Abstract
We present the case of a female patient who developed persistently elevated levels of cardiac troponin (cTn) after a previous episode of clinically presumed myocarditis. Extensive investigation concluded that the presence of heterophile antibodies was causing false positive cTn elevation. (Level of Difficulty: Intermediate.)
Key Words: cardiac magnetic resonance, echocardiography, myocardial infarction, speckle tracking
Abbreviations and Acronyms: CAD, coronary artery disease; CK, creatine kinase; CMR, cardiac magnetic resonance; CRP, C-reactive protein; cTn, cardiac troponin; ECG, electrocardiogram; hAb, heterophile antibody; MINOCA, myocardial infarction with nonobstructive coronary arteries; RL, reference limit
Graphical abstract
We present the case of a female patient who developed persistently elevated levels of cardiac troponin (cTn) after a previous episode of clinically…
History of Presentation
A 57-year-old woman presented to the emergency department with symptoms of new onset of prolonged chest pain radiating to the left upper limb. It started at rest (in the morning), lasted for more than 3 h, was aggravated with deep breathing, and resolved before hospital admission. She reported flu-like symptoms 2 weeks before admission. No further symptoms were reported. Physical examination was unremarkable.
Learning Objectives
-
•
To question the possibility of false positive cTn results whenever discrepancies between cTn levels and clinical, angiographic, and imaging findings occur.
-
•
To perform cTn measurements using a different assay to exclude false positive results; in case of mismatch, the supplier’s laboratory should be contacted.
-
•
To recognize hAb as a rare but yet possible source of false positives in common cTn assays.
Past Medical History
The patient had a history of depression, fibromyalgia, and hypothyroidism. She was receiving long-term levothyroxine therapy (0.1 mg daily).
Differential diagnosis
The differential diagnosis included cardiovascular causes of chest pain such as acute coronary syndrome, acute myocarditis, aortic dissection, and acute pulmonary embolism. Noncardiac causes are numerous and include gastroesophageal reflux disease, pancreatitis, acute cholecystitis, spontaneous pneumothorax, osteochondritis, and anxiety, among others.
Investigations
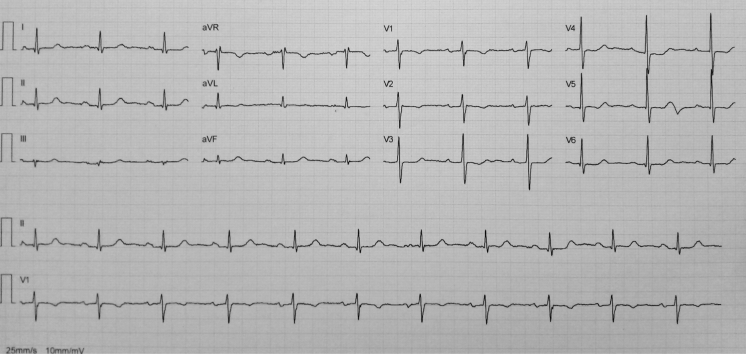
Electrocardiogram (ECG) revealed sinus rhythm at 60 beats/min, T-wave inversion in leads V1 and V2, and slightly biphasic T waves in leads V3 to V5 (Figure 1). Complete blood count and D-dimer were normal. C-reactive protein (CRP) was elevated (35 mg/dl; reference limit (RL) <5.0 mg/dl). Both creatine kinase (CK) (380 U/l; RL 10 to 145 U/l) and cardiac troponin I (cTnI) (6.24 ng/ml; RL <0.04 ng/ml) were increased.
Figure 1.
Electrocardiogram at Admission
Sinus rhythm, T-wave inversion in leads V1 and V2 and slightly biphasic T waves in leads V3 to V5.
A preliminary diagnosis of non–ST-segment elevation acute myocardial infarction (AMI) was assumed, and the patient underwent an early invasive strategy; coronary angiography showed no obstructive coronary artery disease (CAD). Transthoracic echocardiogram (TTE) revealed preserved biventricular systolic function with no regional wall motion abnormalities and no evident pericardial effusion.
Management
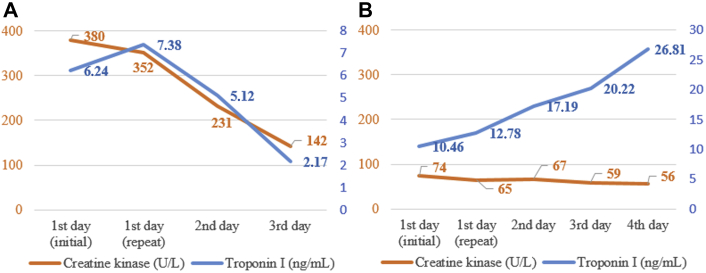
On the basis of the described findings, a presumable diagnosis of myopericarditis was considered, and symptomatic treatment with ibuprofen 600 mg (3 times daily) was initiated. The following days, levels of CRP and both cTnI and CK started to decrease consistently (Figure 2A); ECG normalized. The patient was discharged on a drug-tapering regimen.
Figure 2.
Temporal Evolution of the Biomarkers of Myocardial Necrosis
(A) During the first and (B) the second hospital admissions.
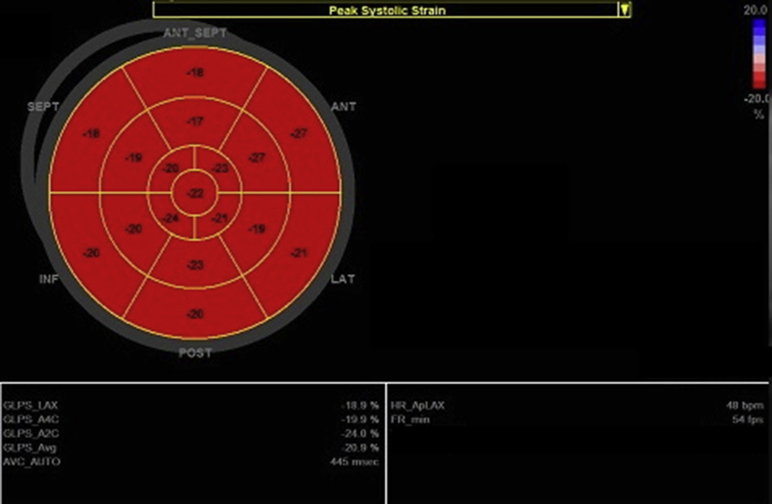
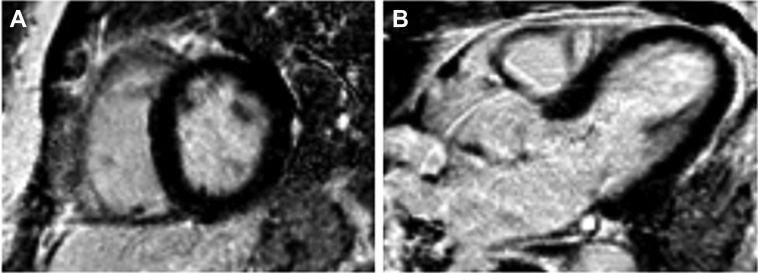
Four weeks later, she returned to the hospital because of a new episode of brief chest discomfort. Physical examination was unremarkable. ECG was normal. Routine blood tests revealed isolated cTnI elevation (10.46 ng/ml); kidney function was normal. Repeat coronary angiography excluded CAD, and a new transthoracic echocardiogram was unremarkable, with completely normal left ventricular global longitudinal peak systolic strain (Figure 3). Cardiac magnetic resonance (CMR) was normal, and late gadolinium enhancement was absent (Figures 4A and 4B). Surprisingly and despite a clear lack of symptoms, cTnI kept rising, to 26.81 ng/ml on the fourth day of hospitalization, whereas CK remained normal (Figure 2B). Given these discrepancies, we considered the possibility of falsely elevated cTnI levels from the routine assay used in our hospital (Beckman Coulter Access AccuTnI+3; Beckman Coulter, Inc., Brea, California). By using a point-of-care assay, normal results were obtained. The same blood sample was sent to the AccuTnI+3 supplier's laboratory, and it was treated with interference blocking proteins. The conclusion reached was that the presence of heterophile antibodies (hAbs) caused abnormally elevated cTnI levels. We reported this finding to the patient and reinforced the absence of heart disease.
Figure 3.
Left Ventricular Global Longitudinal Strain: “Bull’s Eye” Plot
Global peak systolic strain is normal (−20.9%; normal range <−16.0%); all segments show preserved strain. ANT = anterior; Ap = apical; AVC = aortic valve closure; Avg = average; A2C = apical 2-chamber; A4C = apical 4-chamber; FR = frame rate; GLPS = global longitudinal peak systolic strain; HR = heart rate; INF = inferior; LAT = lateral; LAX = long-axis; POST = posterior; SEPT = interventricular septum. (General Electric Healthcare, Little Chalfont, United Kingdom.)
Figure 4.
Cardiac Magnetic Resonance
T1-weighted (A) short-axis and (B) 5-chamber views showing no myocardial late gadolinium enhancement.
Discussion
The fourth universal definition of AMI denotes the presence of acute myocardial injury detected by abnormal cardiac biomarkers in the setting of clinical, electrocardiographic, imaging, or angiographic evidence of acute myocardial ischemia (1). However, some of these cases (varying from 1% to 13%) occur without angiographic CAD (≥50% diameter stenosis in a major epicardial vessel). In the absence of any clinically overt specific cause for the acute presentation, the term myocardial infarction with nonobstructive coronary arteries (MINOCA) is used to classify this condition (2). Conditions frequently associated with such an entity include Takotsubo cardiomyopathy, myocarditis, and cardiac emboli (3). There is an important overlap between the diagnostic criteria for MINOCA and clinically suspected myocarditis, the diagnosis first assumed in our patient. According to expert consensus from the European Society of Cardiology, although endomyocardial biopsy should be taken as the gold standard for the diagnosis of definite myocarditis, CMR may be considered in clinically stable patients, and findings should be based on the Lake Louise criteria (4).
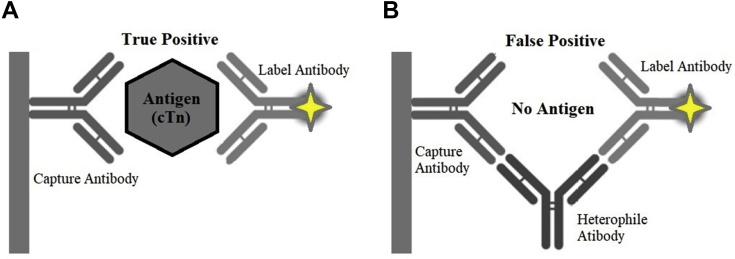
In the current case and regarding the second admission, despite the persistence of elevated cTnI, there were no associated consistent symptoms or evidence of an obvious cardiovascular cause for such phenomenon because coronary angiograms and other cardiac images were consistently normal (1). Nonetheless, cTn may also be elevated in a broad spectrum of noncardiovascular conditions such as strenuous exercise, chronic kidney disease, infectious diseases, sepsis, severe anemia, stroke, and subarachnoid hemorrhage (1). Our patient did not present with any condition that could explain isolated and persistent cTn elevation. Therefore, we suspected false positive results. Like most commercially available cTnI assays, AccuTnI+3 is a 2-site immunoenzymatic “sandwich” assay in which a capture antibody specifically binds to the human cTnI present in the sample, and then the captured cTnI reacts with a second antibody that is coupled to an indicator molecule detected by chemiluminescent methods (5). Erroneous cTnI results are uncommon (<0.5%) (1). As for other laboratory tests, they may emerge from a variety of pre-analytical issues (such as misidentification) and analytical constraints, including interference from spurious hemolysis, hyperbilirubinemia or turbidity, fibrin clots, rheumatoid factor, calibration bias, reagent deterioration, analyzer malfunction, instrumental carryover, and inappropriate sample dilution, as well as the presence of microparticles or immunocomplexes, hAbs, human antimouse antibodies, and autoantibodies (6). In cases of remarkable discrepancy between clinical findings and cTn levels, cTn should be measured using an alternative assay. If a cTn mismatch is present, the supplier’s laboratory must be contacted to rule out analytical interferences resulting in real but very rare false positive measurements (7). In our patient, normal cTnI levels were obtained with a point-of-care test that uses different antibodies for cTnI quantitation, thus confirming the presence of false positive results. Suspecting an analytical problem, we sent blood from the same sample to the Beckman Coulter laboratory, where it was treated with a preparation containing specific hAb blocking reagents (PolyMak 33, HBR-1) that, added to immunoassay reagents, have the ability to block the binding of hAbs and prevent interference (Figures 5A and 5B) (8). This test reduced the cTnI result by 74% of the original value, a finding indicating both the presence and the successful blockage of hAbs.
Figure 5.
Schematic Presentation
(A) Normal 2-site “sandwich” immunoassay. (B) Interference by heterophile antibodies.
The hAbs, present in 0.1% to 3% of the general population, can usually develop after a viral infection, animal exposure, certain diets and medications, vaccines, rheumatoid factors, blood transfusions, autoimmune diseases, and dialysis (6,8). They exhibit weak multispecific activity against poorly defined antigens, binding not specifically to portions of the assay antibodies, thus representing a rare yet possible source of false positives in common cTn assays. This interference may be transient or become permanent (6).
Follow-Up
The patient was referred to internal medicine consultation to search for an underlying organic disease, namely, autoimmune, given her history of fibromyalgia. No cardiovascular symptoms or events were recorded since that time.
Conclusions
The present case draws attention to the association between circulating serum hAbs and the possibility of falsely elevated cTn using current immunoassays. Although it is a rare phenomenon, all cardiologists must be aware of it, especially when diagnostic investigations are discrepant or inconclusive, to avoid misdiagnosis.
Footnotes
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, or patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Thygesen K., Alpert J.S., Jaffe A.S. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 2.Agewall S., Beltrame J.F., Reynolds H.R. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. 2017;38:143–153. doi: 10.1093/eurheartj/ehw149. [DOI] [PubMed] [Google Scholar]
- 3.Sá F.M., Ruivo C., Santos L.G. Myocardial infarction with nonobstructive coronary arteries: a single-center retrospective study. Coron Artery Dis. 2018;29:511–515. doi: 10.1097/MCA.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 4.Caforio A.L., Pankuweit S., Arbustini E. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 5.Tate J.R., Panteghini M. Measurement of cardiac troponin revisited. Biochim Clin. 2008;32:535–546. [Google Scholar]
- 6.Lippi G., Aloe R., Meschi T., Borghi L., Cervellin G. Interference from heterophilic antibodies in troponin testing: case report and systematic review of the literature. Clin Chim Acta. 2013;426:79–84. doi: 10.1016/j.cca.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Mair J., Lindahl B., Muller C. What to do when you question cardiac troponin values. Eur Heart J Acute Cardiovasc Care. 2018;7:577–586. doi: 10.1177/2048872617708973. [DOI] [PubMed] [Google Scholar]
- 8.Ghali S., Lewis K., Kazan V. Fluctuation of spuriously elevated troponin I: a case report. Case Rep Crit Care. 2012;2012:585879. doi: 10.1155/2012/585879. [DOI] [PMC free article] [PubMed] [Google Scholar]