Abstract
Statin-induced necrotizing autoimmune myopathy (SINAM) is an exceptionally rare yet devastating complication of statin therapy that can occur at any time after initiation. It should be considered in patients who develop proximal muscle weakness and marked elevated creatine phosphokinase while taking statin therapy. (Level of Difficulty: Beginner.)
Key Words: autoimmune, myopathy, peripheral vascular disease, statins
Abbreviations and Acronyms: CPK, creatine phosphokinase; ELISA, enzyme-linked immunosorbent assay; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; IVIG, intravenous immune globulin; SINAM, statin-induced necrotizing autoimmune myopathy
Graphical abstract
Statin-induced necrotizing autoimmune myopathy (SINAM) is an exceptionally rare yet devastating complication of statin therapy that can occur at any…
Presentation
A 55-year-old African-American female presented with 7 months of progressive proximal muscle weakness. Over the previous several months, she became unable to climb stairs, get up from a seated position, and lift objects over her head without significant assistance. She also admitted to shortness of breath and hoarseness with exertion. She denied oral ulcers, arthralgias, rashes, hair loss, vasospasms, and reflux. Medication review was notable for atorvastatin therapy, which had been started 4 years previously. A physical examination was notable for fine pulmonary crackles and asymmetric right upper extremity bicep extension weakness and right lower extremity hip extension weakness.
Learning Objectives
-
•
Objectives are to understand the clinical course of statin-induced necrotizing autoimmune myopathy to allow for prompt evaluation if suspected at any point during statin administration.
-
•
An additional objective is to identify evidence-based steps that will aid in reversing this fatal myopathy.
Medical history
The patient had a history of hypertension, peripheral artery disease with an iliac stent, and tobacco use.
Differential diagnosis
The differential included statin-induced necrotizing autoimmune myopathy (SINAM), polymyositis, dermatomyositis, antisynthetase syndrome, inclusion body myositis, drug-induced myositis, and paraneoplastic syndrome.
Investigations
On admission, her creatine phosphokinase (CPK) level was 9,259 IU/l. Electromyography showed generalized irritable myopathy. Laboratory study results revealed an antinuclear antibody of 1:160, negative extended myositis panel results, and a 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) antibody level >200 (normal <20), thus providing a diagnosis of SINAM. No muscle biopsy was pursued, given her initial rapid response to treatment.
Management
The patient was started on oral prednisone, but at 6-week follow-up, her lower extremity weakness worsened. She was unable to stand without assistance or abduct her arms past 90°. She had no bulbar symptoms. Due to disease progression, she was given intravenous immune globulin (IVIG) and intravenous methylprednisolone and was started on adjunctive methotrexate and folic acid. She was discharged on oral prednisone, methotrexate, and monthly IVIG therapy.
Discussion
Statins are widely used lipid-lowering drugs with significant benefit in prevention and treatment of vascular disease. Although they are generally regarded as safe, adverse muscle-related events secondary to statin use are well documented, with presentations ranging from myalgias to rhabdomyolysis (Table 1) (1).
Table 1.
Comparison of Common Statin-Induced Myopathies and SINAM
| Statin-Associated Muscle Symptoms With or Without CK Elevation | Self-Limited Toxic Myopathy | SINAM | |
|---|---|---|---|
| Symptoms | No muscle weakness Myalgia (++) |
Proximal muscle weakness (++) Myalgia (+) |
Proximal muscle weakness (+++) Myalgia (++) Dysphagia (+/++) Respiratory failure (+/-) |
| CPK | <1,000 IU/l | <20,000 IU/l | 1,000–20,000 IU/l |
| Anti-HMGCR antibody | Negative | Negative | Positive |
| Clinical course after withdrawal of statin | Variable but with good outcome | Improvement with discontinuation or dose reduction of statin | Persistent/progressive symptoms |
| Recommended therapy | None | Discontinue or reduce statin dose | Discontinue statin and start immunosuppression |
CPK = creatine phospokinase; HMGCR = 3-hydroxy-3-methylglutaryl-coenzyme A reductase; SINAM = statin-induced necrotizing autoimmune myopathy.
Occurring in 1 of 100,000 patients taking a statin, SINAM is a rare and potentially life-threatening complication characterized by acute, severe onset of progressive proximal muscle weakness. It presents with serum creatine kinase concentration >1,000 IU/l following the induction of statin therapy and is thought to be mediated by autoantibodies to the enzymatic target of statins, HMGCR (2,3). Given the rarity of SINAM, the natural history of untreated disease is not well described. Even in the setting of aggressive treatment, case reports have described mortality due to respiratory complications in the setting of bulbar involvement (4).
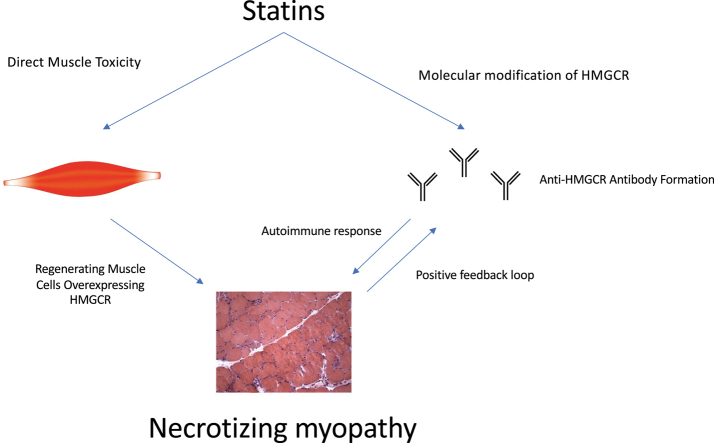
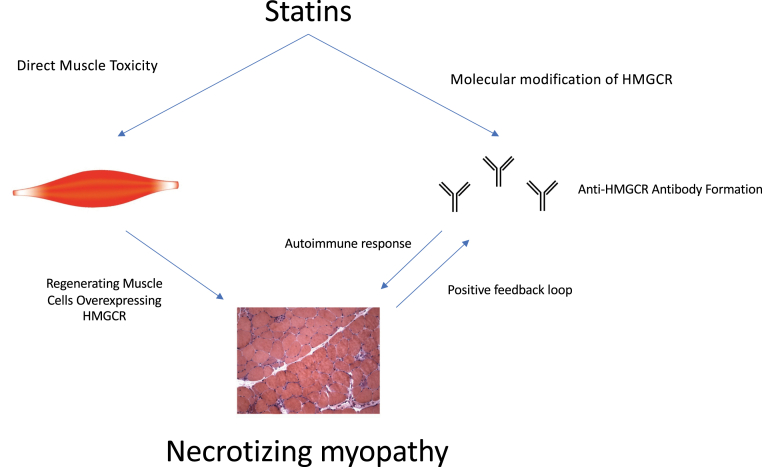
The pathophysiological mechanism of SINAM is poorly understood. It is hypothesized that statins trigger an overexpression of HMGCR in genetically susceptible patients. This is thought to cause the development of autoimmunity against HMGCR (Figure 1) (5). It is not known whether the autoantibodies have a direct pathological effect on myocytes or if there are intermediary factors involved in the immune-mediated muscle injury (6). Recent investigations have found that introduction of anti-HMGCR antibodies can induce muscle weakness in mice through a complement-mediated mechanism. Thus, therapy in the future could be centered on plasma exchange and inhibition of complement activation (7).
Figure 1.
Proposed Pathogenesis of SINAM
Statins trigger overexpression of HMGCR in genetically susceptible patients, which propagates a positive bio feedback loop of autoimmune myopathy. HMGCR = 3-hydroxy-3-methylglutaryl-coenzyme A reductase; SINAM = statin-induced necrotizing autoimmune myopathy.
Although the mechanism remains unclear, certain epidemiological patterns have emerged, providing guidance for management of patients who are at increased risk. The most notable factors stratifying the risk of development include age >50 years and African American descent. Genetic association studies have demonstrated a strong correlation between SINAM and HLA-DR11 in both white and African American patients and found decreased levels of HLA-DQA1 and HLA-DQB6 alleles in white patients with anti-HMGCR antibodies (8). Of note, African American patients present with a higher serum CPK level and are less responsive to treatment (7).
Making a timely diagnosis is often difficult because SINAM has a variable time course, with symptoms often presenting years after initial statin exposure (7). One hallmark feature of SINAM is the persistence of myositis and elevated CPK despite discontinuation of the statin. In contrast, statin-related myositis resolves following discontinuation of the drug (9).
Physical examination typically demonstrates symmetrical proximal muscle weakness, without skin involvement, which is more often seen in dermatomyositis (10). Additionally, statins commonly exacerbate underlying metabolic conditions, thus evaluation for Addison, Cushing, McArdle, and thyroid diseases should be considered.
SINAM is pathologically characterized by myonecrosis, thus CPK levels are usually elevated near the 10,000 IU/l concentration, with normal inflammatory markers. Electromyography commonly shows a nonspecific irritable myopathy pattern indistinguishable from other inflammatory myopathies (9). Detection of anti-HMGCR antibodies using enzyme-linked immunosorbent assay (ELISA) has a sensitivity of 94.4% and a specificity of 99.3%. Anti-HMGCR antibodies have been found in patients without prior statin exposure, thus further immunoprecipitation testing may be necessary. The negative predictive value is >0.999, indicating that a negative ELISA screen provides strong evidence against antibody presence.
Management of patients in whom SINAM has been diagnosed is centered on aggressive immunotherapy, with first-line therapy using high-dose corticosteroids and/or IVIG. Adjuvant therapy is introduced based on consideration of a patient’s individual risk factors and includes methotrexate, azathioprine, mycophenolate mofetil, and rituximab. The initial level of anti-HMGCR antibody in response to ELISA positively correlates with the serum CPK level and clinical severity at presentation. Falling antibody levels correlate with clinical improvement, thus serving as a way to monitor treatment efficacy (11). Earlier and more intense treatment is associated with improved outcomes, as the prolonged increase of CPK predisposes an individual to renal dysfunction (12).
Follow-up
At 15 weeks’ follow-up, the patient reported substantial clinical improvement. She was able to lift her hands above her head, comb her hair, and independently get up from a sitting position without assistance. CPK level normalized at 21 weeks’ follow-up while the patient was on a gradual prednisone taper and methotrexate.
Conclusions
Prompt clinical evaluation of the patient taking statins who develops myalgias, proximal muscle weakness, and CPK levels in the 1,000s at any point during administration is warranted to assess for the onset of SINAM. With recent advances and increased availability in ELISA technology, relatively cheap and time-efficient screening for these antibodies can aid with earlier diagnosis and intervention. Further stratification of an individual’s risk of developing SINAM prior to starting statin therapy could help prevent unnecessary morbidity and mortality from an otherwise commonly used medication. Future ambitions should be directed toward determining if patients with SINAM can be rechallenged with a statin or if they would benefit from other cholesterol-lowering therapies such as ezetimibe, PCSK9 inhibitors, or bempidoic acid.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, or patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Jacobson T.A. NLA task force on statin safety—2014 update. J Clin Lipidol. 2014;8(Suppl):S1–S4. doi: 10.1016/j.jacl.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Grable-Esposito P., Katzberg H.D., Greenberg S.A., Srinivasan J., Katz J., Amato A.A. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve. 2010;41:185–190. doi: 10.1002/mus.21486. [DOI] [PubMed] [Google Scholar]
- 3.Mammen A.L., Chung T., Christopher-Stine L. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63:713–721. doi: 10.1002/art.30156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaclavik L. Feel the burn: a case report of statin-induced necrotizing myositis. Chest Journal. 2016;150:409. [Google Scholar]
- 5.Kassardjian C.D., Lennon V.A., Alfugham N.B., Mahler M., Milone M. Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol. 2015;72:996–1003. doi: 10.1001/jamaneurol.2015.1207. [DOI] [PubMed] [Google Scholar]
- 6.Selva-O'Callaghan A., Alvarado-Cardenas M., Pinal-Fernández Statin-induced myalgia and myositis: an update on pathogenesis and clinical recommendations. Expert Rev Clin Immunol. 2018;14:215–224. doi: 10.1080/1744666X.2018.1440206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergua C., Chiavelli H., Allenbach Y. In vivo pathogenicity of IgG from patients with anti-SRP or anti-HMGCR autoantibodies in immune-mediated necrotising myopathy. Ann Rheum Dis. 2019;78:131–139. doi: 10.1136/annrheumdis-2018-213518. [DOI] [PubMed] [Google Scholar]
- 8.Mammen A.L. Statin-associated autoimmune myopathy. N Engl J Med. 2016;374:664–669. doi: 10.1056/NEJMra1515161. [DOI] [PubMed] [Google Scholar]
- 9.Mammen A.L., Gaudet D., Brisson D. Increased frequency of DRB1*11:01 in anti-hydroxymethylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Care Res. 2012;64:1233–1237. doi: 10.1002/acr.21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christopher-Stine L., Casciola-Rosen L.A., Hong G., Chung T., Corse A.M., Mammen A.L. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis and Rheumatism Journal. 2010;62:2757–2766. doi: 10.1002/art.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christopher-Stine L., Basharat P. Statin-associated immune-mediated myopathy. Curr Opin Lipidol. 2017;28:186–192. doi: 10.1097/MOL.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 12.Casciola-Rosen L., Mammen A.L. Myositis autoantibodies. Curr Opin Rheumatol. 2012;24:602–608. doi: 10.1097/BOR.0b013e328358bd85. [DOI] [PMC free article] [PubMed] [Google Scholar]