Abstract
Type A intramural hematoma (IMH) constitutes a variant of acute aortic syndrome. Western guidelines support an aggressive surgical approach, whereas Asian centers propose initial conservative treatment. Further expanding on this notion, we present a case of conservative subacute type A IMH management, resulting in radical hematoma resorption within 4 weeks. (Level of Difficulty: Beginner.)
Key Words: anti-impulse treatment, aortic dissection, intramural hematoma
Abbreviations and Acronyms: AD, aortic dissection; CTA, computational tomography angiography; HTN, hypertension; IMH, intramural hematoma; IRAD, International Registry of Acute Aortic Dissection; MDT, multidisciplinary team; TAA, thoracic aortic aneurysm
Graphical abstract
Type A intramural hematoma (IMH) constitutes a variant of acute aortic syndrome. Western guidelines support an aggressive surgical approach, whereas…
A 68-year-old man was transferred to our center after the diagnosis of a subacute aortic intramural hematoma (IMH) following an episode of back pain, associated with left lower limb weakness and paresthesia, and urinary retention 5 days before presentation. In specific, his pain was of acute onset, mainly located on the left lumbar region with radiation to the left thigh (T12-L3 dermatomal distribution), and described as aching. On admission he was hemodynamically stable with no signs of malperfusion complications.
Learning Objectives
-
•
To summarize the differential diagnosis of acute aortic syndrome variants, including classic aortic dissection, penetrating ulcer disease, and intramural hematoma.
-
•
To describe the clinical and radiological criteria pertaining to and safeguarding early conservative management of acute/subacute type A intramural hematomas.
The first reading of blood pressure in emergency department was 160/90 mm Hg in sinus tachycardia of 110 beats/mini. After prompt diagnosis and initiation of beta-blockers, his blood pressure was effectively reduced to 135/80 mm Hg and heart rate 90 beats/min.
Past Medical History
His past medical history was positive for systemic hypertension (HTN).
Differential Diagnosis
The constellation of symptoms mandated the exclusion of acute radiculopathy and a possible cauda equina syndrome; thus, urgent computed tomography was performed, which demonstrated a Stanford type A IMH extending to the diaphragmatic hiatus and excluded any mechanical causes of radiculopathy. Thereafter, the differential diagnosis included IMH, classic aortic dissection (AD), and penetrating ulcer disease, necessitating an aortic computational tomography angiography (CTA).
Investigations
The first CTA had shown a Stanford type A IMH, extending from the aortic root to the diaphragmatic aortic hiatus; the repeat CTA performed at our hospital demonstrated a nonexpanding IMH and an aneurysmatic thoracic aorta (TAA) (maximum IMH diameter: 4.6 mm on the ascending and 4.7 mm on the descending aorta; maximum TAA diameter: 44.7 mm at the sinuses of Valsalva, 42.4 mm at the sino-tubular junction, and 39.2 mm at the ascending aorta) (Figure 1). The initial laboratory results showed an inflammatory response (white blood cells: 11.6 × 109/l; C-reactive protein 145 mg/l) with an associated renal insult (creatinine 222 μmol/l; urea 13.4 mmol/l).
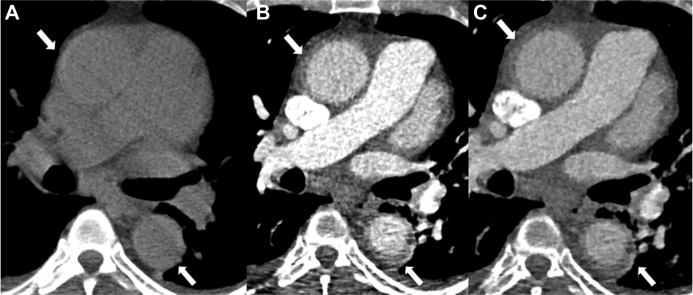
Figure 1.
CT of IMH
Computed tomography (CT) scan of the aorta before (A), and after the administration of iodinated contrast (arterial [B], delayed [C]). On noncontrast CT, there is high-density thickening of the ascending and descending aortic wall (arrow), indicating hematoma. Post contrast, there is no significant change in the enhancement, typical of intramural hematoma (IMH).
Management
After a multidisciplinary (MDT) discussion, a conservative treatment approach was decided with close clinical surveillance, tight blood pressure control, and weekly reassessment of the IMH via repeat CTA. An intensive anti-impulse regimen was instituted (beta-blocker; loop, thiazide, and potassium-sparing diuretics; alpha antagonist; calcium-channel blocker), aimed at maximum systolic pressure of 100 mm Hg; an angiotensin-converting enzyme inhibitor was added on the confirmation of renal function improvement. The patient’s back pain quickly subsided on mild analgesia, with concomitant improvement of his left lower limb and urinary neurological signs. After a trivial increase in diameter, follow-up imaging demonstrated a progressive decrease in IMH volume and radiological density, with no signs of a dissection flap or intimal entry point development (Figure 2).
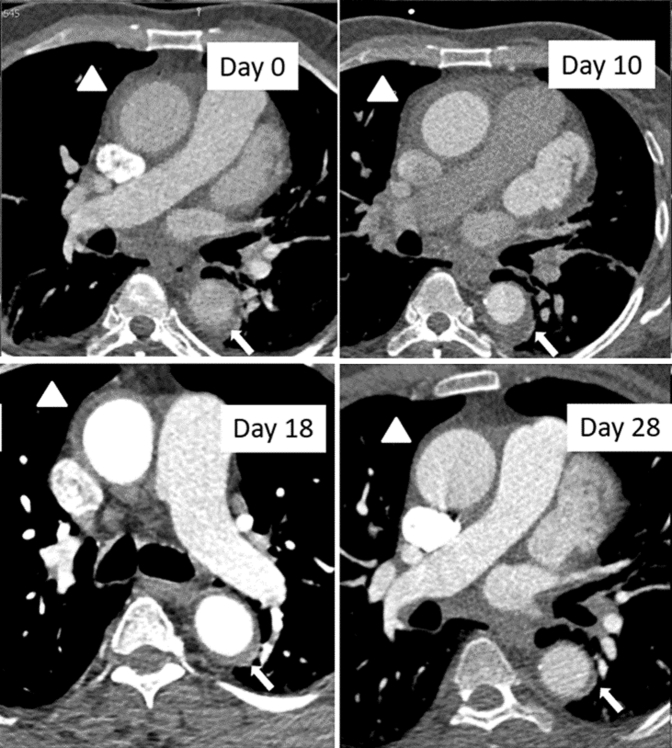
Figure 2.
Evolution of Aortic IMH on CT Angiogram
The arrowhead shows intramural hematoma (IMH) in the ascending aorta and the arrow in the descending aorta. A trivial increase in IMH thickness was demonstrated on day 10, followed by progressive decrease in volume on the follow-up computed tomography (CT) scans.
On the fourth CTA performed, exactly 1 month after the initial diagnosis, there was a noticeable improvement of the extent of the IMH, namely from the sinuses of Valsalva to the level of the superior aspect of the pulmonary arteries (Figure 2). MDT assessment once again considered the patient eligible for continued conservative treatment. Lower limb neurological status and urinary function further improved with continuous neurological rehabilitation, whereas a brain and whole spine magnetic resonance imaging assessment excluded any signs of residual ischemic damage.
Discussion
IMH constitutes a variant of acute aortic syndrome, along with AD and penetrating ulcer disease (1). In most cases, rupture of the vasa vasorum with resultant hemorrhage into the aortic media is the inciting event, with severe pain as the commonest presenting symptom, with back pain being reported in 41% of cases (1). By definition, the hematoma is contained within the aortic wall with no intimal penetration; thus, lack of intimal entry point and dissection flap differentiate it from AD and penetrating ulcer disease (1). Furthermore, compared with classic AD, it has a predilection for older ages (mean age at presentation 69.6 years), and more than 80% of patients are hypertensive (1). Consistent with this profile, our patient was 68 years of age, with uncontrolled HTN, and presented with excruciating back pain. The lack of intimal entry points, as well as of any dissection flap or false lumen on repeat CTA examinations secured the diagnosis of IMH.
Stanford classification of AD also pertains to IMH, with 15% to 50% of patients presenting with Stanford A hematomas (1). Compared with classic AD, the superficial development of IMH closer to the adventitia makes it more susceptible to mediastinal rupture, periaortic hematoma, or pericardial effusion; on the contrary, aortic regurgitation and malperfusion complications are relatively less common (renal failure, mesenteric, or peripheral ischemia) and overall survivability is better (1). In this context, although hemodynamic instability unequivocally mandates surgical intervention, the optimal treatment strategy of stable type A IMH cases has not been defined. On the one hand, centers in Japan and Korea have achieved comparable survivability (<10% in-hospital mortality) with initial medical treatment and surgical intervention reserved for complications (persistent pain, uncontrolled HTN, IMH progression, or intimal disruption development) (1). The largest related study to date prospectively compared this early conservative treatment of type A IMH (n = 101) with surgically treated type A AD patients (n = 256) and demonstrated similar in-hospital mortality rates (7.9% vs. 10.7%, respectively, p = 0.56), and nondiffering 1-, 2-, and 3-year survivability; patients with stable IMH with early conservative treatment had an 86.3% 3-year survival rate (2). Similarly, a multicentric study in 165 consecutive patients with acute IMH found no difference in the in-hospital and 2-year mortality rates in initially medically versus initially surgically treated patients with type A IMH (4.4% vs. 4.3% in-hospital mortality, p = 0.428; 14.9% vs. 7.1% 2-year mortality, p = 0.45) (3). Crucially, delayed surgery has not been found to increase operative mortality (4,5). Moreover, maximum aortic and maximum hematoma diameter have been found to reliably predict adverse outcomes in the context of conservative treatment (2, 3, 4,6), further safeguarding its implementation; this evidence is reflected on Asian guidelines, which advise for anti-impulse therapy provided the IMH is <11 mm and the aortic diameter <50 mm (high-risk markers for progression) (7).
On the other hand, most Western studies have demonstrated improved survivability of surgically, in comparison with medically treated patients (1). Importantly, the most recent analysis from the International Registry of Acute Aortic Dissection (IRAD) highlights a 40% in-hospital mortality in conservatively treated patients with type A IMH (8); however, these results should be interpreted with skepticism. The small reported IMH prevalence (5%) compared with Asian studies (>30%) (2,3,6) raises the specter of possible underdiagnosis. In addition, IMH thickness is not included in the IRAD-registered cases, ignoring a significant prognostic factor (2,3,6); at the same time, at least 60% of the reported patients in the medically treated type A IMH group had an ascending aortic diameter of ≥50 mm, violating a crucial radiological prognostic rule (2,4,6,7). Nevertheless, both European and American guidelines recommend surgery (Class I, Level of Evidence: C, and Class IIa, Level of Evidence: C, respectively), with the exception of medically futile cases (1).
In our case, the patient presented subacutely (5 days after the pain episode), demonstrating consistent resolution of the initial pain and neurological signs. The maximum aortic diameter on presentation was 44.7 mm and IMH diameter was 4.7 mm, with no adverse imaging findings of complications or risk of progression (no dissection flap, no intimal tear). Furthermore, HTN was effectively controlled after commencement on intensive antihypertensive treatment. In addition, local radiographic expertise could provide us with reliable follow-up imaging and early recognition of adverse radiological features. To our knowledge, this is one of the few cases of rapid type A IMH resorption; 2 patients have been reported demonstrating extremely early resolution within 2 days, but in the context of active malignancy and its concomitant hypercoagulability effects (9,10).
Follow-Up
The patient was eventually discharged, with a satisfactory clinical and laboratory assessment, having reached his baseline functional status. Routine IMH clinic follow-up, with the first appointment including repeat CTA and clinical examination 4 weeks after discharge, and community rehabilitation were arranged.
Conclusions
This case report further expands on the notion that in the absence of adverse clinical or radiological features of malperfusion complications or expansion, even type A IMH can be safely treated conservatively, under close surveillance.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Evangelista A., Maldonado G., Moral S., Rodriguez-Palomares J. Uncomplicated type A intramural hematoma: surgery or conservative approach?—surgery. Ann Cardiothorac Surg. 2019;8:556–557. doi: 10.21037/acs.2019.07.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song J.K., Yim J.H., Ahn J.M. Outcomes of patients with acute type a aortic intramural hematoma. Circulation. 2009;120:2046–2052. doi: 10.1161/CIRCULATIONAHA.109.879783. [DOI] [PubMed] [Google Scholar]
- 3.Choi Y.J., Son J.W., Lee S.H. Treatment patterns and their outcomes of acute aortic intramural hematoma in real world: multicenter registry for aortic intramural hematoma. BMC Cardiovasc Disord. 2014;14:103. doi: 10.1186/1471-2261-14-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitai T., Kaji S., Yamamuro A. Clinical outcomes of medical therapy and timely operation in initially diagnosed type A aortic intramural hematoma: a 20-year experience. Circulation. 2009;120:S292–S298. doi: 10.1161/CIRCULATIONAHA.108.843615. [DOI] [PubMed] [Google Scholar]
- 5.Baikoussis N.G., Apostolakis E.E., Siminelakis S.N., Papadopoulos G.S., Goudevenos J. Intramural haematoma of the thoracic aorta: who's to be alerted the cardiologist or the cardiac surgeon? J Cardiothorac Surg. 2009;4:54. doi: 10.1186/1749-8090-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song J.M., Kim H.S., Song J.K. Usefulness of the initial noninvasive imaging study to predict the adverse outcomes in the medical treatment of acute type A aortic intramural hematoma. Circulation. 2003;108:S324–S328. doi: 10.1161/01.cir.0000087651.30078.38. [DOI] [PubMed] [Google Scholar]
- 7.JCS Joint Working Group Guidelines for diagnosis and treatment of aortic aneurysm and aortic dissection (JCS 2011) Circ J. 2013;77:789–828. doi: 10.1253/circj.cj-66-0057. [DOI] [PubMed] [Google Scholar]
- 8.Harris K.M., Braverman A.C., Eagle K.A. Acute aortic intramural hematoma: an analysis from the International Registry of Acute Aortic Dissection. Circulation. 2012;126:S91–S96. doi: 10.1161/CIRCULATIONAHA.111.084541. [DOI] [PubMed] [Google Scholar]
- 9.Velasquez C.A., Bin Mahmood S.U., Zafar M.A. Precipitous resolution of type-A intramural hematoma with medical management in a patient with metastatic stage 4 renal cell carcinoma. Int J Angiol. 2017;26:267–270. doi: 10.1055/s-0037-1604333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohmi M., Tabayashi K., Moizumi Y., Komatsu T., Sekino Y., Goko C. Extremely rapid regression of aortic intramural hematoma. J Thorac Cardiovasc Surg. 1999;118:968–969. doi: 10.1016/s0022-5223(99)70075-9. [DOI] [PubMed] [Google Scholar]