a patient’s history of controlled substance use, but more specifically, opioid history. In most jurisdictions, PMPs can be used by health care providers when making prescribing or dispensing decisions, ensuring opioids are prescribed in appropriate quantities and when necessary, following best-practice guidelines [10] and reducing the number of individuals at risk of subsequent dependence and harm [11, 12]. PMPs also aim to regulate opioid exposure in the community by monitoring patient behaviors, such as use of multiple providers, and other potential flags for diversion of supply or misuse. Finally, PMPs monitor health care provider prescribing practices and habits and may use this information to inform and educate providers on appropriate prescribing practices [13, 14]. Variations of PMPs have been implemented in 49 states, the District of Columbia, and two territories across the United States and in nine provinces and territories across Canada [15–17]. Few distinct PMPs exist in Europe, as many jurisdictions instead integrate the functions of PMPs into their broader drug distribution systems [18].
The evidence on whether PMPs are effective in reducing inappropriate prescribing is mixed [19–21]. This may be explained by the varying use of PMPs, where there is often suboptimal use of PMP data by health care providers to inform their prescribing and dispensing decisions [22]. In many jurisdictions, health care providers are not aware that a PMP exists where they practice, or similarly, some are aware of PMPs but do not know how to make use of the data in their practice. For example, in a recent study of physicians in jurisdictions with PMPs from across the United States, approximately 72% of providers knew that a PMP was present in their jurisdiction, but only 53% reported actually using one of the programs [23]. It is important to understand the extent to which these programs are being used, and in the case that they are not being frequently used, it is also important to understand why. Health care professionals often identify the presence of too many barriers as a reason why they may not use PMPs [23–25]. Identifying and acknowledging potential barriers to PMP use is an essential first step to optimize utilization [16, 24, 26–28].
We completed a systematic review to, first, determine what proportion of health care providers access and use PMP data in their practice in jurisdictions where a PMP is available, and second, identify common barriers to accessing PMP data that have been identified by health care providers.
Methods
We used a standard systematic review approach [29], following a predefined protocol, and structured the report according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [30]. This research received ethics approval from the research ethics board at Dalhousie University.
Data Sources and Search Strategy
To retrieve all relevant publications, we developed our search strategy in consultation with a medical librarian and applied this strategy to a range of data sources (see Supplementary Appendix A for the Ovid MEDLINE search strategy). Our search had no restrictions on language, country, or study design and included both quantitative and qualitative findings to provide evidence about factors associated with PMP use; we searched up until January 22, 2018. Only fully published peer-reviewed reports of study data were included, except for theses or dissertations. We searched five electronic databases (Medline, Embase, CINAHL, PsychInfo, and Web of Science), as well as dissertation and theses databases. Additionally, we manually searched the reference lists of all included studies and identified relevant reviews. EndNote X8.1 was used to de-duplicate and manage citations [31]. All identified studies were imported to Covidence, a Web-based software developed by Cochrane to facilitate the systematic review process [32].
Study Selection
All titles and abstracts were screened independently for eligibility by two of four investigators (AR, MW, ER, MA) and were pushed forward to full-text review if consensus was achieved on relevance. Two of the four reviewers (same as above) also independently screened full-text articles to determine eligibility for data extraction. Rates of discordance between reviewers for the title/abstract and full-text review were 8.9% and 9.7%, respectively. At all stages, any discrepancies in eligibility between reviewers were discussed to reach consensus. If no consensus could be reached, a third investigator reviewed and assessed the eligibility. Studies were included for full review if they met the following criteria: 1) The publication was a primary research study in full text (i.e., not a systematic review, editorial, abstract, or commentary), 2) the jurisdiction being studied had an operational PMP (not pending or forthcoming), 3) the study had a measure of PMP use (with a denominator for the measure) and/or had identified barriers to PMP use, and 4) the study population consisted of health care providers. Multiple publications presenting data on the same group of respondents are identified as linked in Supplementary Appendix B. Overlapping outcomes from these publications were included only so as to not over-weight the findings.
Data Extraction and Quality Assessment
We used a pre-tested data extraction form developed in Covidence to extract relevant study details (authors, year, jurisdiction, study design, sample size, response rate), population characteristics (provider or dispenser type), data sources (focus group, survey, administrative), information on data use, and any barriers identified (including whether these barriers were identified by <50%, ≥50%, or an unknown percentage of the study population) for included studies. Two reviewers completed the data extraction for each study independently, and discrepancies were discussed. When consensus could not be reached by the two initial reviewers, the assessment of a third reviewer was sought. The quality of studies was assessed with the Appraisal tool for Cross-Sectional Studies (AXIS) critical appraisal tool for quantitative studies [33] and the Critical Appraisal Skills Programme (CASP) tool for qualitative studies [34].
Data Analysis
Prevalence of PMP data use was expressed as the proportion of respondents reporting having ever used or always using PMP data. We used Stata version 15 (the metaprop command) to combine proportions of ever using PMP data under a random effects model due to the heterogeneity between studies, using the Freeman-Tukey Double Transformation to stabilize variances. We present the proportion of providers using PMP as a range and a pooled estimate with 95% confidence interval (CI). We also examined rates of PMP data use by provider type. Finally, we present data on the proportion of providers who reported “always using PMP data while prescribing” in our meta-summary. Always using PMP data was presented as mean and standard deviation. Other measures of frequency of use were not pooled due to significant heterogeneity.
Barriers associated with using PMP data were synthesized with qualitative meta-summary methods [35]. Barriers (and the percentage of respondents reporting them) were first extracted at the study level in the context of each individual study and were then coded, compared, and combined by theme through the use of data maps. For syntheses and ease of interpretation, we categorized barriers, as mentioned above, into: those reported by <50% of the study sample (defined as a less common barrier), those reported by ≥50% of the study population (defined as a more common barrier), and those reported by an unknown percentage (i.e., those identified in a qualitative meta-summary, labeled as a general barrier) of the study population.
Results
Searches
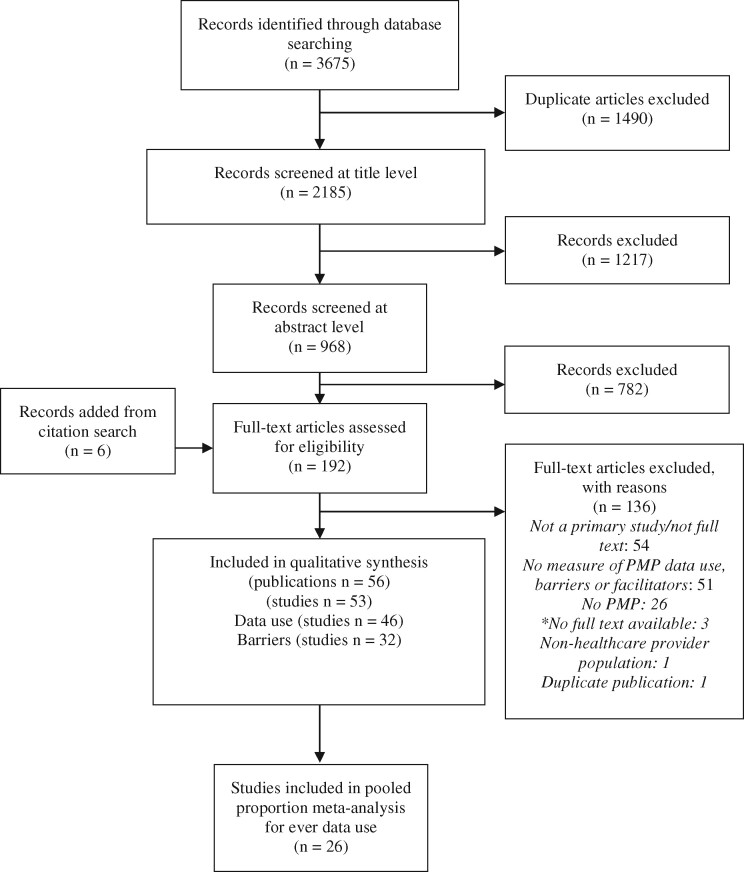
Using the search strategy outlined above, we initially identified 1,490 unique records (Figure 1) and reviewed 192 of these publications in full text. In total, we identified 53 studies (56 publications) that met the inclusion for our review, of which 46 reported on PMP data use and 32 identified barriers to PMP data use (Supplementary Appendix B) [36–38].
Figure 1.
PRISMA diagram for study screening and inclusion process. ES = effect size.
*Reasons for full texts being unavailable to authors: embargoed (1); not yet digitized and informed it would take at least a month (1); not enough information available for authors or library services to find it (1).
Study Characteristics
All included studies were conducted in the United States and covered a range of health care professionals: prescriber populations, including physicians (23 studies), dentists (1 study), nurse practitioners (5 studies); administrative populations (i.e., PMP and health care administrators; 3 studies); and pharmacists (13 studies). Many studies identified the health care professionals included in their study broadly, using terms such as providers (7 studies), clinicians (2 studies), or prescribers (7 studies), and 13 studies looked at more than one specific group of health care professionals. Study participation/response rates varied greatly between studies, from 4% to 100%. Specific characteristics of the PMPs and their implementation were not well described in the studies and were therefore not included in our analysis. However, to add context when discussing specific studies, up-to-date information on the current status of PMPs in different jurisdictions within the United States was collected from “PDMP Assist” and is presented in Supplementary Appendix C.
Methodological Quality
The included studies were of variable methodological quality (Supplementary Appendix D). The highest area of concern for study quality among quantitative studies was poor response rates, which were reported to be as low as 4%. Other areas of concern across studies included whether the data were adequately described, replicability of methods, the use of validated instruments to collect observations, justification of the sample size, and the selection of a representative sample, particularly given the use of convenience sampling approaches in many studies (Table 1).
Table 1.
Summary of critical appraisal of studies with quantitative data using AXIS and CASP tool
| Total studies yes, n* | Total studies yes, % | |
|---|---|---|
| AXIS Item | ||
| 1. Were the aims/objectives of the study clear? | 41 | 93.2% |
| 2. Was the study design appropriate for the stated aim(s)? | 41 | 93.2% |
| 3. Was the sample size justified? | 8 | 18.2% |
| 4. Was the target/reference population clearly defined? (Is it clear whom the research was about?) | 43 | 97.7% |
| 5. Was the sample frame taken from an appropriate population base so that it closely represented the target/reference population under investigation? | 36 | 81.8% |
| 6. Was the selection process likely to select subjects/participants that were representative of the target/reference population under investigation? | 26 | 59.1% |
| 7. Were measures undertaken to address and categorize nonresponders? | 6 | 15.4% |
| 8. Were the risk factor and outcome variables measured appropriate to the aims of the study? | 41 | 93.2% |
| 9. Were the risk factor and outcome variables measured correctly using instruments/measurements that had been trialed, piloted, or published previously? | 30 | 68.2% |
| 10. Is it clear what was used to determine statistical significance and/or precision estimates? (e.g., P values, CIs) | 28 | 80.0% |
| 11. Were the methods (including statistical methods) sufficiently described to enable them to be repeated? | 30 | 68.2% |
| 12. Were the basic data adequately described? | 28 | 63.6% |
| 13. Does the response rate raise concerns about nonresponse bias?† | 33 | 86.8% |
| 14. If appropriate, was information about nonresponders described? | 5 | 13.2% |
| 15. Were the results internally consistent? | 35 | 79.5% |
| 16. Were the results for the analyses described in the methods presented? | 38 | 86.4% |
| 17. Were the authors’ discussions and conclusions justified by the results? | 39 | 88.6% |
| 18. Were the limitations of the study discussed? | 42 | 95.5% |
| 19. Were there any funding sources or conflicts of interest that may affect the authors’ interpretation of the results?† | 0 | 0.0% |
| 20. Was ethics approval or consent of participants attained? | 42 | 95.5% |
| CASP Item | Total studies yes, n | Total studies yes, % |
| 1. Was there a clear statement of the aims of the research? | 14 | 100.0% |
| 2. Is a qualitative methodology appropriate? | 14 | 100.0% |
| 3. Was the research design appropriate to address the aims of the research? | 12 | 85.7% |
| 4. Was the recruitment strategy appropriate to the aims of the research? | 9 | 64.3% |
| 5. Were the data collected in a way that addressed the research issue? | 14 | 100.0% |
| 6. Has the relationship between researcher and participants been adequately considered? | 8 | 57.1% |
| 7. Have ethics issues been taken into consideration? | 13 | 92.9% |
| 8. Was the data analysis sufficiently rigorous? | 10 | 71.4% |
| 9. Is there a clear statement of findings? | 14 | 100.0% |
| 10. How valuable is the research?‡ | 12 | 85.7% |
Because of certain questions being inapplicable for some studies, N is not always 44: Question 7, N = 39; Question 10, N = 35; Question 13, N = 38; and Question 14, N = 38.
Higher rate of responding yes can be interpreted as of concern.
Yes answer indicates that value was demonstrated.
We identified three main areas of concern for qualitative study results: the appropriateness of the recruitment strategy, whether the relationship between researcher and participants was addressed, and the rigor of data analysis (Table 1). In all, qualitative studies were good with respect to their comprehensive assessment of barriers to PMP. Of the 13, six studies used some sort of framework, approach, or method from the literature to describe barriers, and the rest either thematically coded barriers (four studies) or had predefined groupings for barriers based on the structure of questions (three studies).
Prevalence of PMP Data Use
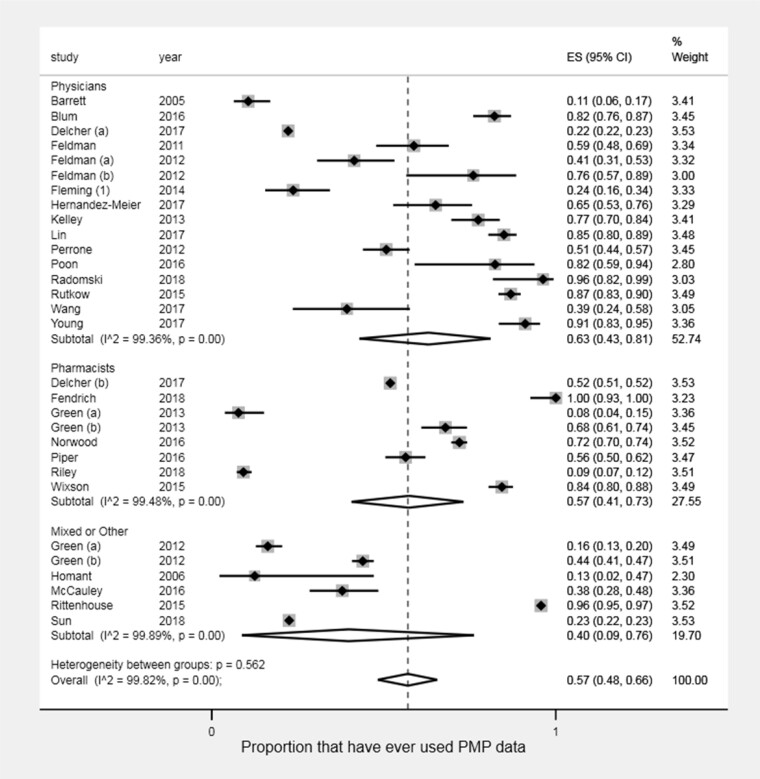
Among the 46 included studies (131,587 participants) reporting data use, the most common outcome reported was the proportion of prescribers or dispensers who have “ever” used PMP data. This outcome was reported in 26 studies representing 30 unique prescriber/dispenser groups (107,998 participants), with proportions of “ever use” of PMP data ranging from 0.08 to 1.00 (Figure 2) [23–25, 27, 28, 39–59]. Overall, the pooled proportion of health care providers who have ever used PMP data was 0.57 (95% CI: 0.48–0.66). Among the health care providers included in these studies, physicians and pharmacists were the two specific groups studied most often, in fourteen and seven studies, respectively. The pooled proportions of physicians (0.63; 95% CI: 0.43–0.81) and pharmacists (0.57; 95% CI: 0.41–0.73) ever using PMP data did not differ significantly. There were no observable differences in data use by year of study publication (Supplementary Appendix E).
Figure 2.
Results of pooled proportion meta-analysis of ever PMP data use by physicians, pharmacists, and mixed or other populations.*
*Feldman (a) represents the subgroup of residents, whereas Feldman (b) represents the subgroup of attending physicians. Green (a) represents pharmacists in Rhode Island, and Green (b) represents pharmacists in Connecticut.
Another 24 studies (108,327 participants) reported on other measures of frequency of PMP data use [22, 23, 27, 28, 39, 41, 43, 46, 47, 50, 56, 57, 60–71]. Frequency of use was defined either within a specified time frame (i.e., past week, past month, over study period) or in specific scenarios (i.e., when prescribing to a new patient or to high-risk patients). A common measure of frequency of use was the proportion of health care professionals who “always” checked or used PMP data when making a prescribing or dispensing decision, which was reported by 11 studies (4,919 participants) [23, 24, 43, 46, 52, 56, 60, 61, 65, 67, 71].
Across these 11 studies, there was a low percentage of health care providers who always used PMP data when making prescribing or dispensing decisions, ranging from 0% to 68% (mean [SD]: 18.7% [18.4%]). Three studies reported a percentage of health care providers who always used PMP data that was markedly higher than the mean: Blum (2016), Fleming (2014), and Chaudhary (2017) [56, 60, 61].
Barriers to PMP Data Utilization
From the 32 studies (11,266 participants) that discussed barriers to PMP data use, we identified 18 groups of barriers (Table 2) [23–25, 27, 28, 41–47, 49, 50, 54–56, 58, 59, 64, 66, 67, 71–80].
Table 2.
Reporting barriers to PMP use identified from included studies
| Study IDs and Frequency of Barrier Reporting |
||||
|---|---|---|---|---|
| Barrier Group | Minor (Reported by <50% of Study Sample) | Major (Reported by ≥50% of Study Sample) | General (No Frequency Reported) | Examples of Barrier Group |
| Not seeing value in PMP data | Rutkow 2015 (23), Perrone 2012 (47), McCauley 2016 (45), Lin 2017 (44), Feldman 2012 (58), Barrett 2005 (45), Hernandez-Meier 2017 (41), Blum 2016 (56), Green 2012 (28), Ulbrich 2010 (79) | NA | Warren 2016 (71), Carnes 2017 (72), Homant 2006 (42), Deyo 2015 (74) | Feel it would not impact or change clinical practice, did not want to use PMP, feel they can rely on their own instinct. |
| Availability of technology | Perrone 2012 (47), Hernandez-Meier 2017 (41), Green 2012 (28), Green 2013 (27), Wixson 2015 (25) | Ulbrich 2010 (79) | Pugliese 2018 (77), Hildebran 2014 (64) | Limited access to phone, internet, or computers at work, not having access to PMP during all hours of the dazy. |
| Usability issues | Rutkow 2015 (23), Blum 2016 (56), Perrone 2012 (47), McAllister 2015 (67), Fazio 2017 (75), Hernandez-Meier 2017 (41), Green 2012 (28), McCauley 2016 (45), Kelley 2013 (43), Barrett 2005 (55) | Deyo 2015 (74), Wang 2017 (54) | Pugliese 2018 (77), Poon 2016 (49), Worley 2015 (80), Click 2017 (73) , Carnes 2017 (72), Smith 2015 (78), Hildebran 2014 (64) | Difficulty interpreting PMP data, difficulty accessing and navigating the PMP, format of information is not easy to use, lack of confidence in performing PMP tasks. |
| Time constraints to using PMP data | McCauley 2016 (45), McAllister 2015 (67), Hernandez-Meier 2017 (41), Blum 2016 (56), Green 2013 (27), Young 2017 (24), Wang 2017 (54), Barrett 2005 (55) | Rutkow 2015 (23), Perrone 2012 (47), Norwood 2016 (46), Kelley 2013 (43), Deyo 2015 (74) | Radomski 2018 (50), Hildebran 2014 (64), Worley 2015 (80), Warren 2016 (71), Smith 2015 (78) | Time consuming to log in, to retrieve information, increase in burden or workload. |
| Lack of awareness of PMP | Perrone 2012 (47), Feldman 2011 (59), Ulbrich 2010 (79), Lin 2017 (44) | McCauley 2016 (45), Green 2012 (28), Green 2013 (27) | NA | Unaware of PMP or availability of PMP data among non-users. |
| System slowness | Young 2017 (24), Barrett 2005 (55), Green 2013 (27), Lin 2017∗ (44), Hernandez-Meier 2017 (41) | Ulbrich 2010 (79), Lin 2017∗ (44), Deyo 2015 (74) | Radomski 2018 (50), Carnes 2017 (72), Naiman 2013 (76), Hildebran 2014 (64), Worley 2015 (80), Warren 2016 (71), Smith 2015 (78) | Delay in receiving requested reports, lag time in system updates from when a prescription is dispensed to when it shows up in the PMP, delays in reporting to the system, inability to directly query the system in real time, requests not processing or timing out, system slowness. |
| Concerns with privacy, monitoring, and autonomy | LeMire 2012 (66), Kelley 2013 (43), Blum 2016 (56), Feldman 2012 (58), Barrett 2005 (55), Norwood 2016 (46) | NA | Hildebran 2014 (64), Carnes 2017 (72) | Concerns with patient privacy, feel they are being policed, feel they are being inhibited in prescribing, fear of legal ramifications. |
| Lack of training/education or policies/guidelines | Ulbrich 2010 (79) | Deyo 2015 (74) | Hildebran 2014 (64), Homant 2006 (42), Warren 2016 (71) | Lack of training on how to use PMP or interpret findings, no guidance on how to integrate PMP into workflow, lack of knowledge on PMP policies or laws. |
| Inability to delegate access | Green 2012 (28), Green 2013 (27), Deyo 2015 (74) | NA | Carnes 2017 (72), Smith 2015 (78), Click 2017 (73) | Lack of staff available to access the system, inability of residents to query the system, unable to share account or delegate access. |
| Lack of integration and data sharing (between systems and jurisdictions) | Blum 2016 (56), Deyo 2015 (74) | NA | Radomski 2018 (50), Naiman 2013 (76), Worley 2015 (80), Carnes 2017 (72), Click 2017 (73) | No interstate data sharing, no integration with electronic health/medical record, inability to search outside of one’s jurisdiction. |
| Patient satisfaction concerns | Kelley 2013 (43), Ulbrich 2010 (79) | NA | Hildebran 2014 (64), Smith 2015 (78) | Worried about patient satisfaction rating (which may impact salary), concern with confronting patients, detracting from patient flow. |
| PMP does not meet provider data needs | Blum 2016 (56), Barrett 2005 (55) | NA | Radomski 2018, (50) Carnes 2017 (72), Homant 2006 (42) | Does not cover certain populations such as Veteran's Affairs or homeless, does not monitor drugs of interest, inability to access information on ones own prescribing history. |
| Problems with log-in credentials | Perrone 2012 (47), Hernandez-Meier 2017 (41), Blum 2016 (56), Young 2017 (24) | NA | Poon 2016 (49), Naiman 2013 (76), Hildebran 2014 (64), Click 2017 (73) | Difficulty remembering login credentials, frequent password changes required. |
| Problems with registration process | Perrone 2012 (47), Norwood 2016 (46), McAllister 2015 (67), Lin 2017 (44), Hernandez-Meier 2017 (41), Green 2012, Blum 2016 (56), Green 2013 (27) | Ulbrich 2010 (79) | Hildebran 2014 (64), Smith 2015 (78) | Too time consuming to register, do not know how to register, having to register on each new computer. |
| No incentive to use PMP | Perrone 2012 (47) | NA | Hildebran 2014 (64), Smith 2015 (78) | No reimbursement for task or incentive (financial or otherwise) to use PMP data. |
| Data not reliable | Lin 2017∗ (44), Blum 2016 (44) | Lin 2017 ∗ (44) | Hildebran 2014 (64), Click 2017 (73) | Patient reports filed under more than one ID, missing data, inaccurate data or errors in the system, not all clinicians use the system so patient history may not be comprehensive.. |
| Lack of support from administration | Green 2012 (28), Green 2013 (27), Kelley 2013 (43) | NA | NA | PMP not required or promoted by administration, or PMP use is discouraged by administration. |
| Potential for under-treatment | Blum 2016 (56) | NA | Carnes 2017 (72) | Restrictions in providing opioids to patients who patients feel really need them (i.e. cancer, palliative care, surgery, etc.). |
Identifies studies that have reported barriers within the barrier group for more than one category of frequency of reporting.
Time constraints were the most commonly identified barrier. Time constraints were identified as a less common barrier in eight studies [24, 27, 41, 45, 54–56, 67], a more common barrier in five studies [23, 43, 46, 47, 74], and a general barrier in another five studies [50, 64, 71, 78, 80]. This was represented by reports from health care providers that the process of obtaining information from the PMP to advise prescribing or dispensing situations, specifically in the processes of logging in or retrieving information, was too time consuming.
Issues with system slowness were identified as a less common barrier in five studies [24, 27, 41, 44, 55], a more common barrier in three studies [44, 74, 79], and a general barrier in seven studies [50, 64, 71, 72, 76, 78, 80]. Specific issues included lag times in system updates, as well as infrequent updates, the “timing out” of data access requests, and the lack of timely receipt of information from the system (including inability to query in real time). These issues impact the utility of PMPs for health care providers in a prescribing or dispensing situation.
Related to system issues are issues with usability and a lack of training or guidance in using the PMP. Issues with usability were identified as a less common barrier in ten studies [23, 28, 41, 43, 45, 47, 55, 56, 67, 75], a more common barrier in two studies [54, 74], and a general barrier in seven studies [49, 64, 72, 73, 77, 78, 80]. The issue of a lack of training or guidance in using the PMP was identified as a less common barrier in one study [79], a more common barrier in one study [74], and a general barrier in three studies [42, 64, 71]. Usability issues, including difficulty accessing or navigating the PMP and interpreting the data, could promote a lack of confidence in using the PMP that may prompt health care providers to identify the need for better training or guidance.
Not seeing the value in PMP data was identified as a less common barrier in ten studies [23, 28, 41, 44, 45, 47, 55, 56, 58, 79] and a general barrier in four studies (it was not identified in any study as a more common barrier) [42, 71, 72, 74]. Most often, this was reported as providers feeling that the use of PMPs would not impact their clinical practice. Other reports included not wanting to use PMP data or feeling that they could rely on their instincts in prescribing or dispensing situations.
Along with this, health care professionals expressed that PMPs inhibited their ability to prescribe, impairing their autonomy and raising concerns about patient privacy. This was identified as a less common barrier in six studies [43, 46, 55, 56, 58, 66] and a general barrier in two studies (it was not identified in any study as a more common barrier) [64, 72]. This is linked to a concern that PMPs may result in patients being under-treated (one less common and one general) [56, 72].
Health care providers were also concerned about patient satisfaction ratings and patient flow. This was identified as a less common barrier in two studies [43, 79] and a general barrier in two studies (it was not identified in any study as a more common barrier) [64, 78].
Less frequently acknowledged barriers included the lack of incentives to use the PMP (one less common [47] and two general [64, 78]), the perception that the data collected for PMPs were not reliable (two less common [44, 56], one more common [44], and two general [64, 73]), and a lack of support from PMP administrators (three less common) [27, 28, 43]. Others included an inability to delegate access to staff (three less common [27, 28, 74] and three general [72, 73, 78]), a feeling that the PMP did not provide the type of data a health care provider needed to make dispensing decisions (two less common [55, 56] and three general [42, 50, 72]), and a lack of integration and data sharing across PMP systems in different jurisdictions (two more common [56, 74] and five general [50, 72, 73, 76, 80]). Further barriers related to usability included the availability of the technology required to use the PMP (five less common [25, 27, 28, 41, 47], one more common [79], and two general [64, 77]) and problems with log-in credentials (four more common [24, 41, 47, 56] and four general [49, 64, 73, 76]). Finally, there was an identified lack of awareness of the PMP system (four more common [44, 47, 59, 79] and three less common [27, 28, 45]), as well as problems in registering for the PMP (eight more common [27, 28, 41, 44, 46, 47, 56, 67], one less common [79], and two general [64, 78]).
Discussion
This systematic review identified a significant body of literature on PMP data use and barriers, with 46 identified studies examining PMP data use and 32 identified studies looking at barriers to PMP data use.
Only half of health care providers have ever used PMP data, and on average only about 20% check the system with each prescribing or dispensing decision. Some studies lacked detail that would allow us to distinguish whether “always use” was measured only within the context that the PMP should be used, or simply for every visit. Therefore, we are unable to distinguish “always use” from “always use in the appropriate scenario,” where the appropriate scenario is use of the PMP in cases in which opioids or other controlled substances are being prescribed or filled. The wide range in health care professionals who had ever used a PMP could be influenced by the unique characteristics of the PMP itself (i.e., mandatory PMP use or training) or the study design.
We were unable to study the unique characteristics of the PMPs because of the insufficiency of information on the PMP itself in the studies; however, we were able to summarize information from “PDMP Assist” (Supplementary Appendix C) to add context and hypothesize about our results below. One characteristic we discuss below is mandatory use. There are many complex factors of mandatory PMP use, particularly with regard to the degree to which mandatory use is expected (i.e., only for certain substances, or certain providers, or under suspicious circumstances) and how it would be enforced. As such, we were unable to fully explore the impact of mandatory PMP use laws/declarations on actual PMP use by health care providers, nor have we included this information in Supplementary Appendix C (characteristics of PMPs). However, some information on mandatory use can be obtained from the “PDMP Assist” website [81]. How information on use was gathered would also affect reported estimates, including whether data was collected from surveys or in a cohort study, while the response rate and those who agree to participate may capture different levels of PMP users. The year of the study did not seem to have an impact on use as per our sensitivity analysis. This may be due to the different timelines for PMP implementation and uptake in each jurisdiction.
When comparing the characteristics of PMPs (see Supplementary Appendix C) in studies in the top 15% of PMP ever use [23, 24, 39, 50, 52] against those in the bottom 15% of PMP ever use [27, 28, 42, 51, 55], the only difference of note is the inclusion of mandatory training. None of the jurisdictions in the studies in the bottom 15% of PMP ever use currently have mandatory training, whereas jurisdictions in three of the five studies in the top 15% of PMP ever use currently have mandatory training, which suggests that it may play a role in facilitating greater PMP use. We also compared whether either solicited, or solicited and unsolicited, reports to prescribers and dispensers had any impact on ever use of PMPs. We determined whether reports were solicited, or solicited and unsolicited, by the jurisdiction in which the study took place, using information in Supplementary Appendix C, for those studies that reported ever use and were conducted in just one jurisdiction (19 out of the 26 studies reporting ever use). For reports to prescribers, there were only slight differences in the percentage of ever use according to whether reports were solicited or solicited and unsolicited (59% vs. 52%, respectively). However, for reports to dispensers, there was a greater difference in the percentage of ever use depending on whether reports were solicited or solicited and unsolicited (53% vs. 69%, respectively); however, the latter figure is based on only four studies.
Three studies reported notably higher frequencies of health care providers always using PMP data. Features of these studies that may have accounted for those high frequencies are mandatory use [82], timeliness of data availability [83], law enforcement connection [84], and provider groups [85]. Mandating the use of PMPs may generate greater use, though it does not generate perfect compliance [56]. Targeting the timeliness and ease of accessing data might increase PMP use by addressing numerous identified barriers (Table 2) [61]. More immediate data availability, as well as law enforcement involvement, may stress the importance of checking PMP data. Finally, though many health care professional groups were assessed, a study of nurse practitioners showed higher levels of always using the PMP [60]. Specific provider groups might be more engaged with using the PMP, and understanding why that is the case could be important in developing interventions to increase PMP data use overall.
Barriers that contribute to the low overall utilization of PMPs are important targets for policy and system planning. Many of the barriers identified by health care providers were strongly interrelated and point largely to system-level changes. The largest of these were related to time and usability. To increase use, PMP administrators must look at ways to simplify and speed up the process of accessing PMP data, to increase confidence and ease of using the system among health care providers. Because health care providers already have such a brief time to interact with patients, any improvements to system speed and usability may alleviate the burden of limited time. An intervention to improve PMP literacy may further support this aim and may reassure health care providers by helping them understand the information that the PMP provides, why it is necessary for public health safety, and how patient privacy/confidentiality is protected.
Although these are not the only barriers identified, finding resolutions to these common perceived barriers might be expected to facilitate an increase in PMP data use. Health care professionals have themselves stated that the removal of barriers would increase their day-to-day use of their PMP [24]. Administrators and policy makers have incentive and justification, and now information, to allocate resources toward removing barriers to PMP use.
Better-controlled prescribing (including the use of PMP data) may help to reduce harms directly, through controlling inappropriate prescribing, and indirectly, by reducing the number of problem users. Our finding that PMP data use is low and that many barriers exist to using PMP data demonstrates that this tool is not being used to its fullest potential and could potentially have a larger positive impact on the opioid crisis in reducing misuse and potential harms.
The data collected on PMP data use may be biased in several ways and should be interpreted with caution. The main concerns identified in our critical appraisal of the literature included sampling methods, low response rates, and whether nonresponders may have been systematically different from responders in terms of the outcome of interest. This low response rate and lack of information on nonresponders is problematic, as it not possible to assess whether responders and nonresponders were systematically different from one another. It is likely that those who responded were more actively engaged in the use of PMPs and hold stronger opinions about PMPs. This could have caused an overestimation of the proportion of health care providers who use PMPs and could have inflated the reporting of barriers. Furthermore, there is the potential for biases to emerge in self-reported studies, including social desirability and recall bias. This should not detract from the large number of studies that were identified and pooled for this analysis, which is a study strength.
It is also important to note that we were unable to distinguish between “PMP use” and “appropriate use of a PMP” because of the lack of detail in the included studies. This is a limitation, as we know all use is not created equal. Nevertheless, through this review, we were still able to synthesize information on patterns of use, which has been a gap in the literature.
Another important consideration is that all studies included in this review are from the United States. Although the identified barriers in U.S. studies may be applicable to similar PMP systems in Canada, they may not be applicable to more integrated prescription systems, as are found in many parts of Europe. As such, primary studies are required in both Europe and Canada, as they are both embedded in different health care systems. Additionally, whether or not a jurisdiction has mandatory PMP checking is likely an important characteristic of the PMP that may have contributed to the varying rates of use reported above. Lastly, it should be acknowledged that the mere existence of a PMP may produce a benefit, regardless of its barriers or usage, as it serves as a reminder to prescribe opioids with careful consideration.
The findings from this review may have implications for other studies and reviews examining aspects of PMP utilization or effectiveness, as we know there are barriers that would directly impact use and indirectly impact effectiveness. Two recent systematic reviews of the impact of PMP status on prescribing and overdose outcomes found unclear and mixed results [86, 87]. In both cases, the barriers and low usage levels of PMPs identified in our review may help explain the uncertainty in relationships between PMPs and outcomes. In terms of future studies, we note that among the studies in this review, PMPs (their implementation and features) were not well described. Rather than assessing simply the presence or absence of a PMP, future research should assess the impact of different features or different modes of implementation of PMPs on use and effectiveness.
Conclusion
This review has described the current use of PMP data among health care providers and has highlighted several key barriers experienced by health care providers in their daily use of PMP data. PMPs remain a vital tool to help combat the potential harms associated with prescription opioid misuse. Although these tools are present in most jurisdictions in North America, PMPs remain sorely underutilized, and many barriers to use have been identified. For PMPs to reach their fullest potential for health care providers, patients, and the public, these barriers must be removed or reduced. This review represents a first step in cataloging PMP use barriers for PMP administrators so that meaningful changes can be implemented.
Supplementary Material
Acknowledgments
We thank Leah Boulos for her assistance with the development and execution of our search strategy.
Funding sources: This research was funded by the Canadian Institutes of Health Research Operating Grant: Opioid Crisis Knowledge Synthesis/Subvention (#397982).
Disclosure and conflicts of interest: The authors have no conflicts of interest to declare.
Supplementary Data
Supplementary Data may be found online at http://painmedicine.oxfordjournals.org.
References
- 1. Seth P, Scholl L, Rudd RA, Bacon S.. Overdose deaths involving opioids, cocaine, and psychostimulants—United States, 2015-2016. Am J Transpl 2018;18(6):1556–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canadian Centre on Substance Use and Addiction. Prescription Opioids (Canadian Drug Summary)—A Summary from the Canadian Center on Substance Use and Addiction. 2017:1–12. Available at: https://www.ccsa.ca/prescription-opioids-canadian-drug-summary (accessed April 2019).
- 3.European Monitoring System for Drugs and Drug Addiction. European Drug Report Trends and Developments 2017 [Internet]. European Union Publications Office. 2017. Available at: http://www.emcdda.europa.eu/system/files/publications/4541/TDAT17001ENN.pdf_en (accessed September 2019).
- 4. Asharani PV,, Abdin E, Wen TJ, Subramaniam M, Cheok C, Song G. Correlates of non-medical prescription drug misuse among a treatment-seeking population: A comparison with illicit drug users. Int J Environ Res Public Health 2018;15(9):1978–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whelan E, Asbridge M.. The OxyContin crisis: Problematisation and responsibilisation strategies in addiction, pain, and general medicine journals. Int J Drug Policy 2013;24(5):402–11. [DOI] [PubMed] [Google Scholar]
- 6. Borwein A, Kephart G, Whelan E, Asbridge M.. Prescribing practices amid the OxyContin crisis: Examining the effect of print media coverage on opioid prescribing among physicians. J Pain 2013;14(12):1686–93. [DOI] [PubMed] [Google Scholar]
- 7. Warner M, Chen LH, Makuc DM.. Increase in fatal poisonings involving opioid analgesics in the United States, 1999-2006. NCHS Data Brief 2009;(22):1–8. [PubMed] [Google Scholar]
- 8. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med 2010;152(2):85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Department of Justice, Drug Enforcement Administration, Diversion Control Division. Controlled Substance Schedules. Available at: https://www.deadiversion.usdoj.gov/schedules/index.html (accessed September 2019).
- 10.Prescription Drug Monitoring Program Training and Technical Assistance Center. History of Prescription Drug Monitoring Programs; 2018:1–7. Available at: https://www.pdmpassist.org/pdf/PDMP_admin/TAG_History_PDMPs_final_20180314.pdf (accessed September 2019).
- 11. Reisman RM, Shenoy PJ, Atherly AJ, Flowers CR.. Prescription opioid usage and abuse relationships: An evaluation of state prescription drug monitoring program efficacy. Subst Abuse 2009;3:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ashburn MA. The evolution of prescription drug monitoring programs. Pharmacoepidemiol Drug Saf 2016;25(7):852–3. [DOI] [PubMed] [Google Scholar]
- 13. Hill JC, Fritz JM.. Psychosocial influences on low back pain, disability, and response to treatment. Phys Ther 2011;91(5):712–21. [DOI] [PubMed] [Google Scholar]
- 14. Fisher JE, Zhang Y, Sketris I, Johnston G, Burge F.. The effect of an educational intervention on meperidine use in Nova Scotia, Canada: A time series analysis. Pharmacoepidemiol Drug Saf 2012;21(2):177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prescription Drug Monitoring Program Training and Technical Assistance Center. Status of PDMPs; 2018. Available at: http://www.pdmpassist.org/pdf/PDMP_Program_Status_20180801.pdf.
- 16. Sproule B. Prescription Monitoring Programs in Canada: Best Practice and Program Review. Ottawa, ON: Canadian Centre on Substance Abuse; 2015. Available at: https://campusmentalhealth.ca/wp-content/uploads/2018/03/CCSA-Prescription-Monitoring-Programs-in-Canada-Report-2015-en1.pdf (accessed September 2019).
- 17.Government of New Brunswick. Prescription Monitoring Program and Drug Information System. Available at: https://www2.gnb.ca/content/gnb/en/departments/health/MedicarePrescriptionDrugPlan/PrescriptionMonitoringProgramandDrugInformationSystem.html (accessed September 2019).
- 18. Leonard K. Is the EU Facing the Next Opioid Epidemic? US News; 2016. Available at: https://www.usnews.com/news/best-countries/articles/2016-08-03/european-union-sees-alarming-rates-of-prescription-drug-abuse (accessed September 2019).
- 19. Curtis LH, Stoddard J, Radeva JI, et al. Geographic variation in the prescription of schedule II opioid analgesics among outpatients in the United States. Health Serv Res 2006;41(3p1):837–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yarbrough CR. Prescription drug monitoring programs produce a limited impact on painkiller prescribing in Medicare Part D. Health Serv Res 2018;53(2):671–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bao Y, Pan Y, Taylor A, et al. Prescription drug monitoring programs are associated with sustained reductions in opioid prescribing by physicians. Health Aff 2016;35(6):1045–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deyo RA, Hallvik SE, Hildebran C, et al. Association of prescription drug monitoring program use with opioid prescribing and health outcomes: A comparison of program users and nonusers. J Pain 2018;19(2):166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rutkow L, Turner L, Lucas E, Hwang C, Alexander GC.. Most primary care physicians are aware of prescription drug monitoring programs, but many find the data difficult to access. Health Aff 2015;34(3):484–92. [DOI] [PubMed] [Google Scholar]
- 24. Young HW, Tyndall JA, Cottler LB.. The current utilization and perceptions of prescription drug monitoring programs among emergency medicine providers in Florida. Int J Emerg Med 2017;10(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wixson SE, Blumenschein K, Goodin AJ, Talbert J, Freeman PR.. Prescription drug monitoring program utilization in Kentucky community pharmacies. Pharm Pract (Granada) 2015;13(2):540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weiner SG, Baker O, Poon SJ, et al. The effect of opioid prescribing guidelines on prescriptions by emergency physicians in Ohio. Ann Emerg Med 2017;70(6):799–808.e1. [DOI] [PubMed] [Google Scholar]
- 27. Green TC, Mann MR, Bowman SE, et al. How does use of a prescription monitoring program change pharmacy practice? J Am Pharm Assoc (2003) 2013;53(3):273–81. [DOI] [PubMed] [Google Scholar]
- 28. Green TC, Mann MR, Bowman SE, et al. How does use of a prescription monitoring program change medical practice? Pain Med 2012;13(10):1314–23. [DOI] [PubMed] [Google Scholar]
- 29. Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5. The Cochrane Collaboration; 2011. Available at: http://handbook.cochrane.org (accessed October 2018).
- 30.Prisma. PRISMA: Transparent Reporting of Systematic Reviews and Meta-Analyses; 2015. Available at: http://prisma-statement.org/prismastatement/Checklist.aspx (accessed October 2018).
- 31.Clarivate Analytics. End Note X8. 2017.
- 32.Cochrane Community. About Covidence. Available at: https://community.cochrane.org/help/tools-and-software/covidence/about-covidence (accessed November 2018).
- 33. Downes MJ, Brennan ML, Williams HC, Dean RS.. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016;6(12):e011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Criticial Appraisal Skills Programme. CASP Qualitative Checklist; 2018. Available at: https://casp-uk.net/casp-tools-checklists (accessed November 2018).
- 35. Dixon-Woods M, Cavers D, Agarwal S, et al. Conducting a critical interpretive synthesis of the literature on access to healthcare by vulnerable groups. BMC Med Res Methodol 2006;6(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kelly S, Johnson GT, Harbison RD.. “Pressured to prescribe” the impact of economic and regulatory factors on South-Eastern ED physicians when managing the drug seeking patient. J Emergencies, Trauma Shock 2016;9(2):58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Norwood CW, Wright ER.. Integration of prescription drug monitoring programs (PDMP) in pharmacy practice: Improving clinical decision-making and supporting a pharmacist’s professional judgment. Res Social Adm Pharm 2016;12(2):257–66. [DOI] [PubMed] [Google Scholar]
- 38. Leichtling GJ, Irvine JM, Hildebran C, et al. Clinicians’ use of prescription drug monitoring programs in clinical practice and decision-making. Pain Med 2017;18(6):1063–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fendrich M, Bryan JK, Hooyer K.. Prescription drug monitoring programs and pharmacist orientation toward dispensing controlled substances. Subst Use Misuse 2018;53(8):1324–30. [DOI] [PubMed] [Google Scholar]
- 40. Fleming ML, Hatfield MD, Wattana MK, Todd KH.. Exploratory study of emergency physicians’ use of a prescription monitoring program using a framework of technology acceptance. J Pain Palliat Care Pharmacother 2014;28(1):19–27. [DOI] [PubMed] [Google Scholar]
- 41. Hernandez-Meier JL, Muscott R, Zosel A.. The use of a statewide prescription drug monitoring program by emergency department physicians. Wisconsin Med J 2017;116(2):64–8. [PubMed] [Google Scholar]
- 42. Homant SF. The war on drugs v. the war on pain: Do controlled prescribing laws have a role? Dissertation. Western Michigan University; 2006.
- 43. Kelley SS. The perception of emergency department physicians regarding economic and regulatory factors impacting management of drug seeking patients. Dissertation. University of South Florida; 2013.
- 44. Lin DH, Lucas E, Murimi IB, et al. Physician attitudes and experiences with Maryland’s prescription drug monitoring program (PDMP). Addiction 2017;112(2):311–9. [DOI] [PubMed] [Google Scholar]
- 45. McCauley JL, Leite RS, Melvin CL, Fillingim RB, Brady KT.. Dental opioid prescribing practices and risk mitigation strategy implementation: Identification of potential targets for provider-level intervention. Subst Abus 2016;37(1):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Norwood CW, Wright ER.. Promoting consistent use of prescription drug monitoring programs (PDMP) in outpatient pharmacies: Removing administrative barriers and increasing awareness of Rx drug abuse. Res Social Adm Pharm 2016;12(3):509–14. [DOI] [PubMed] [Google Scholar]
- 47. Perrone J, DeRoos FJ, Nelson LS.. Prescribing practices, knowledge, and use of prescription drug monitoring programs (PDMP) by a national sample of medical toxicologists, 2012. J Med Toxicol 2012;8(4):341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Piper BJ, Desrosiers CE, Lipovsky JW, et al. Use and misuse of opioids in Maine: results from pharmacists, the prescription monitoring, and the diversion alert programs. J Stud Alcohol Drugs 2016;77(4):556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Poon SJ, Greenwood-Ericksen MB, Gish RE, et al. Usability of the Massachusetts prescription drug monitoring program in the emergency department: A mixed-methods study. Acad Emerg Med 2016. Apr;23(4):406–14. [DOI] [PubMed] [Google Scholar]
- 50. Radomski TR, Bixler FR, Zickmund SL, et al. Physicians’ perspectives regarding prescription drug monitoring program use within the Department of Veterans Affairs: A multi-state qualitative study. J Gen Intern Med 2018;33(8):1253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Riley TB. Pharmacist utilization of opioid misuse and abuse interventions: Acceptability among pharmacists and among patients in detox. Dissertation. Kent State University; 2017.
- 52. Rittenhouse R, Wei F, Robertson D, Ryan K.. Utilization of the Arkansas prescription monitoring program to combat prescription drug abuse. Prev Med Reports 2015;2:524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sun BC, Lupulescu-Mann N, Charlesworth CJ, et al. Variations in prescription drug monitoring program use by prescriber specialty. J Subst Abuse Treat 2018;94:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang GS, Roosevelt G, Fagan K, Hoppe J.. Pediatric emergency physician knowledge and utilization of prescription drug monitoring program. Clin Pediatr (Phila) 2017;56(1):80–2. [DOI] [PubMed] [Google Scholar]
- 55. Barrett K, Watson A.. Physician perspectives on a pilot prescription monitoring program. J Pain Palliat Care Pharmacother 2005;19(3):5–13. [PubMed] [Google Scholar]
- 56. Blum CJ, Nelson LS, Hoffman RS.. A survey of physicians’ perspectives on the New York state mandatory prescription monitoring program (ISTOP). J Subst Abuse Treat 2016;70:35–43. [DOI] [PubMed] [Google Scholar]
- 57. Delcher C, Wang Y, Young HW, et al. Trends in Florida’s Prescription Drug Monitoring Program registration and utilization: Implications for increasing voluntary use. J Opioid Manag 2017;13(5):283–9. [DOI] [PubMed] [Google Scholar]
- 58. Feldman L, Skeel Williams K, Knox M, Coates J.. Influencing controlled substance prescribing: Attending and resident physician use of a state prescription monitoring program. Pain Med 2012. Jul;13(7):908–14. [DOI] [PubMed] [Google Scholar]
- 59. Feldman L, Williams KS, Coates J, Knox M.. Awareness and utilization of a prescription monitoring program among physicians. J Pain Palliat Care Pharmacother 2011;25(4):313–7. [DOI] [PubMed] [Google Scholar]
- 60. Chaudhary S, Compton P.. Use of risk mitigation practices by family nurse practitioners prescribing opioids for the management of chronic nonmalignant pain. Subst Abus 2017;38(1):95–104. [DOI] [PubMed] [Google Scholar]
- 61. Fleming ML, Barner JC, Brown CM, et al. Pharmacists’ training, perceived roles, and actions associated with dispensing controlled substance prescriptions. J Am Pharm Assoc (2003) 2014;54(3):241–50. [DOI] [PubMed] [Google Scholar]
- 62. Fleming ML, Chandwani H, Barner JC, Weber SN, Okoro TT.. Prescribers and pharmacists requests for prescription monitoring program (PMP) data: Does PMP structure matter? J Pain Palliat Care Pharmacother 2013;27(2):136–42. [DOI] [PubMed] [Google Scholar]
- 63. Gershman JA, Gershman JA, Fass AD, Popovici I.. Evaluation of Florida physicians’ knowledge and attitudes toward accessing the state prescription drug monitoring program as a prescribing tool. Pain Med 2014;15(12):2013–9. [DOI] [PubMed] [Google Scholar]
- 64. Hildebran C, Cohen DJ, Irvine JM, et al. How clinicians use prescription drug monitoring programs: A qualitative inquiry. Pain Med 2014;15(7):1179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Irvine JM, Hallvik SE, Hildebran C, et al. Who uses a prescription drug monitoring program and how? Insights from a statewide survey of Oregon clinicians. J Pain 2014;15(7):747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lemire SD, Martner SG, Rising C.. Advanced practice nurses’ use of prescription drug monitoring program information. J Nurse Pract 2012;8(5):383–405. [Google Scholar]
- 67. McAllister MW, Aaronson P, Spillane J, et al. Impact of prescription drug-monitoring program on controlled substance prescribing in the ED. Am J Emerg Med 2015;33(6):781–5. [DOI] [PubMed] [Google Scholar]
- 68. Pomerleau AC, Nelson LS, Hoppe JA, et al. The impact of prescription drug monitoring programs and prescribing guidelines on emergency department opioid prescribing: A multi-center survey. Pain Med 2017;18(5):889–97. [DOI] [PubMed] [Google Scholar]
- 69. Ringwalt C, Garrettson M, Alexandridis A.. The effects of North Carolina’s prescription drug monitoring program on the prescribing behaviors of the state’s providers. J Prim Prev 2015;36(2):131–7. [DOI] [PubMed] [Google Scholar]
- 70. Wallace JR. Chronic pain management: Implementing best strategies. Dissertation. North Dakota State University; 2017.
- 71. Warren GKP. Prescription drug abuse: Implementing an evidence-based pain management protocol. North Dakota State University; 2016.
- 72. Carnes NA, Wright ER, Norwood CW.. A qualitative analysis of prescribers’ and dispensers’ views on improving prescription drug monitoring programs. Res Social Adm Pharm 2017;13(6):1167–74. [DOI] [PubMed] [Google Scholar]
- 73. Click IA, Basden JA, Bohannon JM, Anderson H, Tudiver F.. Opioid prescribing in rural family practices: A qualitative study. Subst Use Misuse 2018;53(4):533–40. [DOI] [PubMed] [Google Scholar]
- 74. Deyo RA, Irvine JM, Hallvik SE, et al. Leading a horse to water: facilitating registration and use of a prescription drug monitoring program. Clin J Pain. 2015;31(9):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fazio V. Reducing harms caused by opioids through physician education: A gap analysis of physician underutilization of the California Controlled Substance Utilization Review and Evaluation System. Dissertation. University of Southern California; 2017.
- 76. Naiman M. Systematically gathering clinician opinions on health care technology. Dissertation. University of Illinois at Chicago; 2013.
- 77. Pugliese JA, Wintemute GJ, Henry SG.. Psychosocial correlates of clinicians’ prescription drug monitoring program utilization. Am J Prev Med 2018;54(5):e91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Smith RJ, Kilaru AS, Perrone J, et al. How, why, and for whom do emergency medicine providers use prescription drug monitoring programs? Pain Med 2015;16(6):1122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ulbrich TR, Dula CAC, Green CG, Porter K, Bennett MS.. Factors influencing community pharmacists’ enrollment in a state prescription monitoring program. J Am Pharm Assoc (2003) 2010;50(5):588–94. [DOI] [PubMed] [Google Scholar]
- 80. Worley J, Johnson M, Karnik N.. Psychiatric prescribers’ experiences with doctor shoppers. J Am Psychiatr Nurses Assoc 2015;21(5):309–18. [DOI] [PubMed] [Google Scholar]
- 81.Prescription Drug Monitoring Program Training and Technical Assistance Center. Available at: https://www.pdmpassist.org/ (accessed September 2019).
- 82. Grecu AM, Dave DM, Saffer H.. Mandatory access prescription drug monitoring programs and prescription drug abuse. J Policy Anal Manage 2019;38(1):181–209. [PubMed] [Google Scholar]
- 83. Winstanley EL, Zhang Y, Mashni R, et al. Mandatory review of a prescription drug monitoring program and impact on opioid and benzodiazepine dispensing. Drug Alcohol Depend 2018;188:169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rutkow L, Smith KC, Lai AY, et al. Prescription drug monitoring program design and function: A qualitative analysis. Drug Alcohol Depend 2017;180:395–400. [DOI] [PubMed] [Google Scholar]
- 85. Franklin GM, Fulton-Kehoe D, Turner JA, Sullivan MD, Wickizer TM.. Changes in opioid prescribing for chronic pain in Washington State. J Am Board Fam Med 2013;26(4):394–400. [DOI] [PubMed] [Google Scholar]
- 86. Fink DS, Schleimer JP, Sarvet A, et al. Association between prescription drug monitoring programs and nonfatal and fatal drug overdoses: A systematic review. Ann Intern Med 2018;168(11):783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wilson MN, Hayden JA, Rhodes E, Robinson A, Asbridge M.. Effectiveness of prescription monitoring programs in reducing opioid prescribing, dispensing, and use outcomes: A systematic review. J Pain 2019;20(12):1383–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.