Abstract
Background.
Inflammation may contribute to the high prevalence of depressive symptoms seen in lung cancer. “Sickness behavior” is a cluster of symptoms induced by inflammation that are similar but distinct from depressive symptoms. The Sickness Behavior Inventory-Revised (SBI-R) was developed to measure sickness behavior. We hypothesized that the SBI-R would demonstrate adequate psychometric properties in association with inflammation.
Method.
Participants with stage IV lung cancer (n = 92) were evaluated for sickness behavior using the SBI-R. Concomitant assessments were made of depression (Patient Hospital Questionniare-9, Hospital Anxiety and Depression Scale) and inflammation [C-reactive protein (CRP)]. Classical test theory (CTT) was applied and multivariate models were created to explain SBI-R associations with depression and inflammation. Factor Analysis was also used to identify the underlying factor structure of the hypothesized construct of sickness behavior. A longitudinal analysis was conducted for a subset of participants.
Results.
The sample mean for the 12-item SBI-R was 8.3 (6.7) with a range from 0 to 33. The SBI-R demonstrated adequate internal consistency with a Cronbach’s coefficient of 0.85, which did not increase by more than 0.01 with any single-item removal. This analysis examined factor loadings onto a single factor extracted using the principle components method. Eleven items had factor loadings that exceeded 0.40. SBI-R total scores were significantly correlated with depressive symptoms (r = 0.78, p < 0.001) and CRP (r = 0.47, p < 0.001). Multivariate analyses revealed that inflammation and depressive symptoms explained 67% of SBI-R variance.
Results.
The SBI-R demonstrated adequate reliability and construct validity in this patient population with metastatic lung cancer. The observed findings suggest that the SBI-R can meaningfully capture the presence of sickness behavior and may facilitate a greater understanding of inflammatory depression.
Keywords: C-reactive protein, Depression, Inflammation, Lung cancer, Sickness Behavior Inventory
Introduction
Sickness behavior is a well-described phenomenon in both animal models and humans (Anisman et al., 2005; Dantzer, 2009; Harden et al., 2015). It refers to a set of specific behaviors that occur in response to systemic inflammation, such as lethargy, decreased appetite, reduced social behaviors and mobility, decreased libido, cognitive impairment reflected in recall and reaction times, weight loss, hyperalgesia, and depressed affect (Shattuck and Muehlenbein, 2016). Interestingly, sickness behavior is thought to provide an adaptive response to illness by conserving energy that would be needed to fight infection (Hart, 1988; Shakhar and Shakhar, 2015). However, many of the symptoms ascribed to sickness behavior overlap with the diagnostic criteria for depression and suggest that this vestigial response to illness may also lead to pathogenic behaviors (American Psychiatric Association, 2013). This suggests that inflammation, sickness behavior, and depression may be interrelated, though the true nature of these relationships is not known (Dantzer, 2004). In the context of serious illness, systemic inflammation may exist due to multiple etiologies and therefore may impress a cumulative effect on the patient. This is especially relevant in cancer settings, where patients may experience inflammation from not only the underlying disease but also its treatments, many of which are immunomodulating, and the psychological stress of coping with cancer. Therefore, understanding the etiology of sickness behavior in the context of advanced cancer is a complex task but has the potential to vastly improve clinical care.
While many studies have established an association between inflammation and depression, the directionality of the relationship is not clear (Stewart et al., 2009; Sotelo et al., 2014; Strawbridge et al., 2015). The relationship between inflammation and depression may be better understood once it is possible to accurately consider sickness behavior as a differential diagnosis. For example, inflammation that stems from a biological cause is thought to lead to depression. However, other evidence suggests that depression in the absence of medical illness (or other cause for inflammation) can directly lead to inflammation (Stewart et al., 2009). In short, depression due to inflammation may represent a distinct subtype of depression that is more common in patients with medical illness (Dantzer et al., 2008). That is, somatic symptoms of depression may become more pronounced in the presence of inflammation from illness but the cognitive symptoms of depression (e.g., low self-esteem, feelings of worthlessness, or guilt) may be less pronounced (Anderson et al., 2014). In this regard, while the association between depression and inflammation is well-established, the causal links and directionality between these constructs remain unclear (Tobias et al., 2015).
The ambiguity in this relationship is further amplified by the criteria used to identify and categorize depression in patients with cancer (Olbert et al., 2014). Differentiating depression that is related to inflammation (i.e., sickness behavior) from depression that may be more psychogenic in origin has important treatment implications (Miller and Raison, 2015; Miller et al., 2017). Depressed patients who exhibit high levels of inflammation may benefit from treatment that addresses the source of inflammation and may show a differential response to antidepressant medications (Uher et al., 2014; Felger et al., 2016; Kappelmann et al., 2018). Given the scarcity of mental health resources in cancer settings, appropriate triaging and targeted treatment of depressive symptoms is critical. Greater specificity in screening and assessment would optimize referrals for depression care and non-mental health professionals could be trained to identify and treat sickness behavior, thus minimizing unnecessary burden on mental health services.
Patients with lung cancer represent an ideal population for exploring the interrelationships between depressive symptoms, inflammation, and sickness behavior. First, lung cancer, and small cell lung cancer (SCLC) in particular, has one of the highest rates of co-morbid depression among all cancer types (Hopwood and Stephens, 2000; Mitchell et al., 2011). Furthermore, cancer (and metastatic cancer in particular) is strongly associated with inflammation, and inflammation has even been used as a prognostic marker to gauge survival. Namely, the pro-inflammatory cytokines tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6), and the acute phase reactant C-reactive protein (CRP) have most commonly been associated with depressive symptoms in patients with lung cancer (Du et al., 2013; McFarland et al., 2019). Co-morbid depression worsens quality of life and is associated with decreased survival rates for patients with lung cancer (Satin et al., 2009). A further understanding of the underlying biology of depression will facilitate the identification of optimal patient-centered depression treatments in cancer (Young and Singh, 2018).
The Sickness Behavior Inventory (SBI) was developed to measure symptoms associated with inflammation-induced sickness behavior (Raison and Miller, 2003; Tobias et al., 2017). This self-report measure was designed to quantify the severity of sickness behavior symptoms, in order to facilitate differentiating those symptoms induced by inflammation vs. depressive symptoms that may be more psychogenic in origin. The original version of the SBI was studied in a sample of patients with pancreatic cancer and was significantly associated with elevated levels of IL-6 (Tobias et al., 2015). Recently, this measure was revised and expanded to improve its reliability and structural validity (the SBI-R), but no published research has addressed the utility of this measure, nor the extent to which the SBI-R can be differentiated from more general measures of depression (Tobias, 2017).
The aim of this study was to assess the preliminary psychometric properties (i.e., reliability and validity) of the SBI-R and to explore the association between sickness behavior, depressive symptoms, and inflammation in a sample of patients with metastatic lung cancer. It was hypothesized that the SBI-R would demonstrate adequate levels of reliability and convergent validity in patients with metastatic lung cancer. Specifically, we anticipated that the SBI-R would have internal consistency (Cronbach’s coefficient alpha) of 0.70 or greater, with inter-item correlations ranging from 0.20 to 0.70, and that the 12 items would load onto a single factor. In addition, we hypothesized that the SBI-R would be significantly correlated with depressive symptoms and inflammation, but that the SBI-R would be more strongly associated with inflammation than depressive symptoms.
Methods
The Memorial Sloan Kettering Cancer Center Institutional Review Board (IRB) approved this study MED18–165, “Survey of Routine Markers of Inflammation and Psychological Variables in Patients with Metastatic Lung Cancer.” Surveys and lab values were collected from May 2017 to November 2017.
Participants and procedures
Patients with stage IV lung cancer confirmed by histology were included. These criteria included patients with non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) as well as other less common types of lung cancer. They were all undergoing active anticancer treatments, spoke English, and had an Eastern Cooperative Group performance status of less than or equal to 2 to be included (Oken et al., 1982). Patients had to be on active treatment for at least 1 month and had to be more than 1 month from receiving the diagnosis of lung cancer to be included.
Patients completed study measures provided by a treating staff member and laboratory values were obtained the same day that the questionnaires were completed. Patients were recruited at routine follow-up medical oncology appointments. Some patients contributed longitudinal data by completing questionnaires and laboratory information subsequently based on convenience and follow-up (n = 39 of 92, 42.4%). Information on available psychological services was provided in the survey, which encouraged patients to raise any concerns with clinic staff and, in particular, to tell a staff member if they felt significantly depressed or had suicidal ideation. Patient participants were not compensated.
Measures
Demographic information
Demographic information was obtained from the electronic medical record. Information that was obtained included sex, age, race/ethnicity, body mass index (BMI), marital status, length of time with disease, systemic treatment type (e.g., chemotherapy, immunotherapy, and targeted therapy), line of treatment (i.e., 1st line, 2nd line, 3rd line, or beyond), and whether an antidepressant medication was on their list of medication at the time of survey.
Inflammation
CRP was used to collect information on Inflammation. CRP values were collected using routine clinical laboratory procedures (Howren et al., 2009; Rashmi et al., 2017). CRP confers several advantages over measuring pro-inflammatory cytokines (PIC). CRP does not exhibit diurnal variation which is inherent to PICs. CRP is a large protein produced by the liver that has a relatively long half-life as an acute phase reactant; it does not exhibit the diurnal variations and fluctuations that are evident by the shorter half lives of PICs. Moreover, CRP responds to multiple PICs, especially IL-6, and therefore acts as an indirect marker of inflammation (Moshage et al., 1987; Steel and Whitehead, 1994). CRP has been most consistently associated with depression when compared to other PIC depression biomarkers (Howren et al., 2009; Wium-Andersen et al., 2013; Misiak et al., 2018) Also, a CRP cut-point has been defined as 1 mg/L to indicate elevated inflammation by the American Heart Association and this cut-point has been used in antidepressant trials (Myers et al., 2004; Uher et al., 2014). In general, CRP levels less than 0.3 mg/dL are considered normal and seen in most healthy adults; 0.3–1.0 mg/dL is normal to minor elevation; 1.0–10.0 mg/dL is moderate elevation; more than 10.0 mg/dL is marked elevation; and more than 50.0 mg/dL is severe elevation (Nehring et al., 2020). In this study, a CRP value was obtained by turbidimetric immunoassay in a Clinical Laboratory Improvement Amendments (CLIA) certified lab (Pineiro et al., 2018). Interand intra-assay coefficient of variation is reliably less than 5%.
Depression
Severity of depression was measured with two scales, the Patient Health Questionnaire-9 (PHQ-9) and the Hospital Anxiety and Depression Scale-Depression (HADS-D). The PHQ-9 is a nine-item measure that elicits responses on a scale from 0 to 3 based on the frequency with which each symptom occurs (i.e., not at all, several days, more than half the days, and nearly every day) over the previous two weeks. Total scores range from 0 to 27, with higher scores indicating greater severity of depressive symptoms (Kroenke et al., 2001). It has been frequently used in the cancer setting and found to perform well as a continuous measure using a cutoff score of ≥10 to signify clinically significant depressive symptoms (Thekkumpurath et al., 2011; Manea et al., 2012). Past research has reported an average sensitivity rate of 0.77 (0.71–0.84) and specificity of 0.94 (0.90–0.97) for identifying a diagnosis of depression (Wittkampf et al., 2007). The rationale underlying the use of PHQ-9 was its high test–retest reliability, which ranges from 0.81 to 0.96 (Lowe et al., 2004). The HADS-D was used as a measure of depression severity that does not utilize physical parameters of depression and was created for purposes of identifying clinically significant cases of depressive disorders among medically ill patients (Bjelland et al., 2002). Physical symptoms related to medical illness (sleep, appetite disturbance, or fatigue) are excluded from the HADS-D to reduce confounding between depression and physical illness specifically. The HADS-D has been validated in lung cancer settings (Bjelland et al., 2002; Schellekens et al., 2016). It is a 7-item symptom rating scale was with a total score that may range from 0 to 21 points based on individual responses rated from 0 to 3 points. A cutoff of 8 confers an average sensitivity and specificity of 0.80 and is most commonly used to identify clinically significant cases of depression (Zigmond and Snaith, 1983; Bjelland et al., 2002).
Sickness behavior
The SBI-R was developed to measure inflammation-induced sickness behavior using symptoms that have been most consistently reported in the literature (Kent et al., 1992; Raison and Miller, 2003; Dantzer and Kelley, 2007), including anhedonia, psychomotor retardation, anorexia/weight loss, decreased libido, fatigue, hyperaglesia, sleep disturbance, cognitive disturbance, and social isolation. Items are rated on a scale of 0–3, corresponding to severity of the symptom in the preceding 48 h.
Statistical analysis
Univariate analyses were used to evaluate associations between SBI and depressive symptoms (PHQ-9 and HADS-D) and CRP using Spearman correlation coefficients. Internal consistency was calculated (Cronbach’s coefficient alpha), along with inter-item correlations. Analyses examining other potential covariates used independent sample t-tests or ANOVAs. CRP values were log-transformed prior to these analyses since CRP data were not normally distributed; however, untransformed values were also reported for ease of interpretation. The unique contributions of SBI and depressive symptoms in predicting CRP levels were examined using multiple regression models, including covariates identified as statistically significant and those identified as potentially relevant a priori. Receiver Operator Curve analysis was performed to identify optimal cutoff scores for SBI-R using known and established cut-points of 1 mg/L for CRP and 10 for PHQ-9. A longitudinal analysis was conducted for a subset of participants based on convenience and follow-up in clinic. These longitudinal analyses are exploratory and measured differences in SBI-R and CRP between two time points using paired t-tests and Spearman rank correlations between two time points. SBI-R and CRP values were log converted prior to measuring differences to account for non-normal distributions.
Results
Out of 120 potential respondents, 92 returned survey questionnaires (77% response rate). Demographic information is presented in Table 1. The average depression score (PHQ-9) was 6.0 (SD = 4.9) with 27.2% falling above the cutoff score for clinically significant depression. The average depression score using the HADS-D was 4.7 (SD 3.6). The mean CRP score was 1.37 mg/L (SD = 2.5) with a range from <0.05 to 15.5 mg/L. Fifty-five patients (60%) had CRP values above 1.0 mg/L and 26 patients (28%) had CRP values above 3.0 mg/L. These cut-points have been used to identify elevated inflammatory levels (Uher et al., 2014; Felger et al., 2020).
Table 1.
Clinical and demographic characteristics of the sample and associations with SBI-R
| Associations with SBI-R | |||
|---|---|---|---|
| Total (n = 92) | |||
| M (SD) | r | p | |
| SBI-R | 8.3 (6.7) | - | - |
| Age (years) | 65.4 (9.2) | −0.03 | 0.75 |
| Body Mass Index | 26.1 (5.3) | 0.00 | 0.99 |
| Time since diagnosis (months) | 14.7 (14.3) | −0.13 | 0.23 |
| C-reactive protein (mg/L) | 1.37 (2.5) | 0.47 | <0.001 |
| Depression (PHQ-9 total score) | 6.0 (4.9) | 0.78 | <0.001 |
| SBI-R score | |||
| N (%) | M (SD) | P | |
| Disease type | 0.49 | ||
| Adenocarcinoma | 66 (71.0%) | 7.7 (6.5) | |
| Squamous cell CA | 7 (7.5%) | 9.3 (7.3) | |
| Small cell lung cancer | 14 (15.1%) | 10.6 (7.3) | |
| Not otherwise specified | 5 (5.4%) | NA | |
| Treatment type | 0.01 | ||
| Chemotherapy | 34 (40.5%) | 10.4 (7.5) | |
| Immunotherapy | 30 (35.7%) | 8.5 (6.4) | |
| Targeted therapy | 20 (23.8%) | 4.9 (3.8) | |
| Missing | 9 (9.7%) | NA | |
| Line of treatment | 0.80 | ||
| 1st | 49 (59.0%) | 8.1 (6.2) | |
| 2nd | 22 (26.5%) | 8.3 (7.1) | |
| 3rd or beyond | 12 (14.5%) | 9.6 (8.3) | |
| Missing | 10 (10.8%) | NA | |
| Gender | 0.65 | ||
| Female | 63 (67.7%) | 8.1 (6.2) | |
| Male | 29 (31.2%) | 8.8 (7.6) | |
| Race/Ethnicity | 0.02 | ||
| White | 79 (85.9%) | 7.5 (5.9) | |
| Non-white | 13 (14.1%) | 13.7 (8.5) | |
| Black | 7 (7.5%) | ||
| Hispanic | 5 (5.4%) | ||
| Asian | 1 (1.1%) | ||
| Married | 0.35 | ||
| Yes | 64 (69.5%) | 7.9 (6.1) | |
| No | 28 (30.4%) | 9.3 (7.8) | |
| Antidepressant | 0.36 | ||
| Yes | 20 (23.0%) | 9.6 (8.4) | |
| No | 73 (77.0%) | 8.0 (6.1) | |
PHQ-9, Patient Health Questionnaire; SBI-R, Sickness Behavior Inventory-Revised.
The sample mean for the 12-item SBI-R was 8.3 (6.7) with a range from 0 to 33 (possible range: 0–36).The SBI-R demonstrated adequate internal consistency with a Cronbach’s coefficient alpha of 0.85. Corrected item-total correlations ranged from 0.27 (item 10, sleep disturbance) to 0.80 (item 7, motivation to engage in enjoyable activities). Cronbach’s coefficient alpha did not increase significantly (>0.01) with the removal of any item. Factor analysis was also used to identify the relative contributions of each item for the construct of sickness behavior. This analysis examined factor loadings onto a single factor extracted using the principle components method. Of the 12 SBI-R items, 11 had factor loadings that exceeded 0.40 (typically used as a threshold for identifying meaningful factor loadings) (Hair et al., 1998). Only item 10 had a factor loading below 0.40, at 0.396.
Concurrent validity of the SBI-R was evaluated by examining the associations between depressive symptoms, inflammation, and SBI-R individual items and total score (Table 2). The SBI-R total was significantly correlated with depressive symptoms (PHQ-9: r = 0.78, p < 0.001; HADS-D: r = 0.77, p < 0.001) and CRP (r = 0.47, p < 0.001). Each item on the SBI-R was also significantly associated with PHQ-9 total score, with correlations ranging from r = 0.27, p = 0.01 (SBI-R item 12: memory functioning) to r = 0.65, p < 0.001 (SBI-R item 7: motivation). However, CRP levels were significantly associated with only 5 of the 12 SBI-R items. The strongest correlation was with item 8 (enjoyment of activities: r = 0.38, p < 0.001). In addition, SBI-R scores were significantly higher in patients receiving chemotherapy (M = 10.4, SD = 7.5) vs. those patients taking oral targeted therapies (M = 4.9, SD 3.8), t = 3.53, p = 0.012. SBI-R total scores were also higher in non-white patients (M = 13.7, SD = 8.5) than white patients (M = 7.4, SD = 5.9), t = −2.52, p = 0.024. However, CRP levels were higher in non-white vs. white patients (3.51 vs. 1.06 mg/dL, respectively) (t = 2.86, p = 0.005). Of note, levels of depressive symptoms were also higher in non-white vs. white patients (8.38 vs. 5.57) but were not significantly different (t = 1.42, p = 0.18). However, the reason why inflammation and depression may be higher in non-white populations is not clear. No significant associations were observed with any other demographic or clinical variables (e.g., age, gender, BMI, and line of treatment; Table 1).
Table 2.
SBI-R item means and association with depression and inflammation
| Mean (SD) | SBI-R Total |
PHQ-9 total |
HADS-D |
CRP |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Range 0–3 | r | p | r | p | R | p | r | p | |
| SBI-R Total | - | - | 0.78 | <0.001 | 0.77 | <0.001 | 0.47 | <0.001 | |
| S1: Psychomotor retardation | 0.68 (0.87) | 0.57 | <0.001 | 0.46 | <0.001 | 0.51 | <0.001 | 0.19 | 0.07 |
| S2: Appetite | 0.81 (1.08) | 0.54 | <0.001 | 0.49 | <0.001 | 0.35 | 0.001 | 0.17 | 0.10 |
| S3: Pain severity | 0.98 (1.06) | 0.47 | <0.001 | 0.32 | <0.001 | 0.28 | 0.006 | 0.28 | 0.01 |
| S4: Decreased eating sweets | 0.44 (0.90) | 0.45 | <0.001 | 0.27 | <0.001 | 0.24 | 0.02 | −0.01 | 0.94 |
| S5: Libido | 0.60 (1.12) | 0.49 | <0.001 | 0.39 | <0.001 | 0.32 | 0.002 | 0.09 | 0.40 |
| S6: Fatigue | 1.40 (0.98) | 0.67 | <0.001 | 0.56 | <0.001 | 0.66 | <0.001 | 0.40 | <0.001 |
| S7: Motivated | 0.93 (0.98) | 0.82 | <0.001 | 0.65 | <0.001 | 0.66 | <0.001 | 0.33 | 0.001 |
| S8: Enjoyment of activities | 0.58 (0.86) | 0.62 | <0.001 | 0.51 | <0.001 | 0.56 | <0.001 | 0.38 | <0.001 |
| S9: Concentration | 0.44 (0.71) | 0.61 | <0.001 | 0.41 | <0.001 | 0.49 | <0.001 | 0.16 | 0.14 |
| S10: Sleep disturbance | 0.69 (0.87) | 0.42 | <0.001 | 0.40 | <0.001 | 0.38 | <0.001 | 0.12 | 0.26 |
| S11: Decreased socializing | 0.73 (0.93) | 0.71 | <0.001 | 0.54 | <0.001 | 0.33 | 0.002 | 0.23 | 0.03 |
| S12: Memory functioning | 0.40 (0.72) | 0.49 | <0.001 | 0.27 | 0.01 | 0.60 | <0.001 | 0.13 | 0.21 |
CRP, C-reactive protein; HADS-D, Hospital Anxiety and Depression Scale-Depression; PHQ-9, Patient Health Questionnaire; SBI-R, Sickness Behavior Inventory-Revised; SD, Standard Deviation.
Associations between depressive symptoms and CRP have been reported previously (McFarland et al., 2019) but are noted here for comparison to the SBI-R; CRP values were significantly correlated with PHQ-9 (r = 0.35, p < 0.001) and HADS-D (r = 0.34, p 0.001). Multiple regression analyses revealed that SBI-R total scores explained significant variance in CRP levels beyond that explained by PHQ-9 total scores, with the two variables explaining 18% of the variance in CRP values (Table 3). However, when the order of entry was reversed, PHQ-9 scores did not add to the variance explained by SBI-R total scores. A similar pattern was demonstrated when PHQ-9 was replaced by HADS-D (Table 4). HADS-D did not add to the variance explained by SBI-R scores. These models did not demonstrate problematic collinearity (tolerance 0.360; variance inflation factor 2.77).
Table 3.
Hierarchical regression models predicting inflammation (CRP) built from PHQ-9 and SBI-R
| B | t | P | |
|---|---|---|---|
| Model One | |||
| Step 1: | |||
| Sickness Behavior (SBI-R) | 0.45 (0.01) | 4.62 | <0.001 |
| F = 21.38, Adjusted | R2 = 0.19 | ||
| Step 2: | |||
| Sickness Behavior (SBI-R) | 0.51 (0.01) | 3.143 | 0.002 |
| Depression (PHQ-9) | −0.08 (0.02) | −0.477 | 0.64 |
| F =10.71, Adjusted | R2 = 0.18 | ||
| Model Two | |||
| Step 1: | |||
| Depression (PHQ-9) | 0.33 (0.01) | 3.23 | 0.002 |
| F =10.45, Adjusted | R2 = 0.10 | ||
| Step 2: | |||
| Depression (PHQ-9) | −0.08 (0.02) | −0.48 | 0.64 |
| Sickness Behavior (SBI-R) | 0.51 (0.01) | 3.14 | 0.002 |
| F =10.71, Adjusted | R2 = 0.18 | ||
PHQ-9, Patient Health Questionnaire-9; SBI-R, Sickness Behavior Inventory-Revised.
Table 4.
Hierarchical regression models predicting inflammation (CRP) built from HADS-S and SBI-R
| B | t | P | |
|---|---|---|---|
| Model One | |||
| Step 1: | |||
| Sickness Behavior (SBI-R) | 0.45 (0.01) | 4.62 | <0.001 |
| F =21.38, Adjusted | R2 = 0.19, p <0.001 | ||
| Step 2: | |||
| Sickness Behavior (SBI-R) | 0.43 (0.02) | 2.569 | 0.01 |
| Depression (HADS-D) | 0.02 (0.03) | 0.146 | 0.89 |
| F =10.58, Adjusted | R2 = 0.18, p <0.001 | ||
| Model Two | |||
| Step 1: | |||
| Depression (HADS-D) | 0.37 (0.02) | 3.70 | <0.001 |
| F =13.66, Adjusted | R2 = 0.14, p <0.001 | ||
| Step 2: | |||
| Depression (HADS-D) | 0.02(0.03) | 0.146 | 0.88 |
| Sickness Behavior (SBI-R) | 0.43 (0.02) | 2.57 | 0.01 |
| F =10.58, Adjusted | R2 = 0.18, p <0.001 | ||
HADS-D, Hospital Anxiety and Depression Scale-Depression; SBI-R, Sickness Behavior Inventory-Revised.
Exploratory analyses
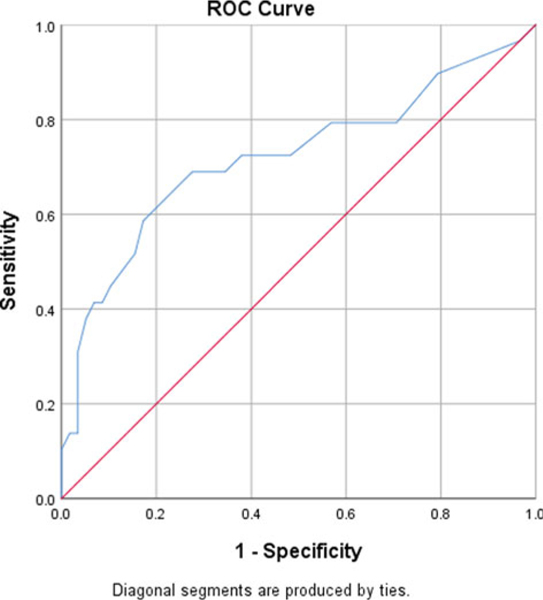
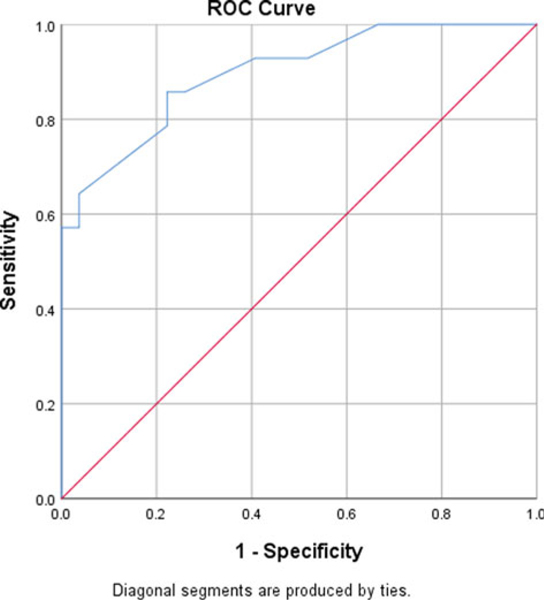
Classification analyses, using Receiver Operating Characteristic Curve (ROC) analysis, was used to identify the optimal cut-point for identifying clinically significant scores on the SBI-R. Given the absence of external indicators of impairment, we utilized the established CRP value of 1.0 to determine the optimal cut-point for maximizing sensitivity and specificity (Figure 1). This analysis suggested that an SBI-R total score of 9 or greater provided the optimal discrimination between those with elevated and non-elevated CRP levels, with sensitivity of 54.1 and specificity of 66.7. The area under the curve (AUC) associated with this model was 0.721 (p = 0.001). Similarly, we utilized the established PHQ-9 value of 10 as a depression screening cut-point to identify clinical depression and found that the SBI-R total score of 9 or greater provided optimal discrimination with a sensitivity of 53% and specificity of 91% for high SBI-R scores (Figure 2).
Fig. 1.
ROC curve of the SBI-R predicting for inflammation: CRP >1 mg/dL.
Fig. 2.
ROC curve of the SBI-R predicting for depression: PHQ-9 >10.
Of the 92 patients for whom baseline data was available, 39 completed all study measures at a second time point. There were no significant differences in demographics, treatment characteristics, depressive symptoms or CRP levels between those who completed measures at a second time point and those who did not. The average time between the surveys was 3 months. There were no significant differences in SBI-R or CRP between the two time points. SBI-R at time 1 was highly correlated to SBI-R at time 2 (r = 0.67, p < 0.001) and CRP values were correlated between the two time points (r = 0.42, p = 0.01) (Table 5). None of the individual items on the SBI-R were significantly different and several were significantly correlated between the two time points (psychomotor retardation, pain severity, libido, fatigue, decreased socializing, and memory function). An analysis of SBI-R correlations with CRP at time point 2 are provided (n = 39) as a point of comparison with the primary analysis (n = 92). SBI-R at time 2 was highly correlated with CRP (r = 0.58, p < 0.001). Changes between time point 1 and 2 on the SBI-R and CRP were also significantly correlated (r = 0.42, p = 0.01).
Table 5.
Exploratory analysis of the SBI-R and inflammation at two time points
| Time Point 1 | Time Point 2 | Correlation | |
|---|---|---|---|
| Inflammation (CRP) | 1.86 (SD 3.14) | 1.67 (SD 3.93) | r = 0.42, p = 0.01 |
| SBI-R | 7.10 (SD 5.24) | 7.85 (SD 6.03) | r = 0.67, p <0.001 |
| Correlation | r =0.42, p = 0.01 | r =0.58, p <0.001 |
CRP, C-reactive protein; SBI-R, Sickness Behavior Inventory-Revised.
Discussion
Sickness behavior is a clinical phenomenon that manifests as several distinct but interrelated symptoms. Although it is well-described, its measurement has received minimal attention, and therefore, the literature on sickness behavior measurement is limited. The development of the SBI-R is one of the first formal attempts to measure sickness behavior in the cancer context. Quantitatively measuring the extent of sickness behavior may inform our understanding of inflammatory depression; simultaneously, it may help explain acute behavioral symptoms secondary to inflammation, even when not associated with depression. Thus, a scale that measures a composite of sickness behavior symptoms and generates an estimate of the severity of sickness behavior is useful for clinical and research purposes. The SBI-R facilitates the description of sickness behavior and may help elucidate the underlying biological mechanism of depression when both depressive symptoms and inflammation are present. This description is particularly apt for medically compromised populations such as patients with metastatic lung cancer where both depression and inflammation are elevated.
This is the first clinical population in which the SBI-R has been psychometrically evaluated. A prior version of this scale was examined in a sample of patients with metastatic pancreatic cancer (Tobias et al., 2015). Results from the initial validation study and the current results share many similarities and support the hypothesis that sickness behavior is a unique clinical entity worthy of study. The prior version of the SBI demonstrated moderate reliability (Cronbach’s alpha = 0.66) and total scores were significantly correlated with IL-6 levels (rs = 0.26, p = 0.03). An expanded version of this scale was developed by Tobias (2017) who found stronger internal consistency (Cronbach’s alpha = 0.80–0.85) but found that there was no significant correlation with IL-6 in that sample, as most patients were in remission and/or had very low levels of inflammation (r = 0.15, p = 0.30). While IL-6 was measured in the first sample, CRP was included in this study as an inflammatory marker due to its several unique advantages over cytokines including measurement stability and clinical utility across clinical populations.
The SBI-R demonstrated strong internal consistency (Cronbach’s coefficient alpha = 0.85) in this sample of patients with metastatic lung cancer as well as a moderate association with inflammation. However, less than half of the SBI-R items were significantly correlated with inflammation, raising questions about whether each of the items are equally salient to the construct of sickness behavior. It is possible that the number of items on the scale could be reduced while still capturing the construct and minimizing measurement burden. At the same time, the longitudinal exploratory analysis found that SBI-R was correlated with CRP at both time points and found that changes in CRP between the two time points were correlated with changes in SBI-R. Future research should utilize larger samples, more sophisticated data analysis techniques (e.g., item response theory), and measure the relative stability of SBI-R responses (i.e., test–retest reliability). These techniques could determine the utility of the SBI-R in measuring the underlying construct of sickness behavior.
The current findings identified a stronger relationship between sickness behavior via the SBI-R and inflammation than between depressive symptoms and inflammation (i.e., β = 0.45 vs. β = 0.33, respectively). Thus, the SBI-R does appear to measure a construct that is distinct from (though partially overlapping with) depressive symptoms and may be useful in helping to answer the question of how inflammatory depression is phenotypically distinct from general, non-inflammation associated depression. Presently, this subtype of depression is best conceived as a manifestation of chronic inflammation related to key symptoms that overlap with depressive symptoms. Also, the SBI may be used to help identify sickness behavior symptoms that may not precipitate over depression and may be clinically distinct from depression (i.e., sickness behavior symptom cluster but without depression). This clinical phenomenon needs further explanation and would benefit from the introduction of this objective measure to help facilitate its study as a unique psychological entity. The SBI-R may help inform the recognition and management of depression since both the diagnosis and treatment of depression is challenging in medical settings (Grassi et al., 2014; Saracino et al., 2018).
In addition to the poor rates of recognizing depression in the lung cancer setting, uptake of depression treatments is also far from ideal, despite its associations with worse overall survival (Sullivan et al., 2016a, 2016b). A further examination of depression as an inflammatory process may actually increase the identification of depression, especially in a medical setting where inflammation can be monitored alongside other medical issues. SBI-R values above 9 were identified as an optimal cut-point for identifying sickness behavior in this exploratory analysis; the modest classification accuracy indicates the need for further research before firm conclusions can be drawn as to how the SBI-R should be used in identifying clinically meaningful sickness behavior. Also, the longitudinal analyses provide further evidence of consistent relationships between variables although the analyses are preliminary based on a convenience sample and subject to potential biases.
Perhaps most importantly, inflammatory depression and associated increases in sickness behavior may respond to differential depression treatments that address either the inflammatory component underlying the depressive symptoms or consequences of inflammation (Haroon et al., 2016; Felger, 2017). For example, patients who receive exogenous pro-inflammatory cytokine therapy such as interferon or IL-2 tend to experience the vegetative symptoms of sickness behavior, which may be followed later by the psychological symptoms of depressed mood, irritability, rage, and anxiety (Capuron et al., 2002). While standard SSRI antidepressants work to ameliorate the psychologically oriented depressive symptoms (e.g., guilt and sense of worthlessness) and can prevent vegetative symptoms (e.g., fatigue and appetite disturbance), other non-SSRI antidepressants may work better for treating inflammation-induced vegetative symptoms of depression (Capuron et al., 2002). For example, inflammation associated depression may be more responsive to agents that upregulate dopamine such as bupropion, in addition to serotonin (Felger, 2017), as inflammation depletes these key neurotransmitters (Muller and Schwarz, 2007). In short, the SBI-R may be useful diagnostically to help determine clinical management strategies for depressive symptoms in the setting of medical illness.
The current study is exploratory and preliminary. As such, its results are limited by a relatively small sample size that precluded some analyses (e.g., item response theory) and the ability to apply outcomes to subgroups. Also, the sample was not ethnically or racially diverse and was therefore dichotomized to enable speculative analysis. While SBI-R was higher in non-white participants, these data are highly exploratory due to the small sample of 13 non-white patients. This difference was likely related to higher rates of inflammation, depression, and possibly stress. The results and conclusions were also limited by the use of self-report depression measures such as the PHQ-9 and the HADS-D instead of using the Structured Clinical Interview for Diagnostic and Statistical Manual-V Disorders (SCID), the gold standard for identifying cases of depression. These two self-report measures of depression were used for the sake of comparison against individual SBI-R questions since some of the SBI-R question domains overlap with the PHQ-9 (e.g., psychomotor retardation, appetite, fatigue, enjoyment of activities, concentration, and sleep disturbance). However, the SBI-R was significantly correlated with both measures to a similar extent. Inflammation was also limited to CRP values, whereas it would have been helpful to compare CRP to other pro-inflammatory cytokines such as IL-6. Future research evaluating the SBI-R should include multiple biomarkers to better elucidate its ability to measure underlying inflammation and perhaps identify differential symptom manifestations of inflammation. Finally, these data were also collected during ongoing anticancer treatments. Although analyses were adjusted for these treatments as a separate variable, it would be important to understand this relationship independent of receiving cancer treatments. For example, chemotherapy precipitates both inflammation and depression and may have exaggerated the relationship between depression and SBI-R. Therefore, the study could be replicated after completing cancer therapies in patients with localized cancers who are undergoing surveillance follow-up for the cancer management.
In summary, the SBI-R performed well as a measure of sickness behavior in patients with metastatic lung cancer. This represents a novel approach to succinctly describe sickness behavior and examine its relationship with both depressive symptoms and inflammation. The quantification of sickness behavior as a distinct entity holds clinical promise. It may provide a mechanism by which the concept of sickness behavior can evolve from a clinical description to a clinical state that can be quantified with useful thresholds that are validated based on depression scales and inflammatory markers.
Acknowledgments and Disclosures.
We thank the Department of Psychiatry and Behavioral Sciences at Memorial Sloan Kettering Cancer Center for assistance with study analysis and manuscript preparation. Authors have reviewed and approved the manuscript as it is submitted and have no conflict of interest to declare. Each author had multiple roles in writing the manuscript including the conception, design, acquisition, analysis, and interpretation of the data. This work was not directly supported by external funding sources.
Funding. This work was supported by the NIH/NCI Cancer Center Support Grant (P30 CA008748) and the NIH Loan Repayment Program L30 CA220778.
Appendix
See Figures A1 and A2.
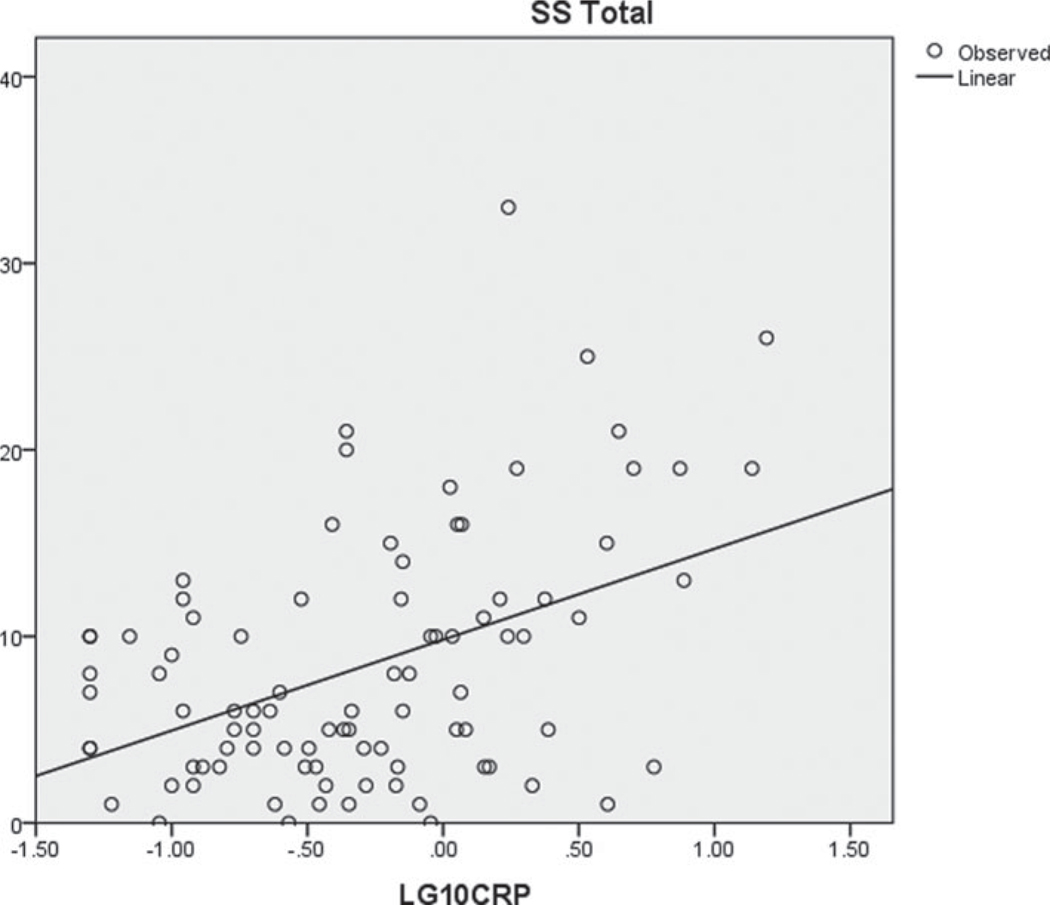
Fig. A1.
Scatterplot of SBI-R and CRP log-transformed.
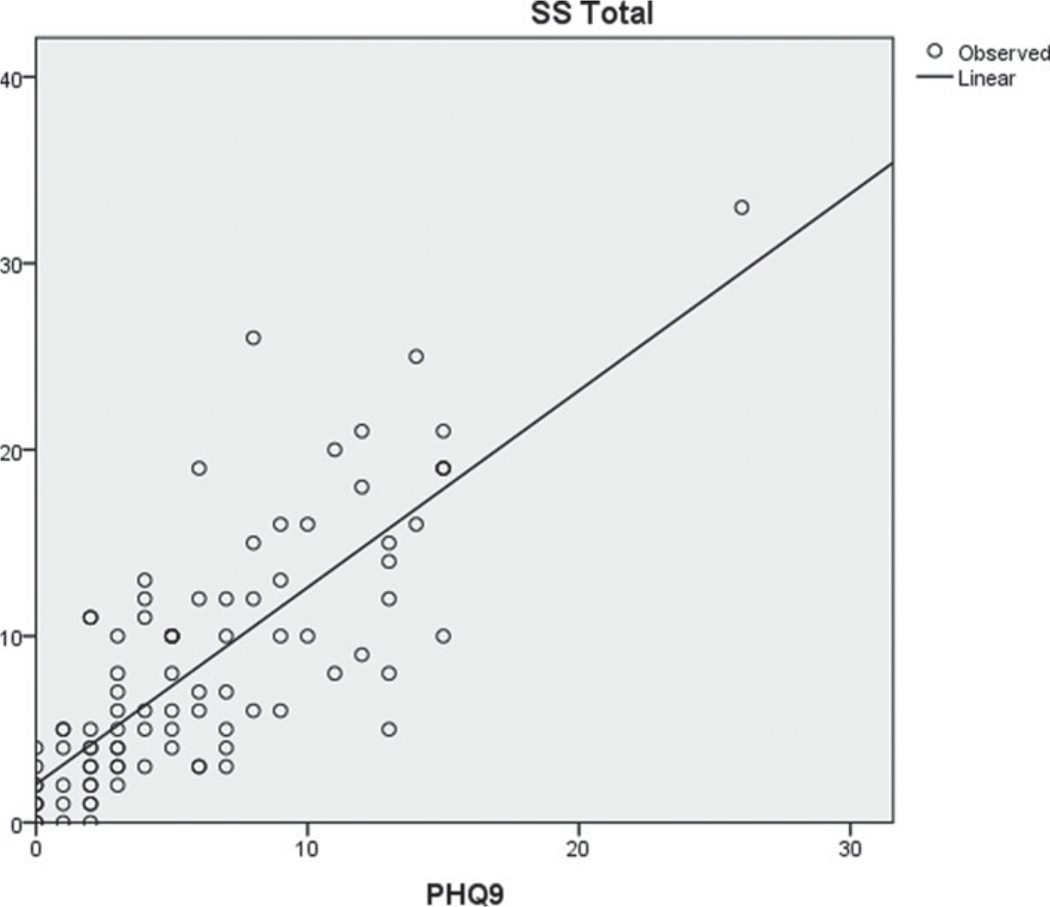
Fig. A2.
Scatterplot of SBI-R and depression (PHQ-9).
Footnotes
Conflict of interests. The authors have no conflict of interest.
References
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorder, 5th ed. Arlington, VA: American Psychiatric Association. [Google Scholar]
- Anderson G, Berk M and Maes M (2014) Biological phenotypes underpin the physio-somatic symptoms of somatization, depression, and chronic fatigue syndrome. Acta Psychiatrica Scandinavica 129(2), 83–97. [DOI] [PubMed] [Google Scholar]
- Anisman H, Merali Z, Poulter MO, et al. (2005) Cytokines as a precipitant of depressive illness: Animal and human studies. Current Pharmaceutical Design 11(8), 963–972. [DOI] [PubMed] [Google Scholar]
- Bjelland I, Dahl AA, Haug TT, et al. (2002) The validity of the hospital anxiety and depression scale. An updated literature review. Journal of Psychosomatic Research 52(2), 69–77. [DOI] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, et al. (2002) Neurobehavioral effects of interferon-alpha in cancer patients: Phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology 26(5), 643–652. [DOI] [PubMed] [Google Scholar]
- Dantzer R (2004) Cytokine-induced sickness behaviour: A neuroimmune response to activation of innate immunity. European Journal of Pharmacology 500(1–3), 399–411. [DOI] [PubMed] [Google Scholar]
- Dantzer R (2009) Cytokine, sickness behavior, and depression. Immunology and Allergy Clinics of North America 29(2), 247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R and Kelley KW (2007) Twenty years of research on cytokine-induced sickness behavior. Brain, Behavior, and Immunity 21(2), 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, et al. (2008) From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience 9(1), 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du YJ, Zhang HY, Li B, et al. (2013) Sputum interleukin-6, tumor necrosis factor-alpha and salivary cortisol as new biomarkers of depression in lung cancer patients. Progress in Neuro-Psychopharmacology & Biological Psychiatry 47, 69–76. [DOI] [PubMed] [Google Scholar]
- Felger JC (2017) The role of dopamine in inflammation-associated depression: Mechanisms and therapeutic implications. Current Topics in Behavioral Neurosciences 31, 199–219. [DOI] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, et al. (2016) Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Molecular Psychiatry 21(10), 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Haroon E, Patel TA, et al. (2020) What does plasma CRP tell us about peripheral and central inflammation in depression? Molecular Psychiatry 25(6), 1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi L, Caruso R, Sabato S, et al. (2014) The UniFe psychiatry working group C. Psychosocial screening and assessment in oncology and palliative care settings. Frontiers in Psychology 5, 1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair JF, Tatham RL, Anderson RE, et al. (1998) Multivariate Data Analysis, 5th ed. Upper Saddle River, NY: Prentice Hall. [Google Scholar]
- Harden LM, Kent S, Pittman QJ, et al. (2015) Fever and sickness behavior: Friend or foe? Brain, Behavior, and Immunity 50, 322–333. [DOI] [PubMed] [Google Scholar]
- Haroon E, Fleischer CC, Felger JC, et al. (2016) Conceptual convergence: Increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Molecular Psychiatry 21(10), 1351–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL (1988) Biological basis of the behavior of sick animals. Neuroscience and Biobehavioral Reviews 12(2), 123–137. [DOI] [PubMed] [Google Scholar]
- Hopwood P and Stephens RJ (2000) Depression in patients with lung cancer: Prevalence and risk factors derived from quality-of-life data. Journal of Clinical Oncology 18(4), 893–903. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM and Suls J (2009) Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosomatic Medicine 71(2), 171–186. [DOI] [PubMed] [Google Scholar]
- Kappelmann N, Lewis G, Dantzer R, et al. (2018) Antidepressant activity of anti-cytokine treatment: A systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Molecular Psychiatry 23(2), 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Kelley KW, et al. (1992) Sickness behavior as a new target for drug development. Trends in Pharmacological Sciences 13(1), 24–28. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL and Williams JB (2001) The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine 16(9), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe B, Kroenke K, Herzog W, et al. (2004) Measuring depression outcome with a brief self-report instrument: Sensitivity to change of the Patient Health Questionnaire (PHQ-9). Journal of Affective Disorders 81(1), 61–66. [DOI] [PubMed] [Google Scholar]
- Manea L, Gilbody S and McMillan D (2012) Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): A meta-analysis. CMAJ: Canadian Medical Association Journal = Journal de l’Association Medicale Canadienne 184(3), E191–E196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland DC, Shaffer K, Breitbart W, et al. (2019) C-reactive protein and its association with depression in patients receiving treatment for metastatic lung cancer. Cancer 125, 779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH and Raison CL (2015) Are Anti-inflammatory therapies viable treatments for psychiatric disorders?: Where the rubber meets the road. JAMA Psychiatry 72(6), 527–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Trivedi MH and Jha MK (2017) Is C-reactive protein ready for prime time in the selection of antidepressant medications? Psychoneuroendocrinology 84, 206. [DOI] [PubMed] [Google Scholar]
- Misiak B, Beszlej JA, Kotowicz K, et al. (2018) Cytokine alterations and cognitive impairment in major depressive disorder: From putative mechanisms to novel treatment targets. Progress in Neuro-Psychopharmacology & Biological Psychiatry 80(Pt C), 177–188. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Chan M, Bhatti H, et al. (2011) Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliativecare settings: A meta-analysis of 94 interview-based studies. The Lancet Oncology 12(2), 160–174. [DOI] [PubMed] [Google Scholar]
- Moshage HJ, Janssen JA, Franssen JH, et al. (1987) Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. The Journal of Clinical Investigation 79(6), 1635–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N and Schwarz MJ (2007) The immune-mediated alteration of serotonin and glutamate: Towards an integrated view of depression. Molecular Psychiatry 12(11), 988–1000. [DOI] [PubMed] [Google Scholar]
- Myers GL, Rifai N, Tracy RP, et al. (2004) CDC/AHA workshop on markers of inflammation and cardiovascular disease: Application to clinical and public health practice: Report from the laboratory science discussion group. Circulation 110(25), e545–e549. [DOI] [PubMed] [Google Scholar]
- Nehring SM, Goyal A, Bansal P, et al. (2020) C Reactive Protein (CRP). Treasure Island, FL: StatPearls. [Google Scholar]
- Oken MM, Creech RH, Tormey DC, et al. (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology 5(6), 649–655. [PubMed] [Google Scholar]
- Olbert CM, Gala GJ and Tupler LA (2014) Quantifying heterogeneity attributable to polythetic diagnostic criteria: Theoretical framework and empirical application. Journal of Abnormal Psychology 123(2), 452–462. [DOI] [PubMed] [Google Scholar]
- Pineiro M, Pato R, Soler L, et al. (2018) A new automated turbidimetric immunoassay for the measurement of canine C-reactive protein. Veterinary Clinical Pathology 47(1), 130–137. [DOI] [PubMed] [Google Scholar]
- Raison CL and Miller AH (2003) Depression in cancer: New developments regarding diagnosis and treatment. Biological Psychiatry 54(3), 283–294. [DOI] [PubMed] [Google Scholar]
- Rashmi N, Galhotra V, Goel P, et al. (2017) Assessment of C-reactive proteins, cytokines, and plasma protein levels in hypertensive patients with apical periodontitis. The Journal of Contemporary Dental Practice 18 (6), 516–521. [DOI] [PubMed] [Google Scholar]
- Saracino RM, Rosenfeld B and Nelson CJ (2018) Performance of four diagnostic approaches to depression in adults with cancer. General Hospital Psychiatry 51, 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satin JR, Linden W and Phillips MJ (2009) Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer 115(22), 5349–5361. [DOI] [PubMed] [Google Scholar]
- Schellekens MPJ, van den Hurk DGM, Prins JB, et al. (2016) The suitability of the Hospital Anxiety and Depression Scale, Distress Thermometer and other instruments to screen for psychiatric disorders in both lung cancer patients and their partners. Journal of Affective Disorders 203, 176–183. [DOI] [PubMed] [Google Scholar]
- Shakhar K and Shakhar G (2015) Why do we feel sick when infected–Can altruism play a role? PLoS Biology 13(10), e1002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck EC and Muehlenbein MP (2016) Towards an integrative picture of human sickness behavior. Brain, Behavior, and Immunity 57, 255–262. [DOI] [PubMed] [Google Scholar]
- Sotelo JL, Musselman D and Nemeroff C (2014) The biology of depression in cancer and the relationship between depression and cancer progression. International Review of Psychiatry 26(1), 16–30. [DOI] [PubMed] [Google Scholar]
- Steel DM and Whitehead AS (1994) The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunology Today 15(2), 81–88. [DOI] [PubMed] [Google Scholar]
- Stewart JC, Rand KL, Muldoon MF, et al. (2009) A prospective evaluation of the directionality of the depression-inflammation relationship. Brain, Behavior, and Immunity 23(7), 936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge R, Arnone D, Danese A, et al. (2015) Inflammation and clinical response to treatment in depression: A meta-analysis. European Neuropsychopharmacology 25(10), 1532–1543. [DOI] [PubMed] [Google Scholar]
- Sullivan DR, Forsberg CW, Ganzini L, et al. (2016a) Depression symptom trends and health domains among lung cancer patients in the CanCORS study. Lung Cancer 100, 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan DR, Forsberg CW, Ganzini L, et al. (2016b) Longitudinal changes in depression symptoms and survival among patients with lung cancer: A national cohort assessment. Journal of Clinical Oncology 34(33), 3984–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thekkumpurath P, Walker J, Butcher I, et al. (2011) Screening for major depression in cancer outpatients: The diagnostic accuracy of the 9-item patient health questionnaire. Cancer 117(1), 218–227. [DOI] [PubMed] [Google Scholar]
- Tobias KG (2017) An Exploration of “Sickness Behavior” in Older Patients with Cancer. ETD Collection for Fordham University Psychology, Fordham University. [Google Scholar]
- Tobias K, Rosenfeld B, Pessin H, et al. (2015) Measuring sickness behavior in the context of pancreatic cancer. Medical Hypotheses 84(3), 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias KG, Lehrfeld J, Rosenfeld B, et al. (2017) Confirmatory factor analysis of the Beck Depression Inventory-II in patients with advanced cancer: A theory-driven approach. Palliative & Supportive Care 15(6), 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Tansey KE, Dew T, et al. (2014) An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. The American Journal of Psychiatry 171(12), 1278–1286. [DOI] [PubMed] [Google Scholar]
- Wittkampf KA, Naeije L, Schene AH, et al. (2007) Diagnostic accuracy of the mood module of the Patient Health Questionnaire: A systematic review. General Hospital Psychiatry 29(5), 388–395. [DOI] [PubMed] [Google Scholar]
- Wium-Andersen MK, Orsted DD, Nielsen SF, et al. (2013) Elevated C-reactive protein levels, psychological distress, and depression in 73, 131 individuals. JAMA Psychiatry 70(2), 176–184. [DOI] [PubMed] [Google Scholar]
- Young K and Singh G (2018) Biological mechanisms of cancer-induced depression. Frontiers in Psychiatry 9, 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond AS and Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 67(6), 361–370. [DOI] [PubMed] [Google Scholar]