Abstract
Patients with systemic lupus erythematosus (SLE) can present with multiple cardiovascular pathologies, including pulmonary hypertension, valvular disease, pericarditis, myocarditis, and premature atherosclerosis. SLE medications can also cause cardiovascular side effects. We present a patient who developed a severe cardiomyopathy secondary to the hydroxychloroquine prescribed to treat her SLE. (Level of Difficulty: Beginner.)
Key Words: cardiomyopathy, heart failure, hydroxychloroquine, systemic lupus erythematosus
Abbreviations and Acronyms: AVB, atrioventricular block; BNP, B-type natriuretic peptide; ECG, electrocardiogram; EF, ejection fraction; EMB, endomyocardial biopsy; HCQ, hydroxychloroquine; hsTnI, high-sensitivity troponin I; SLE, systemic lupus erythematosus
Graphical abstract
Patients with systemic lupus erythematosus (SLE) can present with multiple cardiovascular pathologies, including pulmonary hypertension, valvular disease…
History of Presentation
A 51-year-old female patient presented to the emergency department with 1 week of dyspnea on exertion, abdominal distension, orthopnea, and lower extremity swelling. Her symptoms developed 8 months prior to presentation but worsened 1 week prior to admission. On arrival, the patient’s blood pressure was 102/67 mm Hg, and her heart rate was 62 beats/min. On examination, jugular venous pressure was elevated, and she had ascites and lower extremity edema.
Learning Objectives
-
•
HCQ cardiotoxicity is an underrecognized cause of restrictive cardiomyopathy, with its exact prevalence unknown due to the absence of observational studies.
-
•
Routine annual surveillance for cardiotoxicity should be performed with ECG, echocardiogram, and serum cardiac biomarkers in patients treated with HCQ.
-
•
An EMB should be performed to confirm diagnosis of HCQ cardiotoxicity.
-
•
Treatment includes HCQ discontinuation, diuretics, and afterload reduction for heart failure and a pacemaker for high-degree AVB.
Medical History
The patient had been diagnosed with systemic lupus erythematosus (SLE) at 41 years of age. She had lupus nephritis early in the course of disease that progressed to chronic kidney disease. Her initial treatment regimen included prednisone and hydroxychloroquine (HCQ) titrated to 200 mg twice daily during the first year of her diagnosis. She had 10 years of exposure at the same dose with few flares. She was also diagnosed with common variable immunodeficiencies with multiple infections requiring rotating courses of antibiotics and biopsy-proven inflammatory myositis, treated with rituximab infusions.
Differential Diagnosis
Lupus cardiomyopathy, lupus myocarditis, ischemic cardiomyopathy, constrictive pericarditis, HCQ cardiomyopathy, viral myocarditis, hypertensive cardiomyopathy, hypertrophic cardiomyopathy, infiltrative cardiomyopathies such as amyloidosis, sarcoidosis, and lysosomal storage disorders are differential diagnoses to consider in this patient.
Investigations
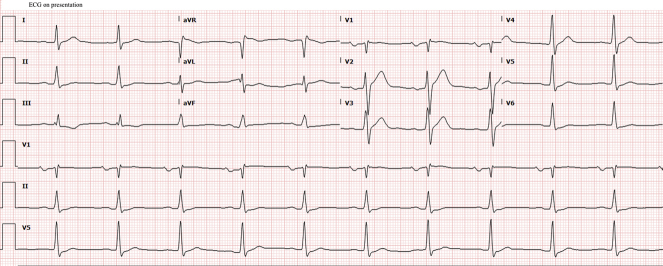
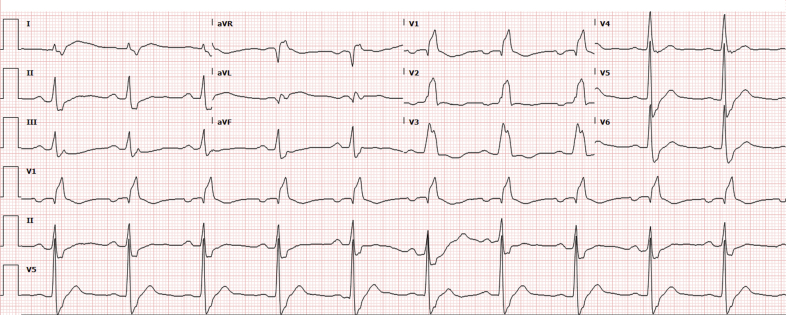
The patient’s electrocardiogram (ECG) showed left atrial enlargement and first-degree atrioventricular block (AVB) (Figure 1). Pertinent laboratory test findings included the following: creatinine level, 1.5 mg/dl; creatinine kinase, 592 U/l; B-type natriuretic peptide (BNP), 4,737 pg/ml; high-sensitivity troponin I (hsTnI), 532 ng/l; erythrocyte sedimentation rate, 18 mm/h; negative anti–double-stranded deoxyribonucleic acid; and normal complement levels.
Figure 1.
Electrocardiogram With Left Atrial Enlargement and First-Degree Atrioventricular Block
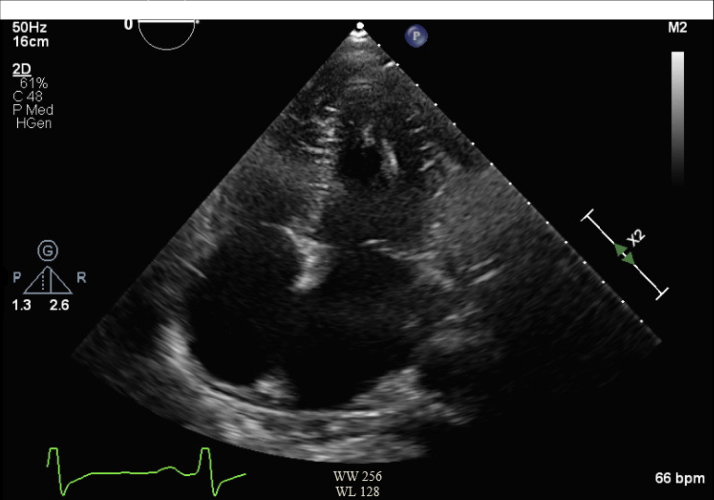
Transthoracic echocardiogram revealed mild left ventricular dysfunction (ejection fraction [EF] 45% to 50%), grade II diastolic dysfunction, mild to moderately reduced right ventricular function, and biatrial enlargement (Figure 2).
Figure 2.
Transthoracic Echocardiogram Apical 4-Chamber View With Biatrial Enlargement and Grade II Diastolic Dysfunction
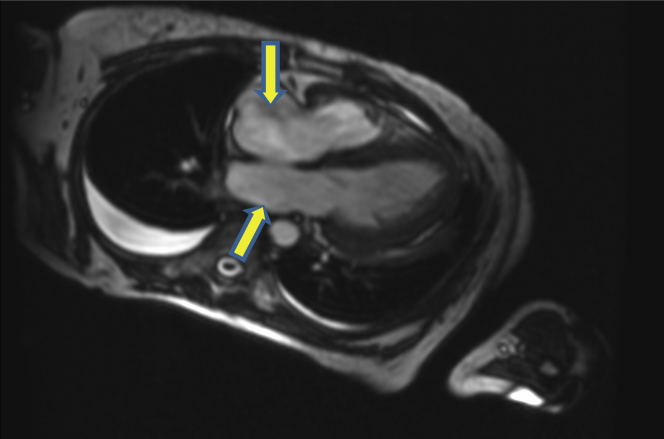
Right heart catheterization after diuresis revealed elevated right atrial pressure but normal pulmonary capillary wedge pressure, pulmonary artery pressures, and a reduced cardiac index (Table 1). Cardiac magnetic resonance imaging showed left ventricular hypertrophy, biatrial enlargement, mildly reduced left ventricular function (EF 52%), moderate right ventricular dysfunction (EF 36%), and no evidence of late gadolinium enhancement (Figure 3).
Table 1.
Invasive Hemodynamic Measurements
| Right atrium, mm Hg | 12 |
| Pulmonary artery, mm Hg | 20/12 (14) |
| Pulmonary artery saturation, % | 53 |
| Pulmonary capillary wedge pressure, mm Hg | 11 |
| Fick cardiac index, l/min/m2 | 1.98 |
| Systemic vascular resistance, dynes/s/cm–5 | 1,755 |
Figure 3.
Cardiac Magnetic Resonance Imaging 4-Chamber View
Cardiac magnetic resonance imaging 4-chamber view with severe biatrial enlargement, hypertrophied left ventricle, and no late gadolinium enhancement.
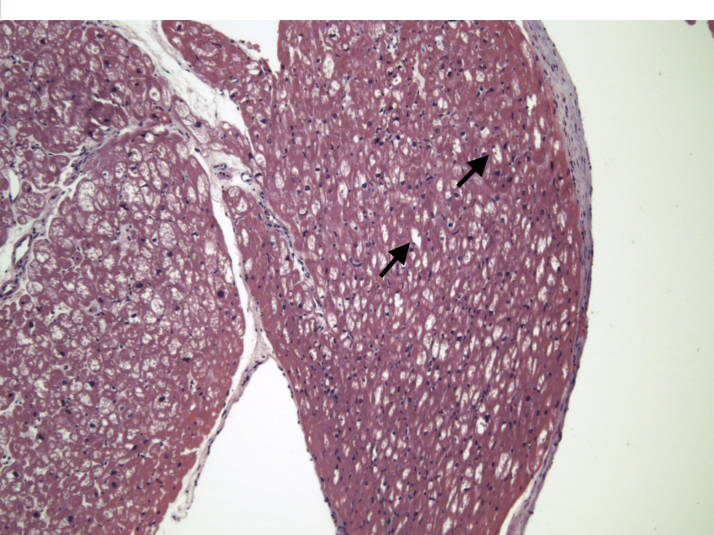
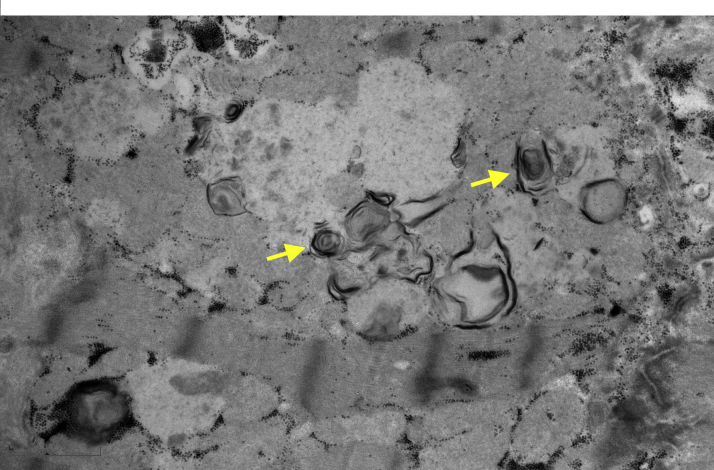
The patient underwent an endomyocardial biopsy (EMB). Hematoxylin and eosin staining revealed prominent myocyte vacuolization (Figure 4), and electron microscopy revealed lamellar bodies highly suggestive of HCQ cardiotoxicity (Figure 5).
Figure 4.
Bright-Field Microscopy of Endomyocardial Biopsy
Hematoxylin and eosin stains with prominent myocyte vacuolization (black arrows).
Figure 5.
Electron Microscopy of Endomyocardial Biopsy Sample
Electron microscopy of endomyocardial biopsy sample, with lamellar bodies suggesting hydroxychloroquine toxicity (yellow arrows).
Management
The patient received intravenous furosemide for fluid overload. Her home HCQ was held on admission. The patient was evaluated by rheumatology, nephrology, general cardiology, and advanced heart failure services. An eye examination did not show a retinal abnormality. Her symptoms improved with diuresis but at the expense of worsening renal function: her creatinine level increased to 1.8 mg/dl from a baseline of 1.2 to 1.4 mg/dl. Diuretics were held with improvement in renal function. The patient was discharged on prednisone, mycophenolate, and an oral diuretic, with close follow-up in the heart failure clinic.
Discussion
HCQ is a 4-aminoquinoline, developed as a less toxic antimalarial drug than chloroquine. It is used as a first-line agent in the management of many rheumatologic diseases because of its relatively safe profile compared with that of other drugs. Retinal damage and skeletal muscle toxicity characterized by proximal muscle weakness are well-recognized complications. Cardiac toxicity can occur in the absence of retinal toxicity; it shares pathological features similar to skeletal muscle toxicity but is less prevalent (1,2).
The proposed mechanism of chloroquine toxicity includes alkalization of the intracellular space by binding directly to the intracellular enzymes of the lysosomes, affecting their degradation function and leading to accumulation of phospholipid, glycogen, myeloid, and curvilinear bodies (3). Risk factors for HCQ cardiotoxicity include female sex, older age, renal dysfunction, lupus nephritis, underlying cardiac disease, and prolonged (>5 years) and high doses of HCQ (5 mg/kg/day) (2). In a systematic review performed by Chatre et al. (4), the median cumulative dose of HCQ was 1,235 g, and median duration of HCQ treatment was 7 years in patients who developed cardiotoxicity. However, HCQ cardiotoxicity has been reported with lower cumulative doses and shorter duration of treatment. Cardiovascular complications include restrictive cardiomyopathy, biventricular systolic dysfunction, and conduction disease. The most frequent conduction abnormalities include AVB (24%) and bundle branch blocks (26%). Torsade de pointes due to QT prolongation can be fatal. Ten percent of cases required a permanent pacemaker for high-degree atrioventricular block or bradyarrhythmias. The conduction system abnormalities are probably associated with the quinidine-like effect, blocking voltage-gated Na+ channels, or direct damage to the conduction system (5,6).
Surveillance for cardiotoxicity may be challenging due to coexisting risk factors for heart failure such as hypertension, valvular heart disease, coronary artery disease, and autoimmune myocardial involvement in patients with SLE and need for invasive testing (e.g., EMB) to confirm diagnosis (4). An electrocardiogram for detection of conduction system abnormalities and echocardiogram with Doppler imaging for structural abnormalities should be performed before initiation of treatment, at month 1, and then as yearly surveillance. A Holter monitor should be used in patients with unexplained syncope and documented evidence of HCQ toxicity. Echocardiographic abnormalities of HCQ cardiotoxicity include ventricular hypertrophy, depressed left ventricular EF, restrictive physiology, and valvular abnormalities such as mitral regurgitation.
The role of cardiac magnetic resonance imaging as a diagnostic tool has not been validated in HCQ toxicity but is commonly performed. It is often used to exclude other infiltrative cardiomyopathies, describe the extent of ventricular hypertrophy, and assess the severity of fibrosis with late gadolinium enhancement (2). The role of T1 mapping (reduced values as seen in Fabry’s disease due to lipid accumulation) needs to be investigated further (4). EMB is the gold standard to diagnose HCQ cardiotoxicity based on findings on light microscopy and electron microscopy. Light microscopy shows hypertrophied myocytes with extensive vacuolization without inflammatory changes. The characteristic findings on electron microscopy include mega-mitochondria, myelin, and curvilinear bodies. Lysosomal storage disorders such as Fabry’s disease that have similar histological findings on EMB need to be ruled with genetic testing (2,4). Cardiac biomarkers (BNP and hsTnI) are nonspecific but may be used routinely to detect myocardial damage.
Treatment for HCQ cardiotoxicity is supportive. Management includes HCQ discontinuation, diuretics, and afterload reduction for heart failure and a pacemaker for high-degree AVB. The prognosis for patients with HCQ toxicity varies. A systematic review of 127 HCQ and chloroquine-related cardiotoxicity cases reported a recovery of normal heart function in 45% of cases, irreversible damage in 13%, and death in 31% of cases despite HCQ/chloroquine drug discontinuation in 78 patients. Heart transplantation was performed in only 2 patients after drug withdrawal (4).
Follow-Up
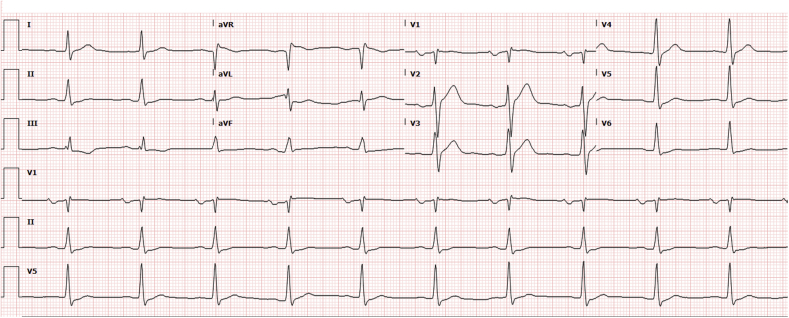
The patient was admitted 1 week after discharge with volume overload. A repeat ECG was concerning for worsening conduction abnormalities (Figure 6). She was readmitted and started on a continuous furosemide infusion. The electrophysiology team was consulted but did not believe that a pacemaker was warranted because of the absence of high-degree AVB. Prednisone was held, leaving mycophenolate as the patient’s only immunosuppressant. The evaluation for cardiac transplantation was initiated because of worsening heart failure symptoms during the hospital admission. The patient had clinical improvement, and the heart transplantation evaluation was halted. In the 4 months since discontinuation of HCQ, the patient has had one admission for volume overload; the conduction system abnormalities persist.
Figure 6.
Follow-Up Electrocardiogram
Follow-up electrocardiogram with right bundle branch block and prolonged QRS duration of 200 ms.
Conclusions
There is no consensus on how to screen for HCQ-induced cardiomyopathy, but here we add another case to the literature to better define the disease and to stress the importance of prompt diagnosis with EMB when a patient with SLE is diagnosed with new-onset heart failure. Routine HCQ surveillance for cardiac toxicity using serial ECG, echocardiogram, and cardiac biomarkers such as BNP and hsTnI should be performed.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, or patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.August C., Holzhausen H.J., Schmoldt A., Pompecki R., Schrӧder S. Histological and ultrastructural findings in chloroquine-induced cardiomyopathy. J Mol Med (Berl) 1995;73:73–77. doi: 10.1007/BF00270580. [DOI] [PubMed] [Google Scholar]
- 2.Joyce E., Fabre A., Mahon N. Hydroxychloroquine cardiotoxicity presenting as a rapidly evolving biventricular cardiomyopathy: key diagnostic features and literature review. Eur Heart J Acute Cardiovasc Care. 2013;2:77–83. doi: 10.1177/2048872612471215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cubero I., Reguero R., Ortega R. Restrictive cardiomyopathy caused by chloroquine. Br Heart J. 1993;69:451–452. doi: 10.1136/hrt.69.5.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatre C., Roubille F., Vernhet H., Jorgensen C., Pers Y.M. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf. 2018;41:919–931. doi: 10.1007/s40264-018-0689-4. [DOI] [PubMed] [Google Scholar]
- 5.Newton-Cheh C., Lin A.E., Baggish A.L., Wang H. Case records of the Massachusetts General Hospital. Case 11-2011. A 47-year-old man with systemic lupus erythematosus and heart failure. N Engl J Med. 2011;364:1450–1460. doi: 10.1056/NEJMcpc1011319. [DOI] [PubMed] [Google Scholar]
- 6.Tӧnnesmann E., Kandolf, Lewalter T. Chloroquine cardiomyopathy—a review of the literature. Immunopharmacol Immunotoxicol. 2013;35:434–442. doi: 10.3109/08923973.2013.780078. [DOI] [PubMed] [Google Scholar]