Abstract
Grapevine downy mildew, evoked by the obligate biotrophic oomycete Plasmopara viticola, is one of the most challenging diseases in viticulture. P. viticola establishes an infection by circumvention of plant immunity, which is achieved by the secretion of effector molecules. One family of potential effectors are the necrosis- and ethylene-inducing peptide 1 (Nep1)-like proteins (NLP). NLP are most abundant in plant pathogenic microorganisms and exist in cytotoxic and non-cyctotoxic forms. Cytotoxic NLP often act as virulence factors and are synthesized in necrotrophic or hemibiotrophic pathogens during the transition from biotrophic to necrotrophic growth. In addition to these cytotoxic NLP, many non-cytotoxic NLP have been identified; their function in biotrophic pathogens is still unknown. In 2020, eight different NLP coding genes were identified in P. viticola and named PvNLP1 to PvNLP8 (Plasmopara viticola NLP 1–8). In the present study, PvNLP4 to PvNLP8 were characterized by using qPCR analysis and transient expression in the model plant Nicotiana benthamiana. Gene expression analysis showed high PvNLP expression during the early stages of infection. Necrosis-inducing activity of PvNLP was not observed in the nonhost N. benthamiana.
Keywords: Plasmopara viticola, NLP, necrosis and ethylene inducing peptide 1, grapevine, downy mildew
1. Introduction
Necrosis- and ethylene-inducing peptide 1 (Nep1)-like proteins (NLP) have been identified in almost 500 different species of bacteria, fungi and oomycetes [1]. Phylogenetic and amino acid sequence analysis revealed conserved patterns that affect the tertiary structure of these proteins and allow for the further division of NLP into three different types [2,3]. With few exceptions, all NLP from oomycetes are type 1 NLP. These NLP share two conserved cysteine residues and can be further divided into type 1 and type 1a by the presence or absence of a cation-binding pocket, respectively. The disulfide bridge between these cysteine residues as well as the cation-binding pocket are essential for the necrosis-inducing ability of these NLP [4,5]. Type 2 NLP, previously observed from fungi and bacteria, were just recently discovered in oomycetes [1]. These NLP share four conserved cysteine residues and, besides the cation-binding pocket, also share a predicted Ca2+-binding site. This Ca2+-binding pocket is necessary to induce necrosis, while the second disulfide bridge has no influence on the cytotoxicity of these proteins [3]. A third class of NLP, classified as type 3, consists of proteins which are less conserved then the other types but normally share three conserved disulfide bridges and have so far only been identified in ascomycete fungi and bacteria [3]. Another conserved region in NLP is a 24 amino acid peptide (nlp24) which is recognized as a pathogen-associated molecular pattern (PAMP) in several plants and consequently induces common pattern-triggered immunity (PTI) responses [6,7]. However, the responsible receptor complex RLP23-SOBIR1-BAK1 was so far only found in some species of Brassicaceae and Asteraceae [8]. Even though NLP are widely distributed in prokaryotic and eukaryotic microorganisms, their majority was discovered in plant pathogenic oomycetes and ascomycetous fungi [1]. While bacteria or non-phytopathogenic microorganisms mostly encode only one or two of these proteins, oomycetes have acquired up to 70 throughout gene duplication [1]. NLP are assumed to have different functions, possibly depending on the lifestyle of the pathogen. Cytotoxic NLP, e.g., from the bacterium Pectobacterium carotovorum or the fungus Verticillium dahliae, can act as virulence factors for their pathogens and are usually expressed during phases of their necrotrophic lifestyle [9,10]. Deletion of these NLP in their hosts clearly reduced their virulence on potato tubers or tomato, respectively. Vice versa, overexpression of a Fusarium oxysporum NLP in Colletotrichum coccodes resulted in increased virulence of the fungus [11]. Furthermore, in Colletotrichum species a correlation of increasing numbers of NLP with a broader host range is assumed [12]. However, inactivation of NLP in Fusarium oxysporum or Magnaporthe oryzae had no effect on the virulence of these pathogens on coca or rice plants, respectively [13,14]. Besides cytotoxic NLP, a large number of non-cytotoxic NLP with a so far unknown function is known from fungi and oomycetes. The obligate biotrophic downy mildew pathogens Hyaloperonospora arabidopsidis (HaNLP) and Plasmopara viticola (PvNLP), for example, have only non-cytotoxic NLP [15,16]. However, characterization of PvNLP was so far limited to PvNLP2 (KC243261), PvNLP3 (MN720268) and the pseudogene PvNLP1 (MN564834). In order to fill this gap, further characterization of PvNLP4–PvNLP8 is presented here.
2. Materials and Methods
The genes of PvNLP4 (MT722075), PvNLP5 (MT722076), PvNLP6 (MT722077), PvNLP7 (MT722078) and PvNLP8 (MT722079) were amplified from an isolate collected in a vineyard in Freiburg (Germany) with the corresponding primers (Table 1) and sequenced as published before [16].
Table 1.
Oligonucleotides used for sequencing PvNLP genes.
| Primer Name | Sequence (5′→3′) |
|---|---|
| PvNLP4-ORF-F | ATGTCGCGGACCATGCAATC |
| PvNLP4-ORF-R | TTAAAAAGGGTACGATTCGTGG |
| PvNLP5-ORF-F | ATGCGCGTCCTAGTATTGTGTG |
| PvNLP5-ORF-R | TTAGAAGGGTCGCGCTTTCTC |
| PvNLP6-ORF-F | ATGCGCACCACGAGCCCATACTC |
| PvNLP6-ORF-R | TTATCGCACACTTTTCGAGGG |
| PvNLP7-ORF-F | ATGCACCTTTGTGCTCTTCTC |
| PvNLP7-ORF-R | TTAAAACGGATACGCCTCCTTC |
| PvNLP8-ORF-F | ATGCACTTGACAGTCTTTTAC |
| PvNLP8-ORF-R | TTAGAATGGATAGGATTTACGAAG |
To identify possible sequence differences in PvNLP between the different isolates, sequence comparisons were performed with the three available genome assemblies, INRA-PV221 (GenBank: GCA_001695595.3), PvitFEM01 (GenBank: GCA_003123765.1), and JL-7-2 (GenBank: GCA_001974925.1)
To investigate PvNLP gene expression during the infection process, experiments were performed as published [16] (pp. 3–4) with the Biozym Blue S’ Green qPCR Kit (Biozym Scientific GmbH, Hessisch Oldendorf, Germany) according to the manufacturer’s instructions and the corresponding primers (Table 2) [16].
Table 2.
Oligonucleotides used for qPCR.
| Primer Name | Sequence (5′→3′) |
|---|---|
| PvNLP4-qPCR-F | CAACCATTCCTTGTTTCTCCTGCTAC |
| PvNLP4-qPCR-R | GAAGGGCGATCTCGTTAATTTGGC |
| PvNLP5-qPCR-F | TCCTAGTATTGTGTGCCGCTGTC |
| PvNLP5-qPCR-R | GTGGCTTGAACATCAGTGCGA |
| PvNLP6-qPCR-F | AGCCCATACTCGCACTGTTCTC |
| PvNLP6-qPCR-R | CAGCGGCTTATACTTGACGGC |
| PvNLP7-qPCR-F | TCAAAGCACACAAAGCTCAAGTCG |
| PvNLP7-qPCR-R | GTCAAGTGTCAAAGCAGTCTGTGC |
| PvNLP8-qPCR-F | TGTCCCACCACTACTTGACTGC |
| PvNLP8-qPCR-R | GATAGGATTTACGAAGAGCGCGGAA |
| PvRXLR28-qPCR-F | AGTAACCAGCCCTTACAGACTCC |
| PvRXLR28-qPCR-R | CTCGTTTGGACTTTGCTCACCTTC |
Gene expression of PvNLP4–PvNLP8 was monitored over the whole course of infection according to stages defined before [16] (pp.3–4) from 1 to 144 h post inoculation (hpi), and PvRXLR28 (KX010958) was monitored as expression pattern reference [17].
To study the cytotoxic characteristics of PvNLP4–PvNLP8 the corresponding genes were amplified with the appropriate primers (Table 3) and transiently expressed in N. benthamiana as published before [16]. To verify the functionality of the experiments, a necrosis-inducing NLP from P. infestans (PiNPP1.1; AY961417) as well as the necrosis-inducing effector RXLR35 (P0CV04) from P. viticola were expressed as positive controls [18,19]. Agrobacteria carrying the respective plasmids were co-infiltrated with the silencing suppressor p19 [20]. To exclude an effect of p19, the plasmid was infiltrated alone as negative control.
Table 3.
Oligonucleotides used for cloning binary vectors for transient expression in N. benthamiana.
| Primer Name | Sequence (5′→3′) |
|---|---|
| XhoI-PvNLP 4–EcoRI-F | AACTCGAGATGTCGCGGACCATGCAATC |
| XhoI-PvNLP 4–EcoRI -R | AAAGAATTCTTAAAAAGGGTACGATTCGTGG |
| XhoI-PvNLP 5–EcoRI-F | AACTCGAGATGCGCGTCCTAGTATTGTGTG |
| XhoI-PvNLP 5–EcoRI-R | AAAGAATTCTTAGAAGGGTCGCGCTTTCTC |
| XhoI-PvNLP 6–HindIII-F | AACTCGAGATGCGCACCACGAGCCCATACTC |
| XhoI-PvNLP 6–HindIII-R | CCCAAGCTTTTATCGCACACTTTTCGAGGG |
| NotI-PvNLP 7–XhoI-F | AATGCGGCCGCATGCACCTTTGTGCTCTTCTC |
| NotI-PvNLP 7–EcoRI-R | AAAGAATTCTTAAAACGGATACGCCTCCTTC |
| NotI-PvNLP 8–XhoI-F | AATGCGGCCGCATGCACTTGACAGTCTTTTAC |
| NotI-PvNLP 8 –EcoRI-R | AAAGAATTCTTAGAATGGATAGGATTTACGAAG |
| XhoI-PvRXLR35–EcoRI-F | AACTCGAGATGCGTGGTGCGTATTACATC |
| XhoI-PvRXLR35–EcoRI-R | AAAGAATTCTTACGAAGCTTTGTCAGTCCTT |
3. Results & Discussion
3.1. Sequence Analysis of PvNLP4–PvNLP8
Sequence analysis of the genes PvNLP4 (MT722075), PvNLP5 (MT722076), PvNLP6 (MT722077), PvNLP7 (MT722078) and PvNLP8 (MT722079) showed several differences compared to the genes inside the reference genomes INRA-PV221 (GenBank: GCA_001695595.3), PvitFEM01 (GenBank: GCA_003123765.1), and JL-7-2 (GenBank: GCA_001974925.1). PvNLP4 shows one silent point mutation (g159 → c159). PvNLP5 is identical to the corresponding sequences in INRA-PV221 and PvitFEM01, but carries the silent mutation c48 → t48 compared to JL-7-2. PvNLP6 was only identified in INRA-PV221 [16]. The gene sequenced during this work carries 12 point mutations: t48 → c48, c52 → g52, c216 → t216, t273 → c273, c372 → t372, a399 → t399, t400 → a400, a427 → g427, a448 → g448, a458 → t458, c654 → t654 and a657 → g657. While eight mutations are silent, five result in the amino acid substitutions R18 → G18, L134 → I134, I143 → V143, K150 → E150, D153 → V153. Only two of these alterations, L134 → I134 and I143 → V143, retain the nonpolar and hydrophobic properties of the original amino acids. The amino acid substitution L134 → I134 is located inside the GHRHDWE heptapeptide motif where G134 is replaced with L134 in INRA-PV221 [16]. Type 1 NLP have been divided into type 1 NLP and non-cytotoxic type 1a NLP based on the occurrence of amino acid substitutions in the GHRHDWE heptapeptide motif [3]. The heptapeptide motif is part of a negatively charged cavity implicated in cation binding (Mg2+) and a specific interaction with the sphingolipid glycosyl inositol phosphorylceramide (GIPC) [5,21]. Therefore, the heptapeptide motif is essential for the cytotoxic activity of NLP [5]. However, the heptapeptide motif is prone to mutations in type 1a NLP, such as PvNLP6, and probably not essential for any additional function other than necrosis induction [3]. The amino acid substitution I143 → V143 in PvNLP6 is also highly conserved within the central region of the NPP1 (necrosis-inducing Phytophthora protein) domain [2]. Gijzen and Nürnberger [2] (p. 1802) highlighted the alterations of the first five amino acids following the heptapeptide motif. The third of these five amino acids corresponds to V143 of PvNLP6. In the majority of all analyzed sequences a valine was conserved at this position, followed by alanine as the second and isoleucine as the third most conserved amino acid [2]. The amino acid substitutions K150 → E150 and D153 → V153 alter the chemical properties of the protein and may therefore have a direct influence on the tertiary structure and the functionality of the protein. However, both amino acids are located in a non-conserved region of type 1 / 1a NLP, which was so far not directly linked to any function [3]. The substitution R18 → G18 is located inside the signal peptide. Since the signal peptide is cleaved off after the secretion process, it has no direct effect on the functionality of the protein. The D-score predicted by SignalP is affected only marginally and changes from 0.8795 to 0.8449 [16] (p.5), therefore this substitution was also assumed as uncritical.
PvNLP7 was identified in INRA-PV221 and JL-7-2 [16]. The PvNLP7 sequenced in this study is identical to the corresponding sequence in INRA-PV221 but shows the seven mutations a112 → t112, g237 → a237, a375 → t375, t492 → c492, t525 → c525, c702 → t702 and c723 → t723 in J-L-7-2. Six of these mutations are silent while one results in the amino acid substitution T112 → S112. This change in PvNLP7 involves two neutral amino acids with hydrophilic characteristics and its influence on the function of the protein is assumed as low. The PvNLP8 amplified during this study is identical to the sequences from INRA-PV221 and PvitFEM01 and was not identified in JL-7-2 [16]. In summary, all genes could be amplified in the present field isolate. Previous work showed that none of the three reference genomes contains all eight PvNLP [16]. However, it is not clear if this divergence is isolate dependent or caused by technical issues during assembling of the reference genomes.
3.2. Gene Expression Analysis
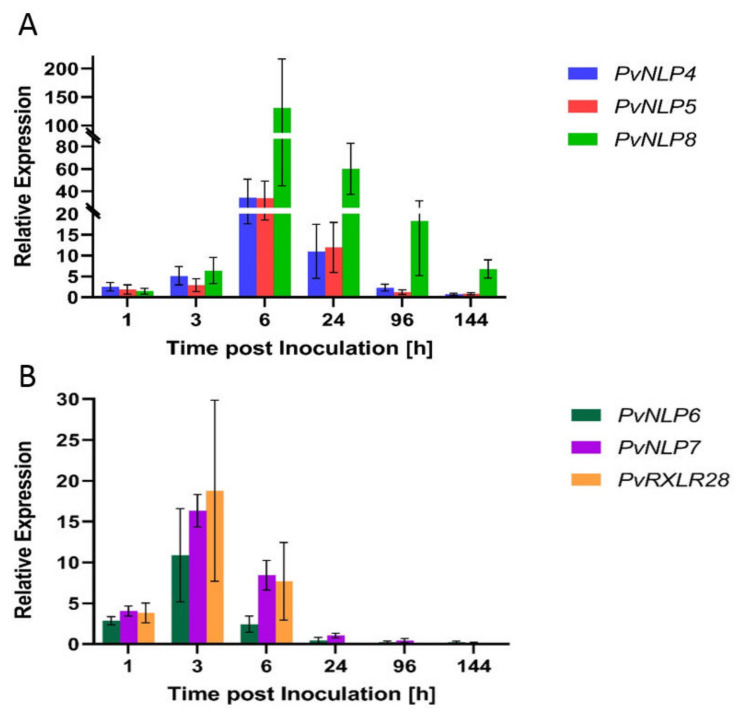
All analyzed genes were activated shortly after contact of sporangia with water (Figure 1). PvNLP4 and PvNLP5 showed increased expression levels over the first 24 hpi. Levels increased from this time point on, when germ tubes entered the leaf (3 hpi), and the maximum expression level of both genes was reached during the formation of first haustoria (6 hpi). Thereafter, the expression levels decreased during branching of hyphae (24 hpi) to a not detectable level. This expression pattern was also detected for PvNLP8, the strongest induced gene during this study. However, the expression of PvNLP8 stayed elevated when P. viticola begins to infect adjacent interveinal leaf zones (96 hpi) until de novo formation of sporangia (144 hpi). Expression over the whole course of infection was also observed for PvNLP2 and PvNLP3 suggesting a different function of these proteins not only at the beginning of the infection [16]. By contrast, PvNLP6 and PvNLP7 as well as PvRXLR28, are expressed during the first six hours after inoculation. Expression starts with the release of zoospores from sporangia (1 hpi) and culminates when germ tubes enter the leaf (3 hpi). Once the formation of first haustoria occurs (6 hpi), expression decreases to a not detectable level. PvRXLR28, which served as reference for early induced effectors [17] (p.4), showed highest expression levels 3 hpi. This time point was not analyzed in the original work but PvRXLR28 is described to be expressed during the first 12 hpi, showing an expression peak at 6 hpi and declining afterwards to an undetectable level at 24 hpi [17]. This change in gene expression was also observed in this study, indicating a similar expression pattern in the isolate used in this study and in the Chinese isolate ZJ-1-1.
Figure 1.
Expression of PvNLP during infection of Vitis vinifera cv. Mueller-Thurgau leaf cells: (A) Relative expression of PvNLP4, PvNLP5 and PvNLP8. (B) Relative expression of PvNLP6, PvNLP7 and PvRXLR28. The displayed graphs represent one of four experiments with similar results. Bars are the mean of four technical replicates ± standard deviation (SD).
3.3. Necrosis-Inducing Activity
Cytotoxic characteristics of PvNLP4-PvNLP8 were studied by agrobacterium-mediated transient expression in N. benthamiana. None of the five PvNLP (PvNLP4, PvNLP5, PvNLP6, PvNLP7 or PvNLP8) were able to induce necrosis in N. benthamiana (Figure 2). Spots infiltrated with the positive control PiNPP1.1 and PvRXLR35 showed beginning chlorosis after 48 h, which developed into necrotic tissue within the next 24 h. Trypan blue staining was performed at 72 h post infiltration to visualize cell death, indicating beginning necrosis formation on the infiltration sites of PiNPP1.1 and PvRXLR35. Infiltration sites with PvNLP were observed for a period of 14 days. During this time no necrosis formation occurred. None of the PvNLP or p19 infiltration sites showed a HR with trypan blue staining. The results are in line with the previous published results [16] (p.10) for PvNLP1, PvNLP2 and PvNLP3, which were also not able to induce necrosis. It should be considered that transient gene expression in tobacco is a non-host-specific method to determine the cytotoxicity of an NLP. However, many NLP were analyzed by transient expression in tobacco in the past, and this method was suggested as highly efficient for this purpose [1,2,3]. Thus, this experiment shows that all analyzed PvNLP that were predicted to be non-cytotoxic are in fact not capable of causing necrosis in plants. Taken together, the exact role of non-cytotoxic NLP remains unclear and further biochemical, and structural studies (e.g., the determination of their crystal structures, Co-immunoprecipitations and binding assays with different substrates) are needed to determine the molecular functions of noncytotoxic NLP [1].
Figure 2.
PvNLP are not able to induce necrosis in N. benthamiana: (a) Transient expression of NLP in leaves of N. benthamiana; (b) Trypan Blue staining for cell death. All pictures were taken 72 h post infiltration. (p19 = silencing suppressor; NPP1 = PiNPP1.1; RXLR35 = PvRXLR35, NLP (4–8) = PvNLP4–8).
Acknowledgments
We thank Katrin Grosser and Alicia Heitzler for excellent technical assistance.
Author Contributions
Conceptualization, R.F. and S.S.; methodology, R.F. and S.S.; software, L.A., R.F. and S.S.; validation, L.A., R.F. and S.S.; investigation, L.A.; resources, R.F.; writing—original draft preparation, S.S.; writing—review and editing, L.A. and R.F.; visualization, L.A.; supervision, R.F. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The sequencing data has been uploaded to GenBank. DNA sequences of PvNLP4, PvNLP5, PvNLP6, PvNLP7 and PvNLP8 can be found using accession numbers MT722075-MT722079.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Seidl M.F., Van Den Ackerveken G. Activity and Phylogenetics of the Broadly Occurring Family of Microbial Nep1-like Proteins. Annu. Rev. Phytopathol. 2019;57:367–386. doi: 10.1146/annurev-phyto-082718-100054. [DOI] [PubMed] [Google Scholar]
- 2.Gijzen M., Nürnberger T. Nep1-like Proteins from Plant Pathogens: Recruitment and Diversification of the NPP1 Domain across Taxa. Phytochemistry. 2006;67:1800–1807. doi: 10.1016/j.phytochem.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Oome S., Van den Ackerveken G. Comparative and Functional Analysis of the Widely Occurring Family of Nep1-Like Proteins. Mol. Plant-Microbe Interact. 2014;27:1081–1094. doi: 10.1094/MPMI-04-14-0118-R. [DOI] [PubMed] [Google Scholar]
- 4.Fellbrich G., Romanski A. NPP1, a Phytophthora-Associated Trigger of Plant Defense in Parsley and Arabidopsis. Plant J. 2002;32:375–390. doi: 10.1046/j.1365-313X.2002.01454.x. [DOI] [PubMed] [Google Scholar]
- 5.Ottmann C., Luberacki B. A Common Toxin Fold Mediates Microbial Attack and Plant Defense. Proc. Natl. Acad. Sci. USA. 2009;106:10359–10364. doi: 10.1073/pnas.0902362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Böhm H., Albert I. A Conserved Peptide Pattern from a Widespread Microbial Virulence Factor Triggers Pattern-Induced Immunity in Arabidopsis. PLoS Pathog. 2014;10:e1004491. doi: 10.1371/journal.ppat.1004491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oome S., Raaymakers T.M. Nep1-like Proteins from Three Kingdoms of Life Act as a Microbe-Associated Molecular Pattern in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2014;111:16955–16960. doi: 10.1073/pnas.1410031111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albert I., Böhm H. An RLP23-SOBIR1-BAK1 Complex Mediates NLP-Triggered Immunity. Nat. Plants. 2015;1:1–9. doi: 10.1038/nplants.2015.140. [DOI] [PubMed] [Google Scholar]
- 9.Mattinen L., Tshuikina M. Identification and Characterization of Nip, Necrosis-Inducing Virulence Protein of Erwinia Carotovora Subsp. Carotovora. Mol. Plant-Microbe Interact. 2004;17:1366–1375. doi: 10.1094/MPMI.2004.17.12.1366. [DOI] [PubMed] [Google Scholar]
- 10.Santhanam P., Van Esse H.P. Evidence for Functional Diversification Within a Fungal NEP1-Like Protein Family. Mol. Plant-Microbe Interact. 2013;26:278–286. doi: 10.1094/MPMI-09-12-0222-R. [DOI] [PubMed] [Google Scholar]
- 11.Amsellem Z., Cohen B.A. Engineering Hypervirulence in a Mycoherbicidal Fungus for Efficient Weed Control. Nat. Biotechnol. 2002;20:1035–1039. doi: 10.1038/nbt743. [DOI] [PubMed] [Google Scholar]
- 12.Baroncelli R., Buchvaldt Amby D. Gene Family Expansions and Contractions Are Associated with Host Range in Plant Pathogens of the Genus Colletotrichum. BMC Genom. 2016;17:1–17. doi: 10.1186/s12864-016-2917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey B.A., Apel-Birkhold P.C. Expression of NEP1 by Fusarium Oxysporum f. Sp. Erythroxyli after Gene Replacement and Overexpression Using Polyethylene Glycol-Mediated Transformation. Phytopathology. 2002;92:833–841. doi: 10.1094/PHYTO.2002.92.8.833. [DOI] [PubMed] [Google Scholar]
- 14.Fang Y.L., Peng Y.L. The Nep1-like Protein Family of Magnaporthe Oryzae Is Dispensable for the Infection of Rice Plants. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-04430-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabral A., Oome S. Nontoxic Nep1-Like Proteins of the Downy Mildew Pathogen Hyaloperonospora Arabidopsidis: Repression of Necrosis-Inducing Activity by a Surface-Exposed Region. Mol. Plant-Microbe Interact. 2012;25:697–708. doi: 10.1094/MPMI-10-11-0269. [DOI] [PubMed] [Google Scholar]
- 16.Schumacher S., Grosser K. Identification and Characterization of Nep1-Like Proteins from the Grapevine Downy Mildew Pathogen Plasmopara Viticola. Front. Plant Sci. 2020;11:1–17. doi: 10.3389/fpls.2020.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiang J., Li X. Studying the Mechanism of Plasmopara Viticola RxLR Effectors on Suppressing Plant Immunity. Front. Microbiol. 2016;7:1–12. doi: 10.3389/fmicb.2016.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanneganti T.-D., Huitema E. Synergistic Interactions of the Plant Cell Death Pathways Induced by Phytophthora Infestans Nep1-Like Protein PiNPP1.1 and INF1 Elicitin. Mol. Plant-Microbe Interact. 2006;19:854–863. doi: 10.1094/MPMI-19-0854. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y., Lan X. In Planta Functional Analysis and Subcellular Localization of the Oomycete Pathogen Plasmopara Viticola Candidate RXLR Effector Repertoire. Front. Plant Sci. 2018;9:1–15. doi: 10.3389/fpls.2018.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakatos L., Szittya G. Molecular Mechanism of RNA Silencing Suppression Mediated by P19 Protein of Tombusviruses. EMBO J. 2004;23:876–884. doi: 10.1038/sj.emboj.7600096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenarčič T., Hodnik V. Eudicot Plant-Specific Sphingolipids Determine Host Selectivity of Microbial NLP Cytolysins. Science. 2017;358:1431–1434. doi: 10.1126/science.aan6874. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequencing data has been uploaded to GenBank. DNA sequences of PvNLP4, PvNLP5, PvNLP6, PvNLP7 and PvNLP8 can be found using accession numbers MT722075-MT722079.