Abstract
Three-dimensional imaging and printed heart models have become increasingly valuable in the management of patients with complex congenital heart disease. We successfully simulated a stenting procedure on a 3-dimensional printed model of a patient with d-transposition of the great arteries status post-Mustard operation with caval baffle atresia and stenosis. (Level of Difficulty: Advanced.)
Key words: congenital heart disease, pre-procedural planning, Mustard operation, transcatheter intervention, transposition of the great arteries
Abbreviations and Acronyms: 3D, 3-dimensional; CT, computed tomography; IVC, inferior vena cava; SVC, superior vena cava
Graphical abstract
Three-dimensional imaging and printed heart models have become increasingly valuable in the management of patients with complex congenital heart…
A 49-year-old woman with d-transposition of the great arteries, status post-Mustard operation at age 2 years, noted exercise intolerance. Computed tomography (CT) angiography and 3-dimensional (3D) reconstructions revealed superior vena cava (SVC) limb atresia and inferior vena cava (IVC) limb stenosis (Figure 1).
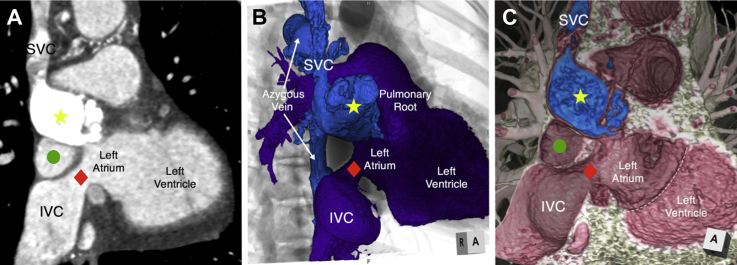
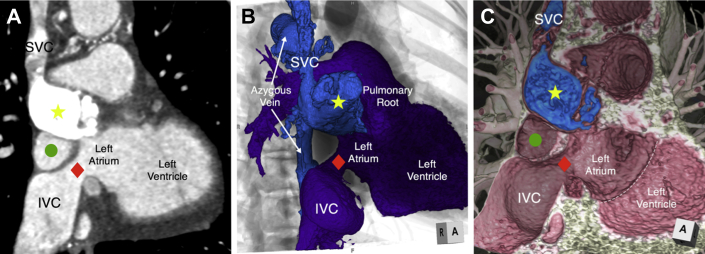
Figure 1.
CT Angiography and 3D Reconstructions Demonstrating SVC Limb Atresia and IVC Limb Stenosis
(A) 2D CT image in a parasagittal plane demonstrating the densely opacified and dilated SVC limb of the systemic venous baffle (yellow star), which is atretic proximally. The IVC limb is stenotic (red diamond). Similar views were recreated with 3D volume-rendering reconstructions using (B) endocast reconstruction with overlay on a fluoroscopic representation and (C) virtual dissection reconstruction techniques, demonstrating not only the 3D relationship of the systemic venous to the pulmonary venous baffle (green circle), but also the relationship to the mitral valve annulus (white dashed line). 2D = 2-dimensional; 3D = 3-dimensional; CT = computed tomography; IVC = inferior vena cava; SVC = superior vena cava.
A 3D printed heart model was generated to facilitate pre-procedural interventional simulation. In the model, after perforation of the SVC atresia, a wire rail was established between the SVC and IVC limbs of the systemic venous baffle. A single 36-mm ev3 LD Max stent (Covidien, Plymouth, Minnesota) was implanted across both stenotic caval lesions, simultaneously relieving the stenosis within each baffle limb, but “jailing” inflow to the subpulmonic mitral valve through the open cells of the stent (Supplemental Figure 1). After crossing a side strut, and establishing a wire course across the mitral valve, serial balloon angioplasty dilated and ultimately fractured the jailing side-strut, thereby generating unobstructed mitral inflow (Video 1). The stent adequately relieved SVC and IVC pathway obstruction, thereby validating feasibility of this proposed 2-stent, 3-lesion strategy.
Online Video 1.
Demonstration of 3D Printed Heart Model Post-Simulated Stent Procedure
Final appearance of the 3-dimensional (3D) model following simulated intervention.
The procedure was successfully replicated in vivo in the cardiac catheterization laboratory, with post-intervention rotational angiography demonstrating unobstructed flow through the SVC and IVC limbs to the subpulmonic left ventricle (Video 2). The patient reported restoration of normal exercise capacity at 6-month follow-up.
Online Video 2.
Systemic Venous Baffle Angiography Post-Stent Procedure
Systemic venous baffle angiography with simultaneous superior vena cava and inferior vena cava power injections. There is unobstructed flow across the stent.
Discussion
Advances in cross-sectional imaging have improved pre-procedural planning in complex congenital heart disease. 3D virtual and printed heart models accurately replicate patient anatomy, serve as an advanced visuospatial planning tool, and facilitate procedural simulation (1,2). This case highlights the benefits of maximizing the obtained 2-dimensional CT data with both virtual and printed models, the latter of which was used for successful procedural planning and simulation.
Footnotes
Dr. Goldstein has been a consultant for Medtronic and W.L. Gore & Associates. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, or patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos and a figure, please see the online version of this paper.
Appendix
3D Printed Heart Model Pre- and Post-Stent Deployment and Modification. Viewed via the mitral valve annulus (red outline) of the 3-dimensional (3D) printed model, a stent is delivered across the obstructed systemic venous baffle (A). After stent implantation, a side strut is dilated (B) to promote unobstructed mitral valve inflow (C). LVOT = left ventricular outflow tract.
References
- 1.Mori S., Tretter J.T., Spicer D.E., Bolender D.L., Anderson R.H. What is the real cardiac anatomy? Clin Anat. 2019;32:288–309. doi: 10.1002/ca.23340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore R.A., Riggs K.W., Kourtidou S. Three-dimensional printing and virtual surgery for congenital heart procedural planning. Birth Defects Res. 2018;110:1082–1090. doi: 10.1002/bdr2.1370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
3D Printed Heart Model Pre- and Post-Stent Deployment and Modification. Viewed via the mitral valve annulus (red outline) of the 3-dimensional (3D) printed model, a stent is delivered across the obstructed systemic venous baffle (A). After stent implantation, a side strut is dilated (B) to promote unobstructed mitral valve inflow (C). LVOT = left ventricular outflow tract.