Abstract
We report the case of a 54-year-old woman who presented for cardiological evaluation having noted a decline in her heart rate on her wearable heart rate monitor. She was found to be in 2:1 atrioventricular block. Subsequent evaluation revealed cardiac and pulmonary sarcoidosis, treated with steroids and implantable cardioverter-defibrillator placement. (Level of Difficulty: Beginner.)
Key Words: bradycardia, cardiac pacemaker, cardiovascular disease
Abbreviations and Acronyms: AVB, atrioventricular block; HR, heart rate
Graphical abstract
We report the case of a 54-year-old woman who presented for cardiological evaluation having noted a decline in her heart rate on her wearable heart…
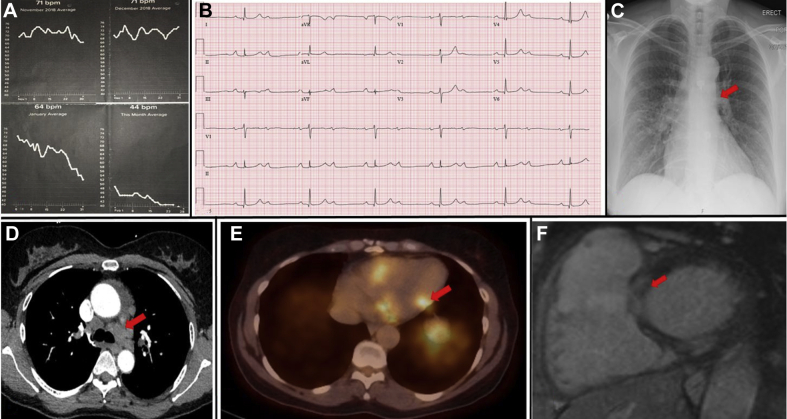
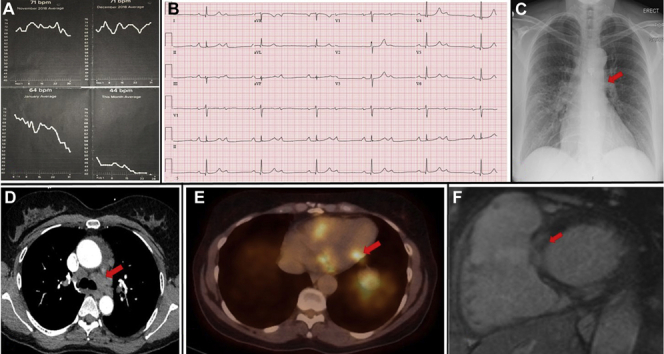
A 54-year-old white woman with a history of hypertension presented for cardiological assessment having noted a gradual decline in resting heart rate (HR) over a 2-month period, as recorded by her smartwatch Fitbit fitness tracker wearable HR monitor (Fitbit, San Francisco, California) (Figure 1A). She reported associated chest tightness and a decline in exercise tolerance, with no syncope. Electrocardiography revealed sinus rhythm with 2:1 atrioventricular block (AVB) (Figure 1B). She was admitted to the hospital for inpatient evaluation.
Figure 1.
Clinical Data
(A) Wearable heart rate monitor trend. (B) Twelve-lead electrocardiogram showing 2:1 atrioventricular block. (C) Chest radiography showing prominent hila. (D) Computed tomography showing mediastinal, bilateral hilar and bilateral intrapulmonary adenopathy. (E) Positron emission tomography showing increased 18F-fluorodeoxyglucose uptake at the inferolateral base, midportion of the inferolateral wall, and lateral wall of the right ventricle, consistent with an active inflammatory process. (F) Cardiac magnetic resonance imaging showing multiple foci of midmyocardial to subepicardial delayed enhancement.
Chest radiography was notable for prominent right hilum (Figure 1C). Chest computed tomography revealed mediastinal, bilateral hilar and bilateral intrapulmonary adenopathy as well as pulmonary nodules and nodular thickening of the interstitium suggestive of pulmonary sarcoidosis (Figure 1D). Results of pulmonary function tests were normal. These findings prompted further investigation for possible cardiac involvement of sarcoidosis, causing AVB. Cardiac positron emission tomography identified cardiac patchy increased 18F-fluorodeoxyglucose uptake at the inferolateral base, midportion of the inferolateral wall, and lateral wall of the right ventricle, consistent with an active inflammatory process (Figure 1E). Cardiac magnetic resonance imaging revealed multiple foci of midmyocardial to subepicardial delayed enhancement in the basal anterior and inferior walls and in the inferior wall at midcavity, in a pattern consistent with cardiac sarcoidosis (Figure 1F). Biventricular function was normal. Inpatient telemetry demonstrated persistent 2:1 AVB and intermittent higher degree AVB. A diagnosis of sarcoidosis with cardiac and limited pulmonary parenchymal involvement was made. The patient was managed by a multidisciplinary team including cardiology, pulmonology, rheumatology, and electrophysiology. The weight of clinical evidence was believed to be strong enough to obviate the need for tissue diagnosis. A Boston Scientific (Marlborough, Massachusetts) dual-chamber implantable cardioverter-defibrillator was placed, as per consensus statement clinical guidelines (1). Two weeks following device implantation, the patient was initiated on a course of steroid therapy, with prednisone 40 mg once daily.
Following 1 month of steroid therapy, the patient returned for device follow-up. The wound had healed well, lead parameters were stable, and no arrhythmia events had been recorded. The patient’s atrioventricular conduction had recovered, and she no longer required ventricular pacing. The RHYTHMIQ algorithm was enabled to minimize unnecessary right ventricular pacing. Further device follow-up with 3-monthly remote home monitoring was arranged. A gradual wean of the steroid dose was planned.
The popularity of wearable technologies including smartwatches has increased. Many wearable devices can monitor HR with clinically acceptable accuracy and can provide important information regarding arrhythmia. Wearable devices are set to become increasingly relevant in cardiac monitoring. Well-designed studies are essential to establish the role of wearable technologies in cardiovascular diagnostics. Recent studies have demonstrated their effectiveness in detecting atrial fibrillation (2). The AliveCor KardiaMobile device (AliveCor, Mountain View, California) was recently approved by the U.S. Food and Drug Administration to detect bradycardia (HR <40 beats/min) as well as tachycardia (HR 100 to 140 beats/min) (3). The field is likely to continue to evolve rapidly. This clinical vignette highlights an interesting case of how recordings from a wearable HR monitor ultimately prompted investigation, diagnosis, and multidisciplinary treatment of sarcoidosis.
Footnotes
This publication was funded in part by the Pennsylvania Steel Company EP Research Fund. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, or patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Al-Khatib S.M., Stevenson W.G., Ackerman M.J. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018;15:e73–e189. doi: 10.1016/j.hrthm.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 2.Turakhia M.P., Desai M., Hedlin H. Rationale and design of a large-scale, app-based study to identify cardiac arrhythmias using a smartwatch: the Apple Heart Study. Am Heart J. 2019;207:66–75. doi: 10.1016/j.ahj.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AliveCor Our devices. https://clinicians.alivecor.com/our-devices/ Available at: