Abstract
We describe the endovascular management of a middle-aged woman who developed a bleeding suprasternal fistula after conventional aortic valve replacement. The patient’s condition was considered inoperable. A customized stent attached to a transcatheter valve was successfully used to treat the individual, this being the first-in-human case of the promising Endo-Bentall procedure. (Level of Difficulty: Advanced.)
Key Words: aorta, aortic valve, coronary circulation, left-sided catheterization, valve replacement
Abbreviations and Acronyms: ASA, acetylsalicylic acid; CT, computed tomography; ECMO, extracorporeal membrane oxygenation; NYHA, New York Heart Association; STJ, sinus tubular junction; TAVR, transcatheter aortic valve replacement
Graphical abstract
This paper describes the endovascular management of a middle-aged woman who developed a bleeding suprasternal fistula after conventional aortic valve…
History of Presentation
A 64-year-old woman presented to the emergency department with repeated “bleeding in her neck” for 2 years. She was hemodynamically stable with no active signs of infection. She reported no other symptoms, including those of heart failure. She was heavily sedentary and engaged in self-limitation. On physical examination, there was a small yet actively bleeding cutaneous orifice in the suprasternal notch. Additionally, she had a 2/6 systolic crescendo-decrescendo murmur on auscultation.
Learning Objectives
-
•
The ascending aorta is one of the last frontiers for endovascular and catheter-based management.
-
•
Innovative procedures developed through solid industry partnerships may allow previously undertreated patients to receive appropriate care if they are carefully selected and procedures are carefully planned and performed.
Surgical history
The patient had undergone conventional surgical aortic valve replacement in 2014. A bioprosthetic Carpentier-Edwards Perimount Magna 23-mm aortic xenograft (Edwards Lifesciences, Irvine, California) was implanted then after a diagnosis of severe aortic stenosis. Symptoms improved from New York Heart Association (NYHA) functional class IV before surgery to NYHA functional class I afterwards. The patient had been lost to follow-up since. The patient presented at the authors’ institution with visible skin bleeding arising from the suprasternal notch.
Differential diagnosis
Possible differential diagnoses included true aneurysm from the aorta (or arch branches) and a pseudoaneurysm arising from the previous aortotomy.
Investigations
A computed tomography (CT) angiogram revealed a pseudoaneurysm in the suture line of the ascending aorta. The pseudoaneurysm had, over time, fistulized with the skin, leading to the bleeding orifice. The same test results showed a severely calcified porcelain aorta. Echocardiography was ordered, given the murmur, and showed a 28-mm Hg mean gradient with reduced leaflet mobility. Further evaluation included a coronary angiogram, which showed no lesions in the coronary arteries, and carotid and vertebral Doppler ultrasonograms which showed no atherosclerosis.
Management
Initially, percutaneous closure of the pseudoaneurysm was attempted, using Amplatzer vascular occluders (Abbott Laboratories, Lake Bluff, Illinois). In total, 7 devices were deployed inside the lesion without success (Figure 1). Given the porcelain aorta and a EuroSCORE of 28.5%, conventional surgery was contraindicated by multiple surgical teams.
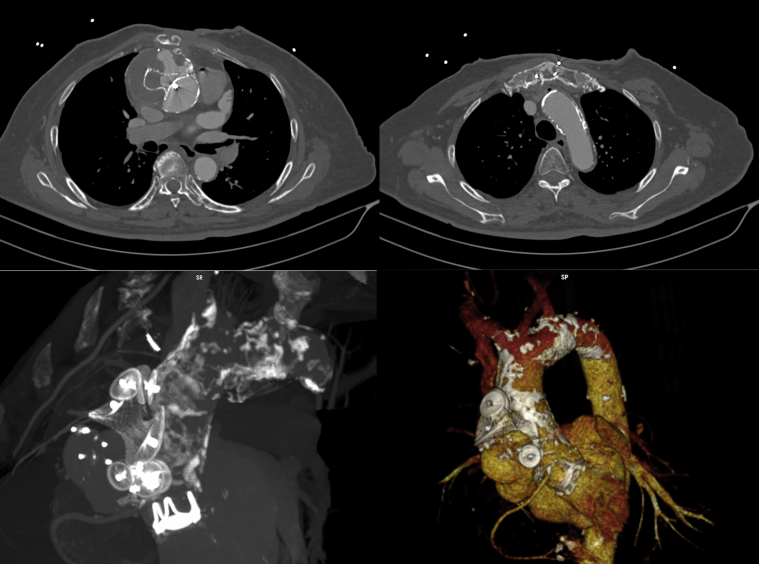
Figure 1.
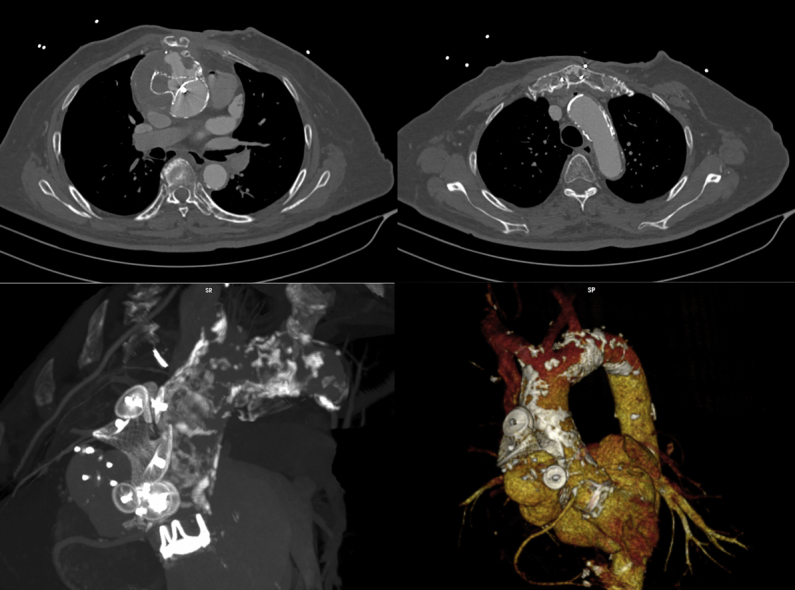
Pre-Operative Computed Tomography
Three-dimensional reconstruction demonstrates an ascending aortic pseudoaneurysm, porcelain aorta, and multiple vascular plugs.
The case was brought to the institutional heart team, to whom the authors proposed a compassionate and completely endovascular procedure that was able to simultaneously treat the failing aortic valve and the pseudoaneurysm in the ascending aorta, mimicking a Bentall-De Bono procedure, dubbed the Endo-Bentall procedure (Figure 2). The patient provided informed consent, and ethical approval of the Institutional Review Board was obtained.
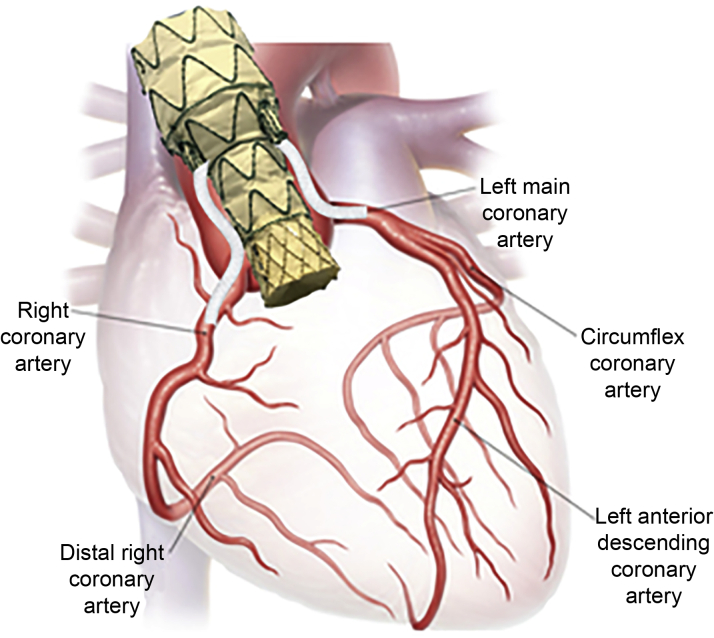
Figure 2.
Endo-Bentall Prosthesis Concept Illustrated
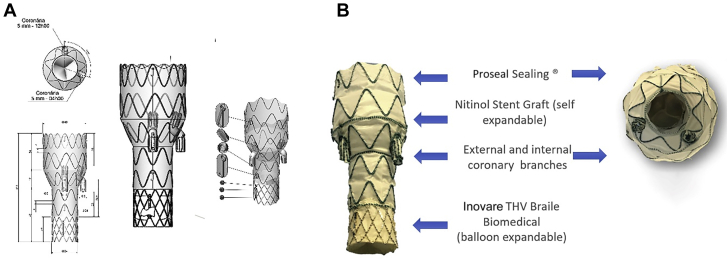
A custom-made device was designed (Figure 3), based on the patient’s CT, including in its proximal segment a balloon-expandable transcatheter prosthesis connected to a self-expandable aortic stent. The transcatheter valve is a modified 22-mm Braile Inovare (Braile Biomedica, São José do Rio Preto, Brazil), with a lozenge chromium-cobalt frame, 20 mm in height, with 3 radiopaque markers identifying the base, valve, and skirt, and a single sheet of bovine pericardium (1).
Figure 3.
Endo-Bentall Prosthesis
(A) Prosthesis design with coronary branches and key reference markers. (B) Prosthesis final characteristics.
The main stent body had 2 branches positioned with internal and external segments, in such a way that after its release, the branches were 2 cm to the coronary ostia. At this height, the graft is reduced in diameter to the same size of the transcatheter aortic valve replacement (TAVR) (22 mm), thus providing additional space for coronary access and bridging stent deployment.
The diameter of the prosthesis at the distal ascending aorta segment was 40 mm, considering an oversize of approximately 20% (33 mm distal aorta). The distance between the sinus tubular junction (STJ) and the brachiocephalic trunk was approximately 6.3 cm, allowing a prosthetic segment length of 34 mm × 40 mm in diameter, to be used to reduce the endoleak risk. Additionally, a proprietary Proseal (Braile Biomedical) sealing was applied to the distal prosthesis segment. The defect was a circular-shaped tear located 6 mm below the STJ, originating posteriorly near the noncoronary sinus and extending anteriorly and superiorly. Distance from the right coronary artery to the defect was just 5 mm, making isolated aortic stenting impossible due to the absence of an adequate proximal landing zone.
Connection stents would then ensure coronary flow. The full set of devices was tested in a 3-dimensional (3D) printed model of the patient’s aorta, using fluoroscopy.
After the patient was pre-medicated with acetylsalicylic acid (ASA) (200 mg) and clopidogrel (75 mg), anticoagulation with unfractionated heparin (4 mg/kg) was initiated. A temporary pacemaker was positioned in the right ventricle. Two 0.014-inch guidewires were positioned in the right and left coronary arteries. A pigtail was also positioned in the aortic root with the aid of a guidewire (Video 1).
Online Video 1.
Pre-deployment aortography.
The left ventricular apex was punctured with a 6-F introducer. A stiff guidewire was used to cross the failed bioprosthetic aortic valve, and the introducer was exchanged for a 30-F introducer. Rapid pacing was used to achieve controlled hypotension. The main body of the stent was slowly deployed in the ascending aorta (Video 2), and afterward, the balloon was expanded to inflate the transcatheter heart valve (Video 3). Pressure was then restored. Both transesophageal echocardiography and angiography showed effective release (Video 4). The ventricular apex was then closed. Extracorporeal membrane oxygenation (ECMO) support was initiated prior to any attempts at coronary cannulation.
Online Video 2.
Main body deployment of a self-expandable portion.
Online Video 3.
Main body deployment of a balloon-expandable portion (transcatheter heart valve).
Online Video 4.
Aortography after the main body is completely deployed with full exclusion of the pseudoaneurysm.
The right coronary artery was cannulated first through the inside of the main stent body by using a 6- × 60-mm Fluency stent (Becton Dickinson, Franklin Lakes, New Jersey) (Videos 5 and 6). In the left coronary artery, the Fluency stent positioning was unsuccessful. The operator switched to a 7- × 57-mm Ventus BX stent (Jotec GmbH, Hechingen, Germany), which worked adequately (Videos 7 and 8).
Online Video 5.
Right coronary connection stent deployment.
Online Video 6.
Right coronary connection stent angiography.
Online Video 7.
Left coronary connection stent deployment.
Online Video 8.
Left coronary connection stent angiography.
A final angiogram showed no leaks and patent ostia. Control echocardiography showed normal valve function with a low mean gradient of 10 mm Hg. Main procedural steps are shown in Figure 4. Total procedural time was 390 min. The patient was transferred to the intensive care unit for 4 days. A new CT angiogram was ordered, documenting zero endoleak and patent coronary connecting stents. After a week of hospital stay, the patient was discharged home with a prescription for ASA (200 mg) and clopidogrel (75 mg). There was no myocardial infarction, vascular complications, stroke, conduction abnormalities, or renal failure.
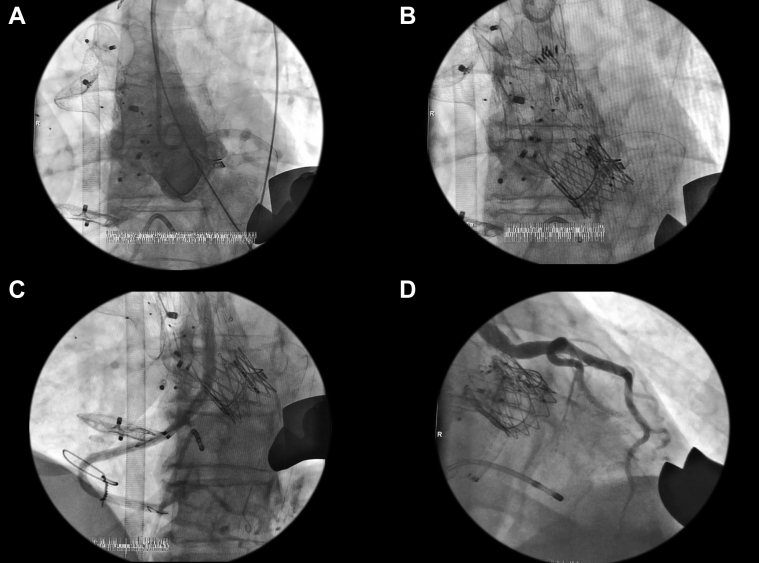
Figure 4.
Main Procedural Steps
(A) Initial angiography shows pseudoaneurysm. (B) Main body is deployed by using a transapical approach. (C) Right coronary stent connection angiogram with no endoleak and adequate flow distal to the right coronary artery. (D) Left coronary stent connection angiogram with no endoleak and adequate flow to distal to the left coronary artery.
Discussion
Conventional management for this case would be the Bentall-De Bono procedure, replacing the ascending aorta and the failed aortic bioprosthesis. The procedure is safe and has worldwide experience (2). However, many patients are denied surgery, particularly for reasons that would preclude aortic cross-clamping (3) and elevated risk scores. The authors’ new technique offers therapeutic opportunity for patients with these seemingly inoperable conditions and was developed through careful procedural planning and close work with industry partners.
There are still issues that may prevent the wide application of this technique. Only customized devices are currently available because of the wide variability in aortic complex anatomy. Some degree of dilation is needed to allow catheter manipulation and cannulation of ostia. Most of the procedural time was spent attempting to cannulate the ostia, as it was necessary to first cannulate the main stent branch and afterward the coronary artery. Also, there are no dedicated devices for bridging the main stent body and coronary arteries. Long-term patency is questionable. TAVR durability is also a concern considering patient age. The use of multiple TAVR-in-TAVR procedures should be an alternative in this case as the initial bioprosthesis is a 23-mm device with a true inner diameter of 21 mm. A supra-annular device should be considered in this scenario in order to provide a lower post-operative gradient if a TAVR-in-TAVR procedure is needed.
Catheter sizing was also a limiting factor. Certainly, smaller devices could have been introduced through the femoral artery. However, coaxial adjustment of the device would have been much more complex. Therefore, in the authors’ early experience with this technique, they would recommend transapical access. Additionally, there was no evidence of instability during the procedure, but it was decided to use ECMO even for a short time to ensure there would be no issues if any complications were to arise. Finally, there is no established antiaggregation protocol for such a procedure. Need for anticoagulation is also not established. It was decided to use double-antiplatelet therapy in light of prior experience and recommendations for both transcatheter devices and coronary interventions.
Follow-up
At 9 months, the patient was alive, without evidence of myocardial infarction, asymptomatic, and well. Control CT (at 4 months) shows sustained results (Figure 5).
Figure 5.
Control Computed Tomography at 4-Month Follow-Up
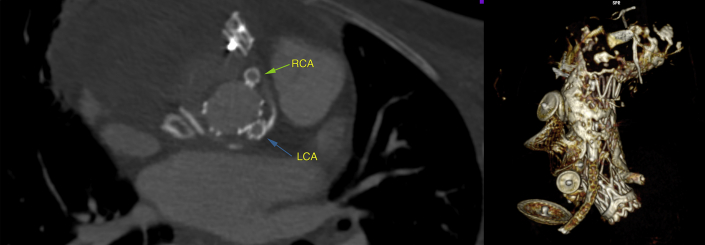
Right and left coronary stents are patent. Additionally, the pseudoaneurysm is fully excluded without endoleaks. Finally, the prosthesis is reconstructed in 3 dimensions. Left main = left coronary connection stent (blue arrow); RCA = right coronary artery connection stent (green arrow).
Conclusions
The Endo-Bentall is a feasible procedure that should be reserved for compassionate use in highly selected patients without a conventional surgical approach. It represents an important step in the management of combined valvular and ascending aortic disease. Follow-up needs to be continued to ascertain benefits and complications and to further improve patient selection.
Footnotes
Drs. Gaia and Palma are proctors for Braile Biomedical Prosthesis. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, or patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
References
- 1.Gaia D.F., Breda J.R., Duarte Ferreira C.B. New Braile Inovare transcatheter aortic prosthesis: clinical results and follow-up. EuroIntervention. 2015;11:682–689. doi: 10.4244/EIJV11I6A136. [DOI] [PubMed] [Google Scholar]
- 2.Urbanski P.P., Heinz N., Zhan X., Hijazi H., Zacher M., Diegeler A. Modified bio-Bentall procedure: 10-year experience. Eur J Cardiothorac Surg. 2010;37:1317–1321. doi: 10.1016/j.ejcts.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Iung B., Cachier A., Baron G. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J. 2005;26:2714–2720. doi: 10.1093/eurheartj/ehi471. [DOI] [PubMed] [Google Scholar]