Abstract
A variety of fenestrated vascular plugs have been used to seal paravalvular leaks with meaningful success; however, incomplete closure and refractory hemolysis remains a common problem. We describe the feasibility and rationale of their first experience using a nonfenestrated Cardioform Septal Occluder (Gore Medical, Flagstaff, Arizona) to treat a giant mitral paravalvular leak. (Level of Difficulty: Advanced.)
Key Words: Cardioform septal occluder, mitral regurgitation, paravalvular leak, percutaneous closure, transapical
Abbreviations and Acronyms: CSO, Cardioform septal occluder; FDA, U.S. Food and Drug Administration; PVL, paravalvular leak; TEE, transesophageal echocardiogram
Graphical abstract
A variety of fenestrated vascular plugs have been used to seal paravalvular leaks with meaningful success; however, incomplete closure and refractory…
Paravalvular leak (PVL) after prosthetic valve replacement is a difficult complication to treat, with an incidence rate of 5% to 32% (1). Reoperation is often required, yet is associated with significant morbidity and PVL recurrence (1,2). Transcatheter PVL closure was introduced as a less invasive alternative to surgery (3, 4, 5), and is presently accepted as a Class IIa recommendation by the American Heart Association/American College of Cardiology for higher-risk patients with symptomatic heart failure and/or intractable hemolysis (6). Despite this, there are presently no U.S. Food and Drug Administration (FDA)–approved PVL closure devices, and although the body of evidence involves the use of fenestrated closure devices, incomplete closure and refractory hemolysis often limits their success (5).
Learning Objectives
-
•
Currently, there are no specific FDA-approved transcatheter closure devices for PVL closure.
-
•
Off-label use of fenestrated vascular plugs have been used to seal PVLs with meaningful success; yet, incomplete closure and refractory hemolysis remains a problem.
-
•
Percutaneous PVL closure using a nonfenestrated Cardioform Septal Occluder can be an option in high-surgical-risk patients with a large mitral PVL.
History of Presentation
A 76-year-old man with a history of coronary artery bypass and bioprosthetic aortic valve replacement followed by redo open aortic valve replacement and mitral valve replacement (Epic bioprosthetic, size #31) was referred for management of a giant mitral PVL resulting in severe regurgitation, refractory New York Heart Association (NYHA) functional class IV heart failure requiring frequent paracentesis, deterioration of renal function, and transfusion-dependent hemolytic anemia (lactate dehydrogenase: 956 U/l, haptoglobin: 370 mg/dl, reticulocyte count: 7.4%). Despite all efforts to manage the condition medically, progressive clinical deterioration ensued.
Investigations
A third redo surgery was considered high risk, with a Society of Thoracic Surgeons score of 12.9%. Transcatheter PVL closure was therefore proposed. Pre-procedural 3-dimensional transesophageal echocardiography (TEE) confirmed the presence of severe (4+) mitral regurgitation arising from a giant posterior PVL involving 35% to 40% of the valve’s circumference between approximately 7 to 11 o’clock (Figure 1, Videos 1, 2, 3, and 4). Despite the presence of a giant continuous PVL, the bioprosthetic valve exhibited mechanical stability with no evidence of rocking motion. TEE examination showed no evidence of interdigitating bridging stitch throughout the single PVL gap. Because of the magnitude of the defect, and concerns for incomplete closure and residual hemolysis with the use of standard fenestrated plugs, the off-label use of a nonfenestrated Cardioform Septal Occluder (CSO) (Gore Medical, Flagstaff, Arizona) was considered.
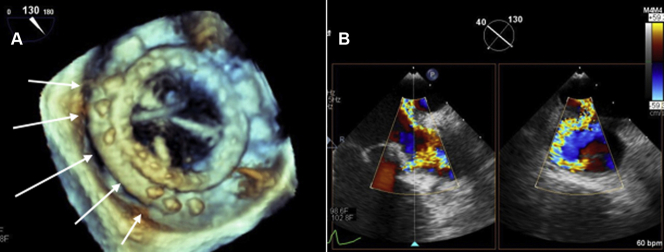
Figure 1.
Giant Mitral Paravalvular Leak
(A) 3-dimensional transesophageal echocardiogram (TEE) examination of the mitral bioprosthesis showing large posterior paravalvular defect (white arrows) involving 35% to 40% of the valve circumference. (B) X-plane TEE color Doppler showing severe mitral paravalvular leak. See Videos 1, 2, 3, and 4.
Online Video 1.
Baseline TEE demonstrating severe PVL with color Doppler
Online Video 2.
Intraoperative TEE color Doppler showing severe mitral PVL
Online Video 3.
Baseline TEE 3D with color Doppler showing severe PVL
Online Video 4.
Baseline TEE 3D showing large PVL
Management
The procedure was performed in a hybrid suite under general anesthesia with intraoperative 3-dimensional TEE guidance. Transapical access was accomplished through a small left lateral thoracotomy between the 5th and 6th intercostal space midclavicular line. The apex of the left ventricle (LV) was exposed under direct visualization and a double pledgeted purse-string suture employed to ensure hemostasis. Weight-adjusted unfractionated heparin was administered intravenously to maintain activated clotting time above 300 s throughout the procedure. Transapical access was obtained with an 11-F 20 cm Cordis sheath (Cordis Corp., Hialeah, Florida). A 5-F multipurpose catheter and Terumo 0.035-inch angled guidewire (Terumo, Doral, Florida) were advanced retrograde through the PVL into the left atrium (LA) (Figure 2A, Video 5). The guidewire was exchanged for an extra-stiff Amplatzer wire (Abbott Vascular, Chicago, Illinois). The 11-F Cordis dilator and sheath were coupled and advanced over the wire, through the PVL, and into the LA. The dilator and wire were removed. A 0.014-inch Mailman coronary “buddy wire” was advanced through the sheath to secure LA access. The 11-F Cordis sheath was used to deliver the first 20-mm CSO under fluoroscopic and TEE guidance. The distal LA and proximal LV discs were deployed while carefully withdrawing the Cordis sheath into the LV. The device was subsequently released once satisfactory results were confirmed on TEE. Access into the LA was regained over the 0.014” “buddy wire.” The same steps were performed to deliver 2 additional 20-mm CSO with satisfactory results (Figure 2B). TEE confirmed device stability, and the absence of a significant residual leak (Figure 3A, Videos 6, 7, 8, and 9). Upon completion the LV apical sheath was removed and protamine administered to reverse the effects of heparin. The LV apical suture pledgets were tied and reinforced to ensure hemostasis. The patient was extubated immediately after the procedure and transferred to the surgical intensive care unit for post-operative management.
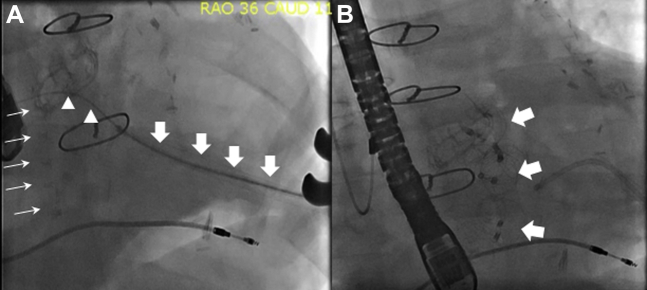
Figure 2.
Transapical PVL Closure With the Cardioform Septal Occluder
(A) Fluoroscopic examination in steep right anterior oblique (RAO) projection showing mitral bioprosthesis (thin arrows) with transapical sheath (thick arrows) and guidewire across the paravalvular leak (PVL) (arrow heads). See Video 5. (B) Fluoroscopic examination in steep RAO projection showing a total of 3 Cardioform Septal Occluders in alignment with the mitral bioprosthesis (arrows).
Online Video 5.
Fluoroscopic examination in steep RAO projection using buddy wire to advance Cardioform septal occluder
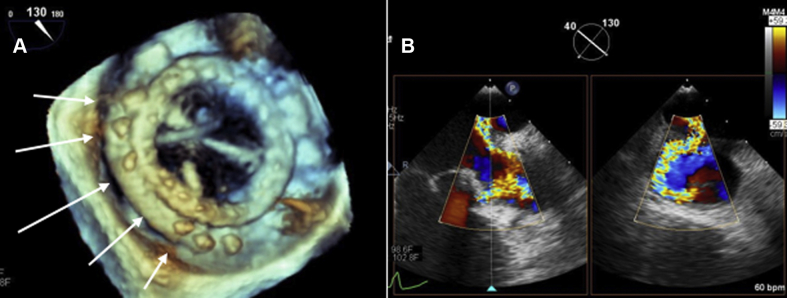
Figure 3.
Intraoperative TEE Following PVL Closure
(A) Intraoperative 3-dimensional TEE image showing mitral valve bioprosthesis with Cardioform Septal Occluder (CSO) (white arrows). See Videos 6, 7, 8, and 9. (B) Transthoracic echocardiogram examination of the mitral bioprosthesis showing CSO with no evidence of residual PVL. See Video 10. Abbreviations as in Figures 1 and 2.
Online Video 6.
Intraoperative 3D TEE plugs noted
Online Video 7.
Intraoperative 3D TEE demonstrating Cardioform device
Online Video 8.
Intraoperative TEE showing Cardioform septal device and trivial leak
Online Video 9.
Intraoperative TEE showing no evidence of PVL
Discussion
In this report, we describe our first experience with the use of a nonfenestrated Cardioform Septal Occluder to treat a giant mitral bioprosthesis PVL in a high-risk patient with refractory heart failure and transfusion-dependent hemolysis. The use of the CSO resulted in successful closure, with meaningful clinical improvement and normalization of hemolytic anemia, demonstrating the safety and feasibility of its use.
Although our patient did remarkably well technically and clinically, it would be unfitting to generalize the safety and efficacy of its use in other PVL cases. The absence of any residual PVL leak or hemolysis in our case is best explained by the soft-conformable nonfenestrated design of the CSO. When compared with the conventional Amplatzer devices, the CSO delivery sheath is more rigid and requires a bigger sheath. This limits access to a transapical open or percutaneous approach only when considered for mitral PVL closure.
Transcatheter PVL closure
Open surgical correction for the treatment of PVL is associated with high morbidity, mortality, and recurrent PVL (5). The advent of advanced percutaneous skills and imaging modalities has allowed transcatheter PVL closure to evolve. In a meta-analysis of over 360 patients, transcatheter PVL closure was associated with high technical and procedural success rates of 87% and 77%, respectively, as well as lower mortality (odds ratio: 0.08; 95% confidence interval: 0.01 to 0.90), fewer surgical reinterventions (odds ratio: 0.08; 95% confidence interval: 0.01 to 0.40), and superior improvement in NYHA functional class or hemolysis (6).
PVL closure: Device choice and percutaneous techniques
Although a variety of endovascular plugs have been tested, none are approved by the FDA for PVL closure. Among commercially available products, the Amplatzer family of plugs (St Jude Medical/Abbott Vascular) have been used most commonly with variable success and operator experience (1,3,5,7,8). Incomplete PVL closure resulting in heart failure and refractory hemolysis remains the most important limitation; this is particularly true among large complex PVL defects, in which multiple plugs are required. Despite ongoing efforts to develop PVL-specific plugs, such as the Occlutech PVL Device (Occlutech, Helsingborg, Sweden) (1,3,5,7,8), the existing limitations and failure rates are best explained by the inherent semirigid multifenestrated nitinol mesh design that results in device underexpansion, malapposition, and incomplete closure, which allows blood to travel across fenestrations at a high velocity resulting in either heart failure or residual hemolysis (4). Predicting post-operative hemolysis is challenging, as even the smallest residual jet may result in severe refractory hemolysis. Therefore, every effort should be made to eliminate any residual flow during PVL closure.
Additionally, a variety of transcatheter mitral PVL closure techniques have been developed to further improve closure rates including an antegrade transseptal, a retrograde transaortic, or a transapical approach. Recognizing the advantages and disadvantages of each is important. Among them, the transseptal approach is least invasive and therefore the most commonly employed (6). Although a thoracotomy can be avoided using this approach, its feasibility may be limited by the size and location of the defect (5). Although more invasive, the transapical approach is associated with greater closure rates regardless of the anatomical location and/or PVL defect size. The transapical approach not only provides direct access to the mitral valve, but also allows for greater use of delivery catheter and closure device options when required (9). Its use should be encouraged in those for whom an initial endovascular approach has failed.
Cardioform Septal Occluder
The Gore Cardioform Septal Occluder (CSO) is unique in its class. It is a transcatheter implant consisting of 2 soft, independent, conformable nitinol discs covered by a thin nonfenestrated expanded polytetrafluoroethylene membrane (Figure 4). It is currently FDA approved for closure of patent foramen ovale and atrial septal defect, and is associated with high closure rates and early endothelization. As with other endovascular plugs, the CSO comes in different sizes (20, 25, and 30 mm) and is delivered percutaneously using fluoroscopy and/or ultrasound imaging. When compared with the Amplatzer occluders, the CSO delivery catheter is less flexible and requires a larger sheath size (11-F). These physical properties limit its use and deliverability in some instances, and explains why in our patient, the device was employed through a more invasive transapical approach. The use of a 20-mm CSO in our case resulted in successful PVL closure with no evidence of prosthetic valve dysfunction. As with other plugs, we encourage a systematic evaluation of the prosthetic mitral valve with TEE before the device is released to ensure the absence of any valvular dysfunction; this may be particularly important in patients with mechanical prosthesis.
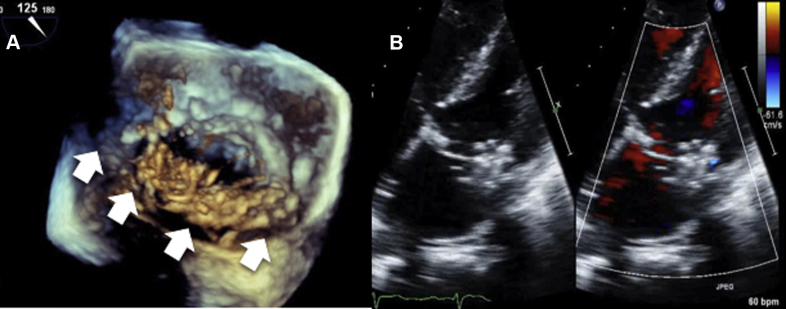
Figure 4.
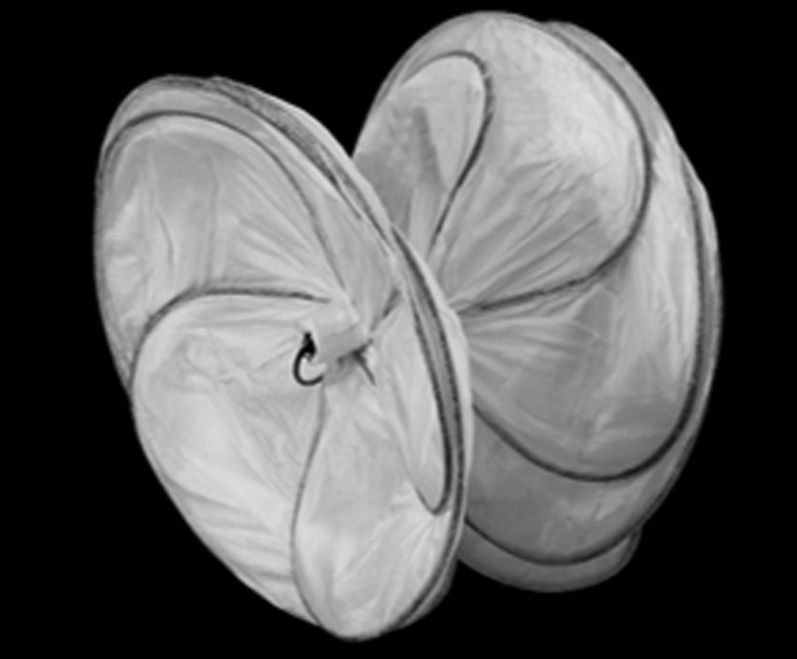
Gore Cardioform Septal Occluder
Follow-Up
The post-operative course was benign, and the patient was discharged on aspirin 81 mg 5 days later. Transthoracic echocardiography on days 1 and 30 showed a trivial residual PVL with no evidence of device migration or thrombus (Figure 3B, Video 10). Remarkable clinical improvement ensued with a reduction in NYHA functional class from IV to II. Serum biomarkers revealed normalization in hemolytic anemia (lactate dehydrogenase: 222 U/l, haptoglobin: 48 mg/dl, reticulocyte count: 2.1%).
Online Video 10.
Transthoracic Echocardiogram 1-month post-op
Conclusions
Percutaneous PVL closure, although challenging, represents an attractive alternative procedure to surgery. The fenestrated vascular plugs that are commonly used often result in incomplete closure and residual hemolysis. This case illustrates the feasibility of a nonfenestrated CSO for the management of large mitral PVL complicated by intractable heart failure and hemolysis.
Footnotes
Dr. Navia is an inventor for and stockholder in NaviGate Cardiac Structures. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, or patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
References
- 1.Calvert P., Northridge D., Malik I. Percutaneous device closure of paravalvular leak: combined experience from the United Kingdom and Ireland. Circulation. 2016;134:934–944. doi: 10.1161/CIRCULATIONAHA.116.022684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hourihan M., Perry S., Mandell V. Transcatheter umbrella closure of valvular and paravalvular leaks. J Am Coll Cardiol. 1992;20:1371–1377. doi: 10.1016/0735-1097(92)90250-q. [DOI] [PubMed] [Google Scholar]
- 3.Nietlispach F., Johnson M., Moss R. Transcatheter closure of paravalvular defects using a purpose-specific occluder. J Am Coll Cardiol Intv. 2010;3:759–765. doi: 10.1016/j.jcin.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura R., Otto C., Bonow R. AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2438–2488. doi: 10.1016/j.jacc.2014.02.537. [DOI] [PubMed] [Google Scholar]
- 5.Okutucu S., Mach M., Oto A. Mitral paravalvular leak closure: transcatheter and surgical solutions. Cardiovasc Revasc Med. 2019 Jun 28 doi: 10.1016/j.carrev.2019.06.012. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Millán X., Skaf S., Joseph L. Transcatheter reduction of paravalvular leaks: a systematic review and meta-analysis. Can J Cardiol. 2015;31:260–269. doi: 10.1016/j.cjca.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Garcia E., Arzamendi D., Jimenez-Quevedo P. Outcomes and predictors of success and complications for paravalvular leak closure: an analysis of the SpanisH real-wOrld paravalvular LEaks closure (HOLE) registry. EuroIntervention. 2017;12:1962–1968. doi: 10.4244/EIJ-D-16-00581. [DOI] [PubMed] [Google Scholar]
- 8.Zorinas A., Janusauskas V., Davidavicius G. Retrospective analysis of single-center early and midterm results of transapical catheter-based mitral paravalvular leak closure with a purpose-specific device. Postepy Kardiol Interwencyjnej. 2018;14:167–175. doi: 10.5114/aic.2018.76408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidavicius G., Rucinskas K., Drasutiene A. Hybrid approach for transcatheter paravalvular leak closure of mitral prosthesis in high-risk patients through transapical access. J Thorac Cardiovasc Surg. 2014;148:1965–1969. doi: 10.1016/j.jtcvs.2014.05.001. [DOI] [PubMed] [Google Scholar]