Corresponding Author

Key Words: closure, device, interventional, leak, paravalvular
Percutaneous closure of paravalvular leaks (PVLs) has surpassed being merely an option for patients ineligible for surgery and now stands as the intervention of choice in the majority of cases, with outcomes that compare favorably to reoperation (1, 2, 3). The success of this technique depends on proper case selection, expert imaging guidance, and choice of a device that will minimize residual post-procedural PVL. In this issue of JACC: Case Reports, Cubeddu et al. (4) describe a case in which they offered symptomatic relief to an unwell patient with a large PVL after bioprosthetic mitral valve replacement by using the Cardioform Septal Occluder device (CSO, Gore Medical, Flagstaff, Arizona).
Pathophysiology of Paravalvular Leaks
Persistent PVLs after prosthetic valve replacement occur at a rate of 5% to 10% in the aortic position and 7% to 17% in the mitral position (5,6). A total of 4% to 6% of patients with PVL will present with symptoms (7), and the development of significant PVL is associated with poor clinical outcomes (8). Complications such as heart failure and hemolysis can arise and are independently associated with poor prognosis (5,9). PVLs tend to occur either because of malapposition of the prosthetic valve (most commonly from annular calcification mismatched with the round shape of the prosthesis) or because of failure of the valve’s anchoring secondary to endocarditis or technical difficulties with suturing (with continuous sutures, uneven sutures, or improper suture tension) (10, 11, 12). Unlike defects in the atrial septum, both mitral and aortic PVLs encounter high (ventricular) pressures, and therefore even small openings can become a source of significant regurgitation. Furthermore, because blood is driven through these small channels at high pressure, hemolysis and transfusion-dependent hemolytic anemia arise.
An Effective Approach to Paravalvular Leak Closure
When faced with a new patient requiring PVL closure, several steps are required to achieve a durable result in a safe and timely manner, as described here.
Case selection
All interventions carry risk, and thus guideline-recommended indications should be adhered to (13):
-
1.
Heart failure with progressive left ventricular dysfunction
-
2.
Hemolysis
Clinical assessment, imaging, and laboratory studies are mandatory. Given the possibility that young, fit patients may receive a more durable result from reoperation, it is essential that a heart team approach is followed. The ratio of surgical to percutaneous treatment in a given center may vary with expertise, and percutaneous PVL closure can be challenging.
Procedural planning
Operators must select the appropriate site of vascular access according to the valve in question and the device to be used. For example, the CSO requires a stiff sheath and therefore essentially mandates transapical access for mitral PVL closure. The most common site of access for aortic PVLs is the femoral artery, and for mitral PVLs it is the femoral vein (1). Transapical access and a hybrid approach with multiple access and wire snaring have been used. Next, the direction of crossing should be decided, and whether it is antegrade or retrograde will depend on the coordinates of the defect. This will also determine the orthogonal angiographic views likely to afford the best vantage points for operators to minimize foreshortening (Figures 1A to 1G). Finally, careful review of pre-operative transesophageal echocardiography (TEE) is essential to characterize the leak. Leaks may be multiple and split by suture lines. It is common for the regurgitant jet of PVLs to follow an irregular course and to travel eccentrically, thus making underestimation of severity on color Doppler imaging a possibility. In those valves where washing jets arise from the sewing ring, further confusion can occur (10).
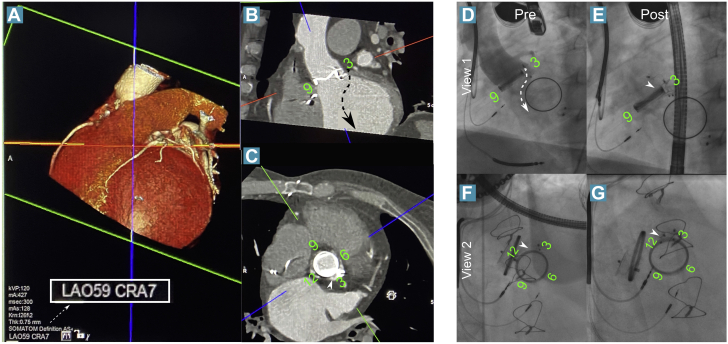
Figure 1.
Planning and Closure of Aortic PVL in a Patient With Previous Percutaneous Closure of Aortic and Mitral PVLs
(A to C) When the crosshairs are aligned with the valve and the left ventricular outflow tract, the third orthogonal plane will be close to the working view en face (the green plane here), and thus the ideal c-arm angle can be derived (left anterior oblique [LAO] 59, cranial [CRA] 7 here). (D) This can then be used procedurally to visualize the expected course of the jet according to transesophageal echocardiographic images (dashed arrow). The orthogonal view (F and G) resolves the overlapping of the old device at 3 o’clock and the paravalvular leak (PVL) at 2 o’clock (white arrowheads). In E and G, the new device can be seen at 2 o’clock. Post = post-procedural; Pre = pre-procedural.
Intraprocedural imaging guidance
Most operators standardize their visualization of the defect position by using a clockface approach (14) (Figure 2, mitral valve assessment). This allows the imager and the interventionist to share a framework. Continuous communication between the imager and operator is essential to facilitate efficient crossing and device delivery, as well as assessing success and complications. The ability to use both echocardiographic and fluoroscopic views to optimize positioning is a key skill, and there may be some benefit to coregistration of computed tomography and TEE images on the fluoroscopy screens.This is an option with the Azurion system (Philips, Amsterdam, the Netherlands) although we do not use it routinely.
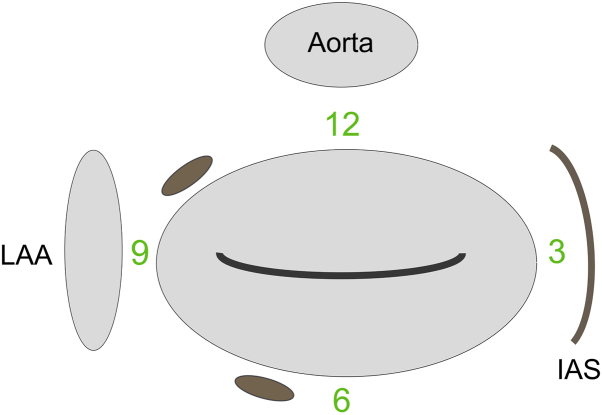
Figure 2.
Schematic Diagram of the Mitral Valve Seen on the TEE Surgical View
Two paravalvular leaks can be seen at the 7 o’clock and 10 o’clock positions. This system allows for standardized definition of the defect location and facilitates communication between the operator and the imager. IAS = intra-atrial septum; LAA = left atrial appendage; TEE = transesophageal echocardiogram.
Device options
Key determinants of device choice are delivery requirements (i.e., what size catheter and sheath can actually be delivered through the defect), the correct waist size to ensure obliteration of the defect itself (and whether multiple devices are needed), and the appropriate disc size and shape to ensure effective anchoring of the device but avoiding prosthetic valve leaflet impingement.
In practice, a range of devices designed by Kurt Amplatzer has been used (Abbott Vascular, Chicago, Illinois). More recently a dedicated PVL closure device, the PLD (PVL device) (Occlutech, Helsingborg, Sweden), has emerged as a dedicated tool. This device is made of a nitinol mesh and has a twisted waist (like PFO closure devices) or a bulkier waist as in the Amplatzer vascular plug (AVP-3) device (Abbott). The twist system has the theoretical disadvantage of leaving space for a residual jet, although flow is often obliterated at the end of the procedure with either device. It comes in square and rectangular forms. Sometimes, however, a row of multiple devices achieves a better result. Table 1 provides an overview of devices in current use.
Table 1.
Properties of the Devices Commonly Used in Percutaneous Closure of PVLs and the Nonfenestrated CSO Device
| Device | Manufacturer | Material | Delivery Sheath | Size Range (mm) |
|---|---|---|---|---|
| Amplatzer duct occluder (ADO) | Abbott | Nitinol mesh | 5-F–7-F | 9–22 |
| ADO-2 | Abbott | Nitinol mesh | 4-F–5-F | 9–12 |
| Amplatzer muscular VSD occluder | Abbott | Nitinol mesh | 5-F–9-F | 9–26 |
| Amplatzer AVP II | Abbott | Nitinol mesh | 5-F–9-F | 3–22 |
| Amplatzer AVP III | Abbott | Nitinol mesh | 3-F | 2×4–5×14 |
| Amplatzer AVP IIII | Abbott | Nitinol mesh | 4-F–5-F | 6–8 |
| PLD-square twist | Occlutech | Nitinol mesh | 6-F–7-F | 3, 5, 7 |
| PLD-square waist | Occlutech | Nitinol mesh | 6-F–7-F | 4, 5, 6, 7 |
| PLD-rectangular twist | Occlutech | Nitinol mesh | 6-F–9-F | 5×4, 5×3, 7×4, 10×4, 12×5 |
| PLD-rectangular waist | Occlutech | Nitinol mesh | 6-F–10-F | 4×2, 6×3, 8×4, 10×4, 12×5, 14×6, 16×8, 18×10 |
| Gore CSO | Gore | Nitinol frame polytetrafluoroethylene membrane | 11-F | 20, 25, 30 |
ADO = Amplatzer duct occluder; AVP = Amplatzer vascular plug; CSO = Cardioform septal occluder; PLD = paravalvular leak device; PVL = paravalvular leak; VSD, ventricular septal defect.
The Promise of a Nonfenestrated Device
We commend Cubeddu et al. (4) for their excellent case presentation and share their interest in the use of a nonfenestrated device. In patients with severe, transfusion-dependent hemolysis, there is a drive to achieve complete resolution of the aberrant flow (although reducing the leak size can paradoxically increase hemolysis). In the atrial septal position, the theoretical benefits of the CSO include the rapidity with which resolution of the shunt is achieved (15). We have some concern that this device is not proven in higher-pressure circulation. There are cases showing its use in an atrioventricular position in closure of mitral leaflet perforation (16,17). In 1 of these cases, the device deformed because of the stiff sheath and oblique angle in the transseptal approach. This complication can, of course, be obviated with the transapical approach, as in the current case. We seek to avoid the transapical access where possible because this approach is associated with a higher rate of complications (18), and, although it is easier for the operator once access is gained, it can be more uncomfortable for the patient, given the prolonged healing duration. Ultimately, this device is a useful addition to the armarium of closure specialists, but we should consider whether fenestrated devices better suited to the task are able to achieve the same result.
Conclusions
Percutaneous closure of paravalvular regurgitation remains in the hands of subspecialist structural interventionalists. In time, better and more dedicated tools will emerge, and the option of a nonfenestrated system may prove to be an important extension of that tool set. We propose that adoption of this feature should not occur at the expense of the valuable traits of existing devices (flexibility, deliverability, waist bulk, and shapeability), but rather, it should be achieved by incorporation of durable membranes into that family of designs.
Footnotes
Both authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, or patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Calvert P.A., Northridge D.B., Malik I.S. Percutaneous device closure of paravalvular leak: combined experience from the United Kingdom and Ireland. Circulation. 2016;134:934–944. doi: 10.1161/CIRCULATIONAHA.116.022684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorajja P., Cabalka A.K., Hagler D.J., Rihal C.S. Percutaneous repair of paravalvular prosthetic regurgitation: acute and 30-day outcomes in 115 patients. Circ Cardiovasc Interv. 2011;4:314–321. doi: 10.1161/CIRCINTERVENTIONS.110.960955. [DOI] [PubMed] [Google Scholar]
- 3.Alkhouli M., Zack C.J., Sarraf M. Successful percutaneous mitral paravalvular leak closure is associated with improved midterm survival. Circ Cardiovasc Interv. 2017;10 doi: 10.1161/CIRCINTERVENTIONS.117.005730. [DOI] [PubMed] [Google Scholar]
- 4.Cubeddu R.J., Sanchez A.M., Perez E., Sleiman J., Lopez D., Navia J.L. First experience using a non-fenestrated Cardioform septal occluder for closure of giant mitral paravalvular leak. J Am Coll Cardiol Case Rep. 2020;2:468–472. doi: 10.1016/j.jaccas.2019.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammermeister K., Sethi G.K., Henderson W.G., Grover F.L., Oprian C., Rahimtoola S.H. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000;36:1152–1158. doi: 10.1016/s0735-1097(00)00834-2. [DOI] [PubMed] [Google Scholar]
- 6.Ionescu A., Fraser A.G., Butchart E.G. Prevalence and clinical significance of incidental paraprosthetic valvar regurgitation: a prospective study using transoesophageal echocardiography. Heart. 2003;89:1316–1321. doi: 10.1136/heart.89.11.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloch G., Vouhe P.R., Menu P. Long-term evaluation of bioprosthetic valves: 615 consecutive cases. Eur Heart J. 1984;5(Suppl D):73–80. doi: 10.1093/eurheartj/5.suppl_d.73. [DOI] [PubMed] [Google Scholar]
- 8.Kodali S., Pibarot P., Douglas P.S. Paravalvular regurgitation after transcatheter aortic valve replacement with the Edwards Sapien valve in the PARTNER trial: characterizing patients and impact on outcomes. Eur Heart J. 2015;36:449–456. doi: 10.1093/eurheartj/ehu384. [DOI] [PubMed] [Google Scholar]
- 9.Cho I.-J., Hong G.-R., Lee S., Byung-Chul C., Ha J.-W., Chung N. Predictors of prognosis in patients with mild to moderate paravalvular leakage after mitral valve replacement. J Card Surg. 2014;29:149–154. doi: 10.1111/jocs.12298. [DOI] [PubMed] [Google Scholar]
- 10.Rallidis L.S., Moyssakis I.E., Ikonomidis I., Nihoyannopoulos P. Natural history of early aortic paraprosthetic regurgitation: a five-year follow-up. Am Heart J. 1999;138:351–357. doi: 10.1016/s0002-8703(99)70124-9. [DOI] [PubMed] [Google Scholar]
- 11.Lee M.K., Choi J.B., Kim N.Y. Continuous suture technique for aortic valve replacement. Tex Heart Inst J. 2018;45:121. doi: 10.14503/THIJ-18-6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair S.K., Bhatnagar G., Valencia O., Chandrasekaran V. Effect of valve suture technique on incidence of paraprosthetic regurgitation and 10-year survival. Ann Thorac Surg. 2010;89:1171–1179. doi: 10.1016/j.athoracsur.2009.12.069. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura R.A., Otto C.M., Bonow R.O. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2438–2488. doi: 10.1016/j.jacc.2014.02.537. [DOI] [PubMed] [Google Scholar]
- 14.Gafoor S., Franke J., Bertog S. A quick guide to paravalvular leak closure. Interv Cardiol. 2015;10:112–117. doi: 10.15420/ICR.2015.10.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gevorgyan Fleming R., Kumar P., West B. Comparison of residual shunt rate and complications across 6 different closure devices for patent foramen ovale. Catheter Cardiovasc Interv. 2019 Oct 26 doi: 10.1002/ccd.28527. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Panaich S., Qazi A., Horwitz P., Staffey K., Rossen J. Transcatheter repair of anterior mitral leaflet perforation: deploy, retrieve, redeploy. J Am Coll Cardiol Case Rep. 2019;1:689–693. doi: 10.1016/j.jaccas.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frisoli T.M., Greenbaum A., O’Neill W.W., Wang D.D., Eng M. Percutaneous repair of mitral valve leaflet perforation. J Am Coll Cardiol Intv. 2019;12:210–213. doi: 10.1016/j.jcin.2018.11.043. [DOI] [PubMed] [Google Scholar]
- 18.Pineda A.M., Rymer J., Wang A. Trends and outcomes of alternative-access transcatheter aortic valve replacement. J Invasive Cardiol. 2019;31:E184–E191. [PubMed] [Google Scholar]