Abstract
Background
Knowledge of the cytotoxicity and bioactivity of endodontic materials may assist in understanding their ability to promote dental pulp stem cell activity and pulp healing in primary teeth.
Materials and methods
This systematic review was carried out by searching the electronic databases such as PubMed, Google Scholar, and Cochrane reviews for the articles published between January 2000 and December 2018 using the appropriate MeSH keywords. An independent investigator evaluated the abstracts and titles for possible inclusion, as per the stipulated inclusion and exclusion criteria. The topics considered for extracting data from each study were: cell lineage, cytotoxicity assay used, and type of material tested.
Results
Seven eligible studies were selected for assessing the quality of evidence on the bioactivity of bioactive endodontic cements (BECs) (1 human cell line, 2 animal cell lines, and 4 in vitro, animal, and human studies) and 13 studies were selected for reviewing the quality of evidence on cytotoxicity (7 human cell lines, 4 animal cell lines, and 2 animal model studies). Very limited studies had been conducted on the bioactivity of materials other than mineral trioxide aggregate (MTA). With regards to cytotoxicity, the studies were diverse and most of the studies were based on MTT assay. Mineral trioxide aggregate is the most frequently used as well as studied root-end filling cement, and the literature evidence corroborated its reduced cytotoxicity and enhanced bioavailability.
Conclusion
There was a lack of sufficient evidence to arrive at a consensus on the ideal material with minimal cytotoxicity and optimal bioactivity. More focused human/cell line-based studies are needed on the available root filling materials.
Clinical significance
The present systematic review provides an update on the available literature evidence on the cytotoxicity and bioactivity of various BECs including MTAs and their influence on the different cells with respect to their composition and strength.
How to cite this article
Maru V, Dixit U, Patil RSB, et al. Cytotoxicity and Bioactivity of Mineral Trioxide Aggregate and Bioactive Endodontic Type Cements: A Systematic Review. Int J Clin Pediatr Dent 2021;14(1):30–39.
Keywords: Bioactive endodontic cements, Bioactivity, Cytotoxicity, Mineral trioxide aggregate
Introduction
Bioactive endodontic cements (BECs) are bioactive materials that form apatite in body fluids, including synthetic body fluids and they are mainly used for pulp capping, pulp therapy, pulpotomy, apexogenesis, apexification, perforation repair, root canal filling, and root canal sealing.1,2 Despite the differences in chemical composition, the bioactivity of BECs is similar.1 The commonly used BECs include calcium-based materials, mineral trioxide aggregate (MTA), Biodentine, root repair material (iRoot), calcium-enriched mixture (CEM), bioaggregate, endosequence root repair material, MTYA1-Ca filler, TheraCal, and bioactive glass.3,4 Mineral trioxide aggregates are the most commonly used BECs owing to their high biocompatibility, sealing ability, and desirable outcomes.5,6 Mineral trioxide aggregate consists of tricalcium silicate, dicalcium silicate, and some traces of tricalcium aluminate and calcium aluminoferrite.7–9 The various MTAs available are ProRoot MTA (gray), tooth-colored ProRoot, Angelus MTA, Biodentine, MTA Bio, and MTA Plus (white and gray). The availability of a various range of bioactive materials needs a proper understanding and guidance for the appropriate use of material for different clinical conditions.10–12 Due to some disadvantages of MTAs such as high cost, long setting time, and tooth discoloration; several newer BECs have been recently introduced to the market.13
Cytotoxicity assays are carried out to study the toxicity of the materials used in BECs, and the damage or irritation they cause when used for various endodontic procedures. Cytotoxicity is tested using in vivo and in vitro methods and the choice of test depends on the chemical composition of the test materials.14,15 The in vitro cytotoxic assays are most relevant and suitable for evaluation due to their reproducibility, simplicity, and cost-effectiveness.16 Cellular viability is influenced by the materials used in filling or treating.17 It is tested by cytotoxicity tests, which measures the biocompatibility of the materials.18
Cell viability and bioactivity tests are significant to assess cellular damage and the biological effect of new biomaterials.19,20 Bioactive endodontic cements materials should possess adequate biocompatibility and bioactivity to promote dental pulp stem cell activity and pulp healing in primary teeth.21 Bioactivity index is the measure of hydroxyapatite formation when used for filling.22,23 Bioactivity index is measured to know the activity, which further depends on the capacity of bone conduction and the material composition22,24 but total cytocompatibility needs to be checked for complete characterization of bioactive materials. However, a critical evaluation and assessment are required to know the complete cytotoxicity and bioactivity of MTA and other BECs.25
The present systematic review is intended to provide an update on the available literature evidence on the cytotoxicity and bioactivity of various BECs including MTAs and their influence on the different cells with respect to their composition and strength.
Materials and Methods
Search Strategy
The protocol for this systematic review has been registered with the PROSPERO International prospective register of systematic reviews, registry No. CRD42021227636 and this review followed PRISMA guidelines.
Electronic databases such as PubMed, Google Scholar, and Cochrane reviews were searched for eligible articles published from January 2000 to December 2018. This particular study period was selected for the following two reasons (1) several studies with a focus on bioactivity and cytotoxicity of BECs had been published during this study period; (2) several newer BECs had been introduced to the market during this period. The search was conducted using all the appropriate MeSH keywords including MTAs and the names of all the available BECs and MTAs for both cytotoxicity and bioactivity. The present review considered in vitro studies using animal and human cells, and those conducted in animal models and pulp tissues. References in the retrieved articles were also explored for potentially relevant studies. Complete versions of all the potentially relevant studies were obtained. The same investigator scrutinized and selected the studies for systematic review based on the inclusion and exclusion criteria.
Inclusion criteria were limited to articles written in English and published in a peer-reviewed journal. All eligible studies were included, regardless of the journal. An independent investigator evaluated the abstracts and titles for possible inclusion. The inclusion and exclusion criteria were as follows:
Inclusion Criteria
Studies on cytotoxicity and bioactivity of BECs.
Original article.
Original data available (results).
English language full-text publication.
Description of methodology completion, which includes usage of multiple dilution and reporting of duration.
Exclusion Criteria
Data not available or only abstract available.
Case reports or letters.
Duplicate studies.
Systematic studies.
Non-English studies.
To extract data from each study, the investigator considered the following topics: cell lineage, cytotoxicity assay used, and type of material tested.
Results
With regard to bioactivity, around 11 full-text articles were selected based on the literature search using the aforementioned keywords (Flowchart 1). Two animal studies and 2 cell line-based studies were excluded due to insufficient data based on the pre-defined inclusion and exclusion criteria. After the exclusion, seven eligible studies were selected for assessing the quality of evidence on the bioactivity of BECs (one human cell line, two animal cell lines, and four in vitro, animal, and human studies).
Flowchart 1.
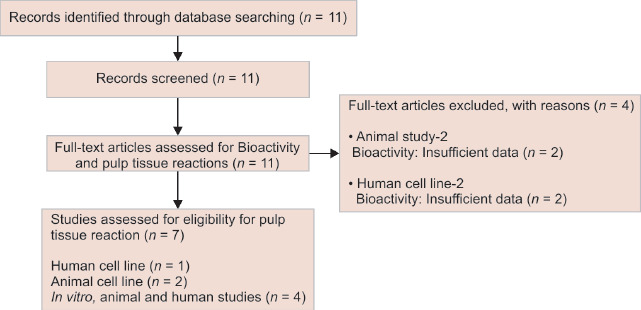
The screening and selection of studies on the bioactivity of various BECs
For evaluating the available literature on cytotoxicity, around 120 articles were identified on a literature search using the specific keywords (Flowchart 2). The total number of articles were 118 after the removal of duplicates and multiple publications. Studies not pertaining to dentistry (1) preliminary report (1) and those with the title (130) and abstract bias (66) were excluded. Out of the 37 full-text articles selected, 24 were excluded due to insufficient data based on the pre-defined inclusion and exclusion criteria. After the exclusion, 13 eligible studies were selected for reviewing the quality of evidence on cytotoxicity of BECs (7 human cell lines, 4 animal cell lines, and 2 animal model studies).
Flowchart 2.
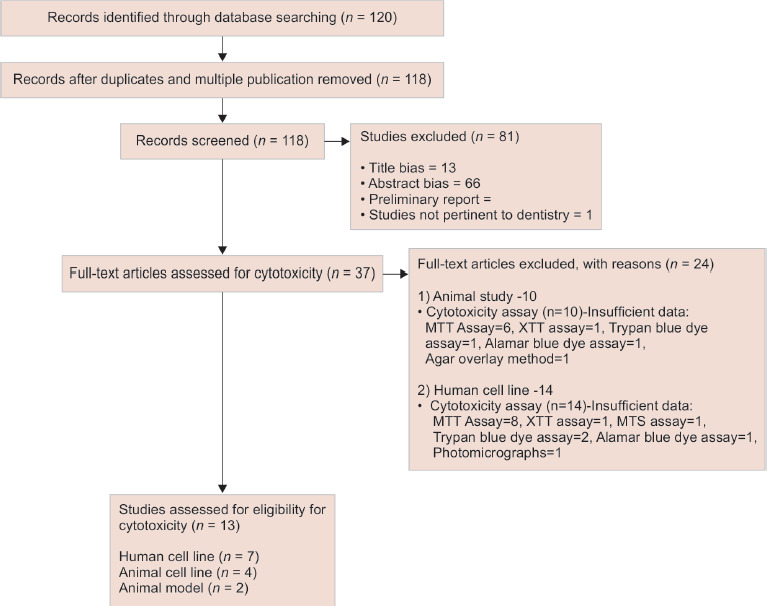
The screening and selection of studies on cytotoxicity of various BECs
Bioactivity
Cell Line-based Bioactivity Studies
Cell line-based studies were distinct with respect to the assay used for the assessment of bioactivity. Güven et al. compared MTA with iRoot SP using ALP and Von Kossa staining. After 14 days of exposure, the iRoot SP-treated group showed less ALP activity and the mineralization through Ca2+ deposits was found to be more for MTA through light microscopic examination.26
Haglund et al. noted that the MTA, IRM, amalgam, and Retroplast had inhibited the cell growth of mouse fibroblasts and macrophages. The cell morphology was examined under a phase-contrast microscope and individually detached cells were counted. The retroplast group showed fewer cell numbers when compared to MTA and amalgam. No cytokine production was detected for the four root filling materials, but this could be attributed to the difference in cell type used.27
Portland cement (PC) is commonly used in clinical practice due to its comparatively less price. It has a similar composition as that of MTA, except for increased calcium aluminate and calcium sulfate levels. Saidon et al. reported the greater tolerability of both MTA and PC and both did not show any cell reaction in, in vivo and in vitro tests conducted using L929 culture cells.28
Table 1 briefs about the bioactivity studies conducted using cell lines.
Table 1.
Characteristics of bioactivity studies conducted using cell lines
| Author and year | Assay | Materials evaluated | Results |
|---|---|---|---|
| Güven et al. (2013)26 | Type of assay (TA): Real-time polymerase chain reaction expression analysis (RT-PCR) and Von Kossa staining | MTA and iRoot SP | MTA was found to be more efficient to mineralize than iRoot SP |
| Cell lineage (CL): Teflon rings cultured with hTGSCs | |||
| Type of contact (TC): Indirect | |||
| Haglund et al. | TA: ELISA testing. | MTA, amalgam, IRM, and | All the materials showed cell |
| (2003)27 | CL: L929 mouse fibroblasts and mouse macrophage cell line RAW 264.7 | Retroplast | growth inhibition |
| TC: Direct | |||
| Saidon et al. (2003)28 | TA: In Vitro: Cell morphology under a phase-contrast microscope | MTA and Portland cement | MTA and PC did not show any cell reaction differentiation and had great tolerability |
| In Vivo: Light microscope evaluation | |||
| CL: L929 mouse fibroblasts | |||
| TC: Direct and indirect |
In Vitro Animal and Human Studies
Studies had validated the effectiveness of MTA and PC in pulp capping. Bidar et al. had performed a histopathological evaluation of direct pulp capping involving MTA and PC in dog premolars (n = 64). Although the researchers had confirmed the pulp protection benefits of these materials, they highlighted the need for conducting extensive research before using them in humans for a longer duration.29
Two studies had reported the superiority of MTA over calcium hydroxide due to the formation of a highly thicker calcified bridge. Leye Benoist et al. had compared the effectiveness of MTA and Dycal in forming dentin bridge using specialized software. The software collected data regarding digitized images and surface length for 3 and 6 months. The researchers observed a statistically significant higher success rate for MTA than Dycal for 3 months, but no difference in dentin thickness was noted after 6 months.30 The randomized control trial by Eskandarizadeh et al. had recommended both white and gray type MTA as the materials of choice for direct pulp capping, in contrast to the hard setting calcium hydroxide cement (Dycal). The study involving 90 intact first and second premolars showed the formation of a significantly thicker calcified bridge with gray MTA when compared to Dycal at 30 and 60 days (p = 0.015 and p = 0.002, respectively), and the same was noted at 90 days for white MTA (p = 0.02).31
Shokouhinejad et al. had evaluated the bioactivity of bioaggregate (BA), endosequence root repair material (ERRM), and white ProRoot MTA. This study conducted on 60 horizontal root sections noted the formation of a substantially greater amount of apatite aggregate after 2 months on the surfaces of all the materials.32 The characteristics of in vitro human and animal studies are summarized in Table 2.
Table 2.
Characteristics of in vitro human and animal studies
| Author and year | Assay | Materials evaluated | Results |
|---|---|---|---|
| Bidar et al. (2017)29 | TA: Light microscope evaluation Animal: 64 dog premolars TC: Direct |
MTA and PC | Chronic inflammation in WMTA, GMTA, white, and gray PC were 45.5, 27.3, 57.1, and 34.1%, respectively |
| Leye et al. (2012)30 | TA: Mesurim Pro(®) software Human: 60 teeth TC: Indirect |
MTA and Dycal | MTA and Dycal success rate 3 months: 93 and 73% 6 months: 89.6 and 73% Dentine thickness increased in both materials with time |
| Eskandarizadeh et al. (2011)31 | TA: Mesurim Pro(®) software Human: 90 intact first and second premolars of human maxillary and mandibular teeth TC: Indirect |
White MTA, Gray MTA, and Dycal | Calcified bridge of GMTA > Dycal at 30 and 60 days WMTA > Dycal at 90 days |
| Shokouhinejad et al. (2012)32 | TA: Scanning electron microscopy (SEM) observation and energy dispersive X-ray (EDX) instrument for elemental analysis Human: 60 horizontal root sections TC: Indirect |
BA, EndoSequence Root Repair Material (ERRM), and white ProRoot Mineral trioxide aggregate (MTA) | MTA, BA, and ERRM showed increased precipitation with time |
Cytotoxicity
Studies on Human Cell Lines
The selected literature on cytotoxicity assessment conducted in human cell lines demonstrated that the studies were diverse, especially with respect to the BECs compared. Whereas, most of the studies had evaluated the cytotoxicity using MTT assay (Table 3). A 2012 study by Hirschman et al. had reported a statistically significant cytotoxic effect with Dycal (Dentsply).33
Table 3.
Characteristics of cytotoxic studies conducted in human cell lines
| Study | Assay | Materials evaluated | Result |
|---|---|---|---|
| Hirschman et al. (2012)33 | TA: MTT-based colorimetric assay | White mineral trioxide aggregate cement (AMTA, MTA-Angelus), | AMTA, ERRM, and UBP had statistically similar cytotoxicity levels. However, Dycal demonstrated a statistically |
| CL: Human dermal fibroblasts | Brasseler ERRM putty, Dycal, and Ultra-blend Plus (UBP) | significant cytotoxic effect | |
| Type of contact (TC): Indirect | |||
| Ma et al. (2011)37 | TA: MTT assay | ERRM Putty and Paste with gray MTA | (ERRM Putty) and Paste (ERRM Paste) showed similar in vitro biocompatibility as that of gray MTA |
| CL: Gingival fibroblast | |||
| Yoshino et al. (2013)34 | TA: MTT assay CL: Periodontal ligament |
White MTA, MTA Fillapex® and Portland cement (PC) | MTA Fillapex demonstrated the highest cytotoxic effect on periodontal ligament fibroblasts followed by white |
| fibroblasts | MTA and PC | ||
| TC: Indirect | |||
| Zhou et al. (2015)35 | TA: Flow cytometry and electron microscopy | EndoSequence BC, MTA Fillapex, and AH Plus (control sealer) | The 2 calcium silicate-containing endodontic sealers demonstrated different cytotoxicities. MTA Fillapex of ≥2 |
| CL: Human gingival fibroblasts | weeks demonstrated more toxicity than fresh/1-week-old cement. MTA Fillapex did not show toxicity at | ||
| TC: Indirect | concentration ≥ 1:32. AH plus was not toxic after setting | ||
| Mukhtar- | TA: MTT assay | BioAggregate and iRoot SP | Both the materials had acceptable biocompatibility and |
| Fayyad (2011)38 | CL: Human fibroblast MRC-5 cells | their cytotoxic effects were concentration-dependent | |
| TC: Indirect | |||
| Zhou et al. (2013)36 | TA: Flow cytometry and electron microscopy | Biodentine, White ProRoot MTA, and glass ionomer cement | No significant difference in cell viability was noted between Biodentine and MTA, and they had a less |
| CL: Human gingival fibroblasts | cytotoxic effect than glass ionomer cement | ||
| TC: Indirect |
Two studies had highlighted the increased cytotoxicity of MTA Fillapex. The cytotoxicity evaluation conducted by Yoshino et al. on human cultured periodontal ligament fibroblasts had noted that MTA Fillapex conferred the highest cytotoxic effect followed by white MTA and Portland cement.34 Similarly, Zhou et al. had reported increased toxicity of MTA Fillapex at ≥2 weeks when compared to fresh/1-week-old cement, but the toxicity was not seen at a concentration of ≥1:32. The study also ruled out the incidence of toxicity of AH plus after setting.35 An earlier study by Zhou et al. had concluded that the toxicity of Biodentine and MTA was less than the ionomer cement.36 The researchers used flow cytometry and electron microscopy-based assays for the evaluation of cytotoxicity in both studies.
Ma et al. had demonstrated that in vitro biocompatibility of ERRM putty and ERRM paste were comparable to that of gray MTA.37 Hirschman et al. had also reported the statistically comparable cytotoxicity levels of AMTA, ERRM putty, and UBP.
One study had identified the acceptable biocompatibility of BioAggregate and iRoot SP, the calcium silicate-phosphate-based ceramic with nano-composition. The study on human fibroblast showed cytotoxicity of BioAggregate was more when compared to iRoot SP and their cytotoxicity was independent of extract concentration.38
Cytotoxicity Studies on Animal Cell Lines
Most of the cytotoxicity studies on animal cell lines were based on MTT assay (Table 4). The genotoxic and cytotoxic study conducted on L929 mouse fibroblast cells by Naghavi et al. had suggested a calcium-enriched mixture (CEM) with comparable biocompatibility as an alternative to MTA. Another major finding was the increased damage of cells by MTA at higher concentrations than CEM (1,000 μg/mL). The researchers speculated high level of arsenic in the medium containing MTA as the reason for increased toxicity at higher concentrations.39
Table 4.
Cytotoxicity studies on animal cell lines
| Author and year | Assay | Material evaluated | Results |
|---|---|---|---|
| Naghavi et al. (2014)39 | Type of assay (TA): MTT assay Cell lineage (CL): L929 mouse |
Calcium enriched mixture (CEM) and MTA | Statistically no difference was found between the materials at concentration 0–500 μg/mL, except |
| fibroblast | at concentration 1,000 μg/mL | ||
| Type of contact (TC): Indirect | |||
| Alanezi et al. | TA: MTT assay | ERRM with gray MTA (GMTA), white | The cell viability of ERRM was comparable to GMTA and WMTA in both set and fresh |
| (2010)40 | CL: L929 mouse fibroblast | MTA (WMTA), and AH26 | conditions |
| TC: Indirect | |||
| Bin et al. (2012) 42 | TA: MTT assay | WMTA (Branco, Angelus), MTA | WMTA cell viability rates were above 70% at all |
| CL: Chinese hamster fibroblasts (V79) | Fillapex (Angelus), and AH Plus (Dentsply) | concentrations but MTA Fillapex and AH Plus were cytotoxic at higher concentrations | |
| TC: Indirect | |||
| Ribeiro et al. (2006)43 | TA: Comet assay using trypan blue staining | MTA Angelus, Portland cement, and white Portland cement | MTA and Portland did not produce or induce any strand breaks in DNA at all concentration |
| CL: Chinese hamster ovary (CHO) cells | |||
| TC: Indirect |
Alanezi et al. had concluded that the cytotoxicity of ERRM was comparable to that of gray and white MTAs at both set and fresh conditions.40 The study also underscored the need for further investigating the solubility, sealing ability, and in vivo endodontic usage of ERRM.41
Ma et al. had noted elevated cytotoxicity of MTA Fillapex at 1:1, 1:2, 1:4, and 1:8 dilutions, and that of AH plus at 1:1, 1:2, and 1:4 dilutions. In addition, both these sealers showed reduced cell viability rates and increased formation of micronuclei when compared to control. The study had concluded white MTA as a less cytotoxic material with cell viability >70%.42
Ribeiro et al. had shown that both MTA and Portland cement do not induce DNA damage and cellular death, which are important events in carcinogenesis. The study conducted on Chinese hamster ovary (CHO) cells using trypan blue staining further corroborated the use of MTA and Portland cement in dentistry.43
Cytotoxic Studies Conducted in Animal Models
Saidon et al. had evaluated the in vitro and in vivo biocompatibility of MTA and PCs using L929 cell lines and guinea pig models. In vitro study did not show any difference in cell reaction between ProRoot MTA and PC. The in vitro study and insertion of materials into the bone cavities of animal models showed bone healing and minimal inflammatory response adjacent to both the material implants. Though the study had suggested PC as a less expensive root-end filling material, the researchers highlighted the need for more human-based studies before the recommendation for unlimited clinical use (Table 5).28
Table 5.
Characteristics of cytotoxic studies conducted in animal models
| Author and year | Assay | Materials evaluated | Results |
|---|---|---|---|
| Saidon et al. (2003)28 | TA: In vitro and in vivo assay In vitro: Millipore culture plate inserts with freshly mixed or set material placed on already attached L929 cell plates In vivo: Freshly mixed materials were inserted into the bone cavities of adult male guinea pigs and histologically evaluated using a light microscope |
ProRoot MTA and Portland cement | Both in vitro and in vivo studies demonstrated that MTA and PC have comparative biocompatibility |
| Batur et al. (2013)44 | TA: In vivo assay The materials were implanted into a dorsal connective tissue of rats for 7, 15, 30, 60, and 90 days |
ProRoot MTA and DiaRoot BA | DiaRoot BioA was found to be more biocompatible than MTA |
The cytotoxicity of ProRoot MTA and DiaRoot BA, a bioceramic nanoparticulate cement, were compared in a study involving 50 Sprague-Dawley rats. The histopathologic evaluation carried out after implanting the materials into a dorsal connective tissue of rats for 7, 15, 30, 60, and 90 days showed that BA is more biocompatible than MTA.44 However, the results were more favorable for MTA in the presence of dystrophic calcification (Table 5).
Discussion
In dentistry, the bioactivity of a material signifies its ability to hydrolyze and produce calcium hydroxide, which in turn contributes to the formation of an interfacial layer and development of an apatite layer.45–49 The activity or bioactivity index is a measure of dental bone regeneration rate and apatite formation level. The dentin bridge is formed by the increased activity of pyrophosphates, which is augmented by the calcium ion release.50–52 The bioactivity assays are intended to evaluate the ability of different materials to form apatite and mineralization based on their composition and strength.53 Alkaline phosphatase (ALP)54,55 and simulating body fluid (SBF) medium are the most commonly used quantitative indicators of mineralization.56,57
Literature search shows that there are very limited studies with sufficient data on the bioactivity of BECs. Such studies have been conducted mainly on cell lines such as human dental pulp cells (HDPCs), human tooth germ stem cells (hTGSCs), MG-63, etc.32,58 The available bioactivity studies are mainly on the comparison of MTAs with other popular sealing materials to characterize their effects and bioactivity. The literature evidence shows that MTA is more efficient with regard to mineralization and improved tolerability;59,60 however, it should be noted that very limited studies have been conducted on the bioactivity of materials other than MTAs.
The study conducted by Guven et al. had concluded that MTA is superior and more bioactive compared to iRoot SP using hTGSCs cell line. Comparison of the same materials by Yuan et al. using RAW 264.7 had provided insights on the mechanism involving the use of MTA as a potential endodontic material for the treatment of persistent apical periodontitis. The researchers reported that both iRoot SP and MTA, induced by lipopolysaccharide, can augment the expression of IL-1β, TNF-α, and IL-6.61
The present review could identify the cell lines (Saidon et al.) and animal studies (Bidar et al.) suggesting comparable bioactivity of Portland cement and MTA. In concurrence with these findings, Bhagat et al. had concluded that the favorable biological response of PC to pulpotomy treatment was comparable to that ProRoot MTA.62 Shahi et al. had also advocated the use of PC as an alternative to MTA. The study reported no statistically significant difference between white MTA, gray MTA, white PC, and gray PC.63
The present review has quoted two human studies comparing the bioactivity of MTA and Dycal (Leye Benoist et al., Eskandarizadeh et al.). Both the studies had highlighted the superiority of MTA over Dycal; but activities such as solubility, dentin bridge formation, and biointeractivity varied between the studies. Similar to these findings, Gandolfi et al. had recommended MTA Plus as a substitute for conventional calcium silicate MTA-like cements owing to its enhanced reactivity, and prolonged potential to release calcium and increase the local pH.64 In contrast, a review by Al-Sabri had concluded calcium hydroxide as the first choice in clinical practice due to the high cost of MTA and the challenges associated with its mixing and handling.65
ProRoot MTA and MTA Angelus were found to be inert and viable.15 ProRoot MTA had demonstrated greater biological properties over OrthoMTA and Endocem MTA in root repair and excellent bioactivity over the traditional cements.66,67
Collado-González et al. reported better cytocompatibility and bioactivity of Biodentine than MTA Angelus, TheraCal LC, and IRM when tested on stem cells from human exfoliated primary teeth.68 Biodentine has been reported to be more advantageous than MTA owing to its consistency, better mechanical properties, and improved handling.3,69
Though in vitro and in vivo studies corroborated the biocompatibility of MTAs, further human studies involving genotoxicity tissue implantation tests and sensitization tests are required to establish a more general outlook on the safety profile of these materials.70
The viability of periradicular cells following retrograde filling, pulp capping, and perforation repair may depend on the cytotoxicity of the root filling material used and they may induce apoptosis or necrosis.71,72 Hence, the use of materials that are toxic to pulpal and periapical tissues may impair the prognosis and clinical outcome.73
The present review has identified literature evidence from both human and animal cell lines suggesting increased cytotoxicity of Fillapex. A comparative study of odontoblast-like cells showed that MTA Fillapex possess more cytotoxicity than AH Plus.74–76 The cellular responses in human dental pulp stem cells noted by Victoria-Escandell et al. had identified MTA-Fillapex as the most cytotoxic oxidative stress inductor in preincubated cell culture medium. The ability of endodontic materials to produce oxidative stress correlates with their cytotoxicity and genotoxicity.77–79
One study had reported a statistically significant cytotoxic effect of Dycal to adult human dermal fibroblasts.33 In concurrence with this finding, a comparative study involving 7 pulp-capping materials had demonstrated the highest cytotoxic effect with Dycal (10% cell viability).80 The study compared the following pulp-capping materials in vitro: TheraCal LC, Dycal, Calcicur, Calcimol LC, ProRoot MTA, MTA-Angelus, and Biodentine. This study also reported the comparable cytotoxic effect of Biodentine with that of MTA, thereby suggesting it as an alternative pulp-capping material.81–83
Biodentine was introduced to the market in 2010 to overcome the limitations of the MTA such as increased cost, slow setting time, and difficulty in manipulation.24 Biodentine and MTA have been reported to be less toxic and more viable than glass isomers at all concentrations.84 Calcium-enriched mixture cement, Biodentine, and MTA exhibited similar cytotoxicity and can be considered equally for root-end surgery procedures.19,85–87 The cytotoxicity is dependent on the chemical composition and varies with the incubation period or setting time. ProRoot MTA and Biodentine with a greater incubation period of 48 hours showed less cytotoxicity than CEM and Biosealer.21,44 In the current review, the 2013 and 2015 studies by Zhou et al. have corroborated the reduced cytotoxicity of MTA-based products. The researchers used flow cytometry and electron microscopy-based assays for the evaluation of cytotoxicity.
The present review has quoted the studies by Hirschman et al. and Jingzhi et al. suggesting the comparable cytotoxicity of ERRM putty with MTA. Contrary to these findings, a rat-model study by Khalil and Abunasef had reported that the implantation of both ERRM and MTA produced injurious effects on subcutaneous tissues.44
The present systematic review holds considerable significance, as to the best of our knowledge, there is no review evaluating the cytotoxicity and bioactivity of available BECs and MTAs. Moreover, following a more rigorous and prospectively defined objective process for the data collection, extraction, and compilation helped in critically scrutinizing the study methodologies and excluding those with vague study designs and unclear protocols. Another area of research is cytotoxicity of the freshly mixed material and its reduction with time. The present review showed that there is very limited data on the cytotoxicity of the freshly mixed materials.
The current study could not perform a meta-analysis of the available literature due to the diversity of the studies including the type of cell lines used and assays conducted. Since a meta-analysis could not be carried out, generalization of the study findings was not possible. Another limitation was the availability of a few studies on all the available BECs in the market, especially pertaining the bioactivity. Moreover, the majority of the studies have used MTA for comparison. Hence, the present literature evidence was inadequate to conduct a more credible review to arrive at a consensus on the ideal material with minimal cytotoxicity and optimal bioactivity.47,88–90 However, the present review confirmed that MTA is the most frequently used as well as studied root-end filling cement, and substantiates its reduced cytotoxicity and enhanced bioavailability.
Conclusion
The current review serves as an update on the available evidence on the cytotoxicity and bioactivity of available BECs and MTAs. It may assist researchers to conduct more focused human-based studies, thereby developing a general agreement on the available root filling materials. The available literature indicates MTA as the most frequently used endodontic filling material with reduced cytotoxicity and improved bioavailability.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Torabinejad M, Parirokh M, Dummer PMH. Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview - part II: other clinical applications and complications. Int Endod J. 2018;51(3):284–317. doi: 10.1111/iej.12843. DOI: [DOI] [PubMed] [Google Scholar]
- 2.Walsh RM, He J, Schweitzer J, et al. Bioactive endodontic materials for everyday use: a review. Gen Dent. 2018;66(3):48–51. [PubMed] [Google Scholar]
- 3.Raghavendra SS, Jadhav GR, Gathani KM, et al. Bioceramics in endodontics – a review. J Istanb Univ Fac Dent. 2017;51(3 Suppl 1):S128–S137. doi: 10.17096/jiufd.63659. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asthana G, Bhargava S. Bioactive materials: a comprehensive review. Sch J App Med Sci. 2014;2(6):3231–3237. [Google Scholar]
- 5.Parirokh M, Torabinejad M, Dummer PMH. Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview - part I: vital pulp therapy. Int Endod J. 2018;51(2):177–205. doi: 10.1111/iej.12841. DOI: [DOI] [PubMed] [Google Scholar]
- 6.Tsai C-L, Ke M-C, Chen Y-H, et al. Mineral trioxide aggregate affects cell viability and induces apoptosis of stem cells from human exfoliated deciduous teeth. BMC Pharmacol Toxicol [Internet] 2018;15(1):19. doi: 10.1186/s40360-018-0214-5. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camilleri J, Montesin FE, Brady K, et al. The constitution of mineral trioxide aggregate. Dent Mat. 2005;21(4):297–303. doi: 10.1016/j.dental.2004.05.010. DOI: [DOI] [PubMed] [Google Scholar]
- 8.Camilleri J. The chemical composition of mineral trioxide aggregate. J Conserv Dent. 2008;11(4):141–143. doi: 10.4103/0972-0707.48834. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camilleri J. Hydration mechanisms of mineral trioxide aggregate. Int Endod J. 2007;40(6):462–470. doi: 10.1111/j.1365-2591.2007.01248.x. DOI: [DOI] [PubMed] [Google Scholar]
- 10.Malhotra N, Agarwal A, Mala K. Mineral trioxide aggregate: a review of physical properties. Compend Contin Educ Dent. 2013;34(2):e25–e32. [PubMed] [Google Scholar]
- 11.Malhotra N, Agarwal A, Mala K. Mineral trioxide aggregate: part 2 - a review of the material aspects. Compend Contin Educ Dent. 2013;34(3):e38–e43. [PubMed] [Google Scholar]
- 12.Srinivasan V, Waterhouse P, Whitworth J. Mineral trioxide aggregate in paediatric dentistry. Int J Paediatr Dent. 2009;19(1):34–47. doi: 10.1111/j.1365-263X.2008.00959.x. DOI: [DOI] [PubMed] [Google Scholar]
- 13.Monisha D, Manish C. MTA as A Revolution in Endodontics-A Review. 2013 In. [Google Scholar]
- 14.Jaberiansari Z, Naderi S, Tabatabaei FS. Cytotoxic effects of various mineral trioxide aggregate formulations, calcium-enriched mixture and a new cement on human pulp stem cells. Iran Endod J. 2014;9(4):271–276. [PMC free article] [PubMed] [Google Scholar]
- 15.Koulaouzidou EA, Economides N, Beltes P, et al. In vitro evaluation of the cytotoxicity of ProRoot MTA and MTA angelus. J Oral Sci. 2008;50(4):397–402. doi: 10.2334/josnusd.50.397. DOI: [DOI] [PubMed] [Google Scholar]
- 16.Ghoddusi J, Tavakkol Afshari J, Donyavi Z, et al. Cytotoxic effect of a new endodontic cement and mineral trioxide aggregate on L929 line culture. Iran Endod J. 2008;3(2):17–23. [PMC free article] [PubMed] [Google Scholar]
- 17.Crespo-Gallardo I, Hay-Levytska O, Martín-González J, et al. Criteria and treatment decisions in the management of deep caries lesions: is there endodontic overtreatment? J Clin Exp Dent. 2018;10(8):e751–e760. doi: 10.4317/jced.55050. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Deus G, Ximenes R, Gurgel-Filho ED, et al. Cytotoxicity of MTA and Portland cement on human ECV 304 endothelial cells. Int Endod J. 2005;38(9):604–609. doi: 10.1111/j.1365-2591.2005.00987.x. DOI: [DOI] [PubMed] [Google Scholar]
- 19.Küçükkaya S, Görduysus MÖ, Zeybek ND, et al. In vitro cytotoxicity of calcium silicate-based endodontic cement as root-end filling materials [Internet]. Scientifica. 2016;2016:9203932. doi: 10.1155/2016/9203932. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomes-Cornélio AL, Rodrigues EM, Salles LP, et al. Bioactivity of MTA plus, biodentine and an experimental calcium silicate‐based cement on human osteoblast‐like cells. Int Endod J. 2017;50(1):39–47. doi: 10.1111/iej.12589. DOI: [DOI] [PubMed] [Google Scholar]
- 21.Khedmat S, Dehghan S, Hadjati J, et al. In vitro cytotoxicity of four calcium silicate-based endodontic cements on human monocytes, a colorimetric MTT assay. Restor Dent Endod. 2014;39(3):149–154. doi: 10.5395/rde.2014.39.3.149. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanomaru-Filho M, Andrade AS, Rodrigues EM, et al. Biocompatibility and mineralized nodule formation of Neo MTA plus and an experimental tricalcium silicate cement containing tantalum oxide. Int Endod J. 2017;50(Suppl 2):e31–e39. doi: 10.1111/iej.12780. DOI: [DOI] [PubMed] [Google Scholar]
- 23.Krishnan V, Lakshmi T. Bioglass: a novel biocompatible innovation. J Adv Pharm Technol Res. 2013;4(2):78–83. doi: 10.4103/2231-4040.111523. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur M, Singh H, Dhillon JS, et al. MTA versus biodentine: review of literature with a comparative analysis. J Clin Diagn Res. 2017;11(8):ZG01–ZG05. doi: 10.7860/JCDR/2017/25840.10374. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pintado LS, Torre E, do N, et al. Development of a dual-cure mineral trioxide aggregate-based cement: biological, physical, and mechanical properties. J Conserv Dent. 2018;21(1):74–79. doi: 10.4103/JCD.JCD_393_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Güven EP, Taşlı PN, Yalvac ME, et al. In vitro comparison of induction capacity and biomineralization ability of mineral trioxide aggregate and a bioceramic root canal sealer. Int Endod J. 2013;46(12):1173–1182. doi: 10.1111/iej.12115. DOI: [DOI] [PubMed] [Google Scholar]
- 27.Haglund R, He J, Jarvis J, et al. Effects of root-end filling materials on fibroblasts and macrophages in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(6):739–745. doi: 10.1067/moe.2003.231. DOI: [DOI] [PubMed] [Google Scholar]
- 28.Saidon J, He J, Zhu Q, et al. Cell and tissue reactions to mineral trioxide aggregate and Portland cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(4):483–489. doi: 10.1067/moe.2003.20. DOI: [DOI] [PubMed] [Google Scholar]
- 29.Bidar M, Naghavi N, Mohtasham N, et al. Mineral trioxide aggregate and Portland cement for direct pulp capping in dog: a histopathological evaluation. J Dent Res Dent Clin Dent Prosp. 2014;8(3):134–140. doi: 10.5681/joddd.2014.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leye Benoist F, Gaye Ndiaye F, Kane AW, et al. Evaluation of mineral trioxide aggregate (MTA) versus calcium hydroxide cement (Dycal(®)) in the formation of a dentine bridge: a randomised controlled trial. Int Dent J. 2012;62(1):33–39. doi: 10.1111/j.1875-595X.2011.00084.x. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eskandarizadeh A, Shahpasandzadeh MH, Shahpasandzadeh M, et al. A comparative study on dental pulp response to calcium hydroxide, white and grey mineral trioxide aggregate as pulp capping agents. J Conserv Dent. 2011;14(4):351–355. doi: 10.4103/0972-0707.87196. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shokouhinejad N, Nekoofar MH, Razmi H, et al. Bioactivity of EndoSequence root repair material and bioaggregate. Int Endod J. 2012;45(12):1127–1134. doi: 10.1111/j.1365-2591.2012.02083.x. DOI: [DOI] [PubMed] [Google Scholar]
- 33.Hirschman WR, Wheater MA, Bringas JS, et al. Cytotoxicity comparison of three current direct pulp-capping agents with a new bioceramic root repair putty. J Endod. 2012;38(3):385–388. doi: 10.1016/j.joen.2011.11.012. DOI: [DOI] [PubMed] [Google Scholar]
- 34.Yoshino P, Nishiyama CK, Modena KC, et al. In vitro cytotoxicity of white MTA, MTA Fillapex® and Portland cement on human periodontal ligament fibroblasts. Braz Dent J. 2013;24(2):111–116. doi: 10.1590/0103-6440201302115. DOI: [DOI] [PubMed] [Google Scholar]
- 35.Zhou H, Du T, Shen Y, et al. In vitro cytotoxicity of calcium silicate-containing endodontic sealers. J Endod. 2015;41(1):56–61. doi: 10.1016/j.joen.2014.09.012. DOI: [DOI] [PubMed] [Google Scholar]
- 36.Zhou H, Shen Y, Wang Z, et al. In vitro cytotoxicity evaluation of a novel root repair material. J Endod. 2013;39(4):478–483. doi: 10.1016/j.joen.2012.11.026. DOI: [DOI] [PubMed] [Google Scholar]
- 37.Ma J, Shen Y, Stojicic S, et al. Biocompatibility of two novel root repair materials. J Endod. 2011;37(6):793–798. doi: 10.1016/j.joen.2011.02.029. DOI: [DOI] [PubMed] [Google Scholar]
- 38.Mukhtar-Fayyad D. Cytocompatibility of new bioceramic-based materials on human fibroblast cells (MRC-5). Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(6):e137–e142. doi: 10.1016/j.tripleo.2011.05.042. DOI: [DOI] [PubMed] [Google Scholar]
- 39.Naghavi N, Ghoddusi J, Sadeghnia HR, et al. Genotoxicity and cytotoxicity of mineral trioxide aggregate and calcium enriched mixture cements on L929 mouse fibroblast cells. Dent Mater J. 2014;33(1):64–69. doi: 10.4012/dmj.2013-123. DOI: [DOI] [PubMed] [Google Scholar]
- 40.Alanezi AZ, Jiang J, Safavi KE, et al. Cytotoxicity evaluation of endosequence root repair material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(3):e122–e125. doi: 10.1016/j.tripleo.2009.11.028. DOI: [DOI] [PubMed] [Google Scholar]
- 41.Nair U, Ghattas S, Saber M, et al. A comparative evaluation of the sealing ability of 2 root-end filling materials: an in vitro leakage study using enterococcus faecalis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(2):e74–e77. doi: 10.1016/j.tripleo.2011.01.030. DOI: [DOI] [PubMed] [Google Scholar]
- 42.Bin CV, Valera MC, Camargo SEA, et al. Cytotoxicity and genotoxicity of root canal sealers based on mineral trioxide aggregate. J Endod. 2012;38(4):495–500. doi: 10.1016/j.joen.2011.11.003. DOI: [DOI] [PubMed] [Google Scholar]
- 43.Ribeiro DA, Sugui MM, Matsumoto MA, et al. Genotoxicity and cytotoxicity of mineral trioxide aggregate and regular and white Portland cements on Chinese hamster ovary (CHO) cells in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(2):258–261. doi: 10.1016/j.tripleo.2005.02.080. DOI: [DOI] [PubMed] [Google Scholar]
- 44.Batur Y-B, Acar G, Yalcin Y, et al. The cytotoxic evaluation of mineral trioxide aggregate and bioaggregate in the subcutaneous connective tissue of rats. Med Oral Patol Oral Cir Bucal. 2013;18(4):e745–e751. doi: 10.4317/medoral.19095. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanz JL, Rodríguez-Lozano FJ, Llena C, et al. Bioactivity of bioceramic materials used in the dentin-pulp complex therapy: a systematic review. Materials (Basel) 2019;12(7):1015. doi: 10.3390/ma12071015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niu L, Jiao K, Wang T, et al. A review of the bioactivity of hydraulic calcium silicate cements. J Dent. 2014;42(5):517–533. doi: 10.1016/j.jdent.2013.12.015. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts HW, Toth JM, Berzins DW, et al. Mineral trioxide aggregate material use in endodontic treatment: a review of the literature. Dent Mater. 2008;24(2):149–164. doi: 10.1016/j.dental.2007.04.007. DOI: [DOI] [PubMed] [Google Scholar]
- 48.Hubbell JA. Bioactive biomaterials. Curr Opin Biotechnol. 1999;10(2):123–129. doi: 10.1016/S0958-1669(99)80021-4. DOI: [DOI] [PubMed] [Google Scholar]
- 49.Hoppe A, Güldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32(11):2757–2774. doi: 10.1016/j.biomaterials.2011.01.004. DOI: [DOI] [PubMed] [Google Scholar]
- 50.Okiji T, Yoshiba K. Reparative dentinogenesis induced by mineral trioxide aggregate: a review from the biological and physicochemical points of view. Int J Dent. 2009;2009:464280. doi: 10.1155/2009/464280. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ravi GR, Subramanyam RV. Possible mechanisms of lack of dentin bridge formation in response to calcium hydroxide in primary teeth. Dent Hypothe. 2015;6(1):6. doi: 10.4103/2155-8213.150863. DOI: [DOI] [Google Scholar]
- 52.Koike T, Polan MAA, Izumikawa M, et al. Induction of reparative dentin formation on exposed dental pulp by dentin phosphophoryn/collagen composite. Biomed Res Int. 2014;2014:745139. doi: 10.1155/2014/745139. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bioactivity and osteoinductivity of glasses and glassceramics and their material determinants [Google Scholar]
- 54.Modareszadeh MR, Di Fiore PM, Tipton DA, et al. Cytotoxicity and alkaline phosphatase activity evaluation of endosequence root repair material. J Endod. 2012;38(8):1101–1105. doi: 10.1016/j.joen.2012.04.014. DOI: [DOI] [PubMed] [Google Scholar]
- 55.Boldrin Mestieri L, Cornélio A, Rodrigues EM, et al. Biocompatibility and bioactivity of calcium silicate-based endodontic sealers in human dental pulp cells. J App Oral Sci. 2015;23(5):467–471. doi: 10.1590/1678-775720150170. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gandolfi M, Siboni F, Polimeni A, et al. In vitro screening of the apatite-forming ability, biointeractivity and physical properties of a tricalcium silicate material for endodontics and restorative dentistry. Dentis J. 2013;1(4):41–60. doi: 10.3390/dj1040041. DOI: [DOI] [Google Scholar]
- 57.Ma R, Guo D. Evaluating the bioactivity of a hydroxyapatite-incorporated polyetheretherketone biocomposite. J Orthop Surg Res [Internet] 2019;14(1):32. doi: 10.1186/s13018-019-1069-1. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mestieri LB, Gomes-Cornélio AL, Rodrigues EM, et al. Biocompatibility and bioactivity of calcium silicate-based endodontic sealers in human dental pulp cells. J Appl Oral Sci. 2015;23(5):467–471. doi: 10.1590/1678-775720150170. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang S-W. Chemical characteristics of mineral trioxide aggregate and its hydration reaction. Restor Dent Endod. 2012;37(4):188–193. doi: 10.5395/rde.2012.37.4.188. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tawil PZ, Duggan DJ, Galicia JC. MTA: a clinical review. Compend Contin Educ Dent. 2015;36(4):247–264. [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan Z, Zhu X, Li Y, et al. Influence of iRoot SP and mineral trioxide aggregate on the activation and polarization of macrophages induced by lipopolysaccharide. BMC Oral Health. 2018;18(1):56. doi: 10.1186/s12903-018-0511-9. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhagat D, Sunder RK, Devendrappa SN, et al. A comparative evaluation of ProRoot mineral trioxide aggregate and Portland cement as a pulpotomy medicament. J Indian Soc Pedod Prev Dent. 2016;34(2):172–176. doi: 10.4103/0970-4388.180448. DOI: [DOI] [PubMed] [Google Scholar]
- 63.Shahi S, Yavari HR, Rahimi S, et al. Comparison of the sealing ability of mineral trioxide aggregate and Portland cement used as root-end filling materials. J Oral Sci. 2011;53(4):517–522. doi: 10.2334/josnusd.53.517. DOI: [DOI] [PubMed] [Google Scholar]
- 64.Gandolfi MG, Siboni F, Primus CM, et al. Ion release, porosity, solubility, and bioactivity of MTA plus tricalcium silicate. J Endodont. 2014;40(10):1632–1637. doi: 10.1016/j.joen.2014.03.025. DOI: [DOI] [PubMed] [Google Scholar]
- 65.Fuad A, Al-Sabri, Elmarakby A, et al. Role of mineral trioxide aggregate (MTA) and calcium hydroxide in conservative dentistry as pulp capping material: a review. Am J Health Res. 2017;5(1):1–6. doi: 10.11648/j.ajhr.20170501.11. DOI: [DOI] [Google Scholar]
- 66.Gandolfi MG, Taddei P, Tinti A, et al. Apatite-forming ability (bioactivity) of ProRoot MTA. Int Endod J. 2010;43(10):917–929. doi: 10.1111/j.1365-2591.2010.01768.x. DOI: [DOI] [PubMed] [Google Scholar]
- 67.Kim M, Yang W, Kim H, et al. Comparison of the biological properties of ProRoot MTA, OrthoMTA, and Endocem MTA cements. J Endod. 2014;40(10):1649–1653. doi: 10.1016/j.joen.2014.04.013. DOI: [DOI] [PubMed] [Google Scholar]
- 68.Collado-González M, García-Bernal D, Oñate-Sánchez RE, et al. Cytotoxicity and bioactivity of various pulpotomy materials on stem cells from human exfoliated primary teeth. Int Endod J. 2017;50(Suppl 2):e19–e30. doi: 10.1111/iej.12751. DOI: [DOI] [PubMed] [Google Scholar]
- 69.Malkondu Ö, Karapinar Kazandağ M, Kazazoğlu E. A review on biodentine, a contemporary dentine replacement and repair material. Biomed Res Int. 2014;2014:160951. doi: 10.1155/2014/160951. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson JM. Future challenges in the in vitro and in vivo evaluation of biomaterial biocompatibility. Regen Biomater. 2016;3(2):73–77. doi: 10.1093/rb/rbw001. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Akbulut MB, Arpaci PU, Eldeniz AU. Effects of four novel root-end filling materials on the viability of periodontal ligament fibroblasts. Restor Dent Endod. 2018;43(3):e24. doi: 10.5395/rde.2018.43.e24. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Samyuktha V, Ravikumar P, Nagesh B, et al. Cytotoxicity evaluation of root repair materials in human-cultured periodontal ligament fibroblasts. J Conserv Dent. 2014;17(5):467–470. doi: 10.4103/0972-0707.139844. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghoddusi J, Forghani M, Parisay I. New approaches in vital pulp therapy in permanent teeth. Iran Endod J. 2014;9(1):15–22. [PMC free article] [PubMed] [Google Scholar]
- 74.Silva EJNL, Rosa TP, Herrera DR, et al. Evaluation of cytotoxicity and physicochemical properties of calcium silicate-based endodontic sealer MTA Fillapex. J Endod. 2013;39(2):274–277. doi: 10.1016/j.joen.2012.06.030. DOI: [DOI] [PubMed] [Google Scholar]
- 75.Collado-González M, Tomás-Catalá CJ, Oñate-Sánchez RE, et al. Cytotoxicity of GuttaFlow Bioseal, GuttaFlow2, MTA Fillapex, and AH Plus on human periodontal ligament stem cells. J Endod. 2017;43(5):816–822. doi: 10.1016/j.joen.2017.01.001. DOI: [DOI] [PubMed] [Google Scholar]
- 76.da Silva EJNL, Santos CC, Zaia AA. Long-term cytotoxic effects of contemporary root canal sealers. J Appl Oral Sci. 2013;21(1):43–47. doi: 10.1590/1678-7757201302304. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Demirci M, Hiller K-A, Bosl C, et al. The induction of oxidative stress, cytotoxicity, and genotoxicity by dental adhesives. Dent Mater. 2008;24(3):362–371. doi: 10.1016/j.dental.2007.06.009. DOI: [DOI] [PubMed] [Google Scholar]
- 78.Chang S-W, Lee S-Y, Kang S-K, et al. In vitro biocompatibility, inflammatory response, and osteogenic potential of 4 root canal sealers: Sealapex, Sankin apatite root sealer, MTA Fillapex, and iRoot SP root canal sealer. J Endod. 2014;40(10):1642–1648. doi: 10.1016/j.joen.2014.04.006. DOI: [DOI] [PubMed] [Google Scholar]
- 79.Victoria-Escandell A, Ibañez-Cabellos JS, de Cutanda SB-S, et al. Cellular responses in human dental pulp stem cells treated with three endodontic materials. Stem Cells Int. 2017;2017:8920356. doi: 10.1155/2017/8920356. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Poggio C, Ceci M, Dagna A, et al. In vitro cytotoxicity evaluation of different pulp capping materials: a comparative study. Arh Hig Rada Toksikol. 2015;66(3):181–188. doi: 10.1515/aiht-2015-66-2589. DOI: [DOI] [PubMed] [Google Scholar]
- 81.Sawicki L, Pameijer CH, Emerich K, et al. Histological evaluation of mineral trioxide aggregate and calcium hydroxide in direct pulp capping of human immature permanent teeth. Am J Dent. 2008;21:262–266. [PubMed] [Google Scholar]
- 82.Cavalcanti BN, Rode SM, Marques MM. Cytotoxicity of substances leached or dissolved from pulp capping materials. Int Endod J. 2005;38(8):505–509. doi: 10.1111/j.1365-2591.2005.00967.x. DOI: [DOI] [PubMed] [Google Scholar]
- 83.Asgary S, Eghbal MJ, Parirokh M, et al. A comparative study of histologic response to different pulp capping materials and a novel endodontic cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(4):609–614. doi: 10.1016/j.tripleo.2008.06.006. DOI: [DOI] [PubMed] [Google Scholar]
- 84.Kaup M, Dammann CH, Schäfer E, et al. Shear bond strength of biodentine, ProRoot MTA, glass ionomer cement and composite resin on human dentine ex vivo. Head Face Med. 2015;11(1):14. doi: 10.1186/s13005-015-0071-z. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saberi A, Farhadmollashahi E, Ghotbi N, et al. Cytotoxic effects of mineral trioxide aggregate, calcium enrichedmixture cement, biodentine and octacalcium pohosphate onhuman gingival fibroblasts. J Dent Res Dent Clin Dent Prospects. 2016;10(2):75–80. doi: 10.15171/joddd.2016.012. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corral Nuñez CM, Bosomworth HJ, Field C, et al. Biodentine and mineral trioxide aggregate induce similar cellular responses in a fibroblast cell line. J Endod. 2014;40(3):406–411. doi: 10.1016/j.joen.2013.11.006. DOI: [DOI] [PubMed] [Google Scholar]
- 87.Köseoğlu S, Pekbağr Yan KT, Kucukyilmaz E, et al. Biological response of commercially available different tricalcium silicate-based cements and pozzolan cement. Microsc Res Tech. 2017;80(9):994–999. doi: 10.1002/jemt.22891. DOI: [DOI] [PubMed] [Google Scholar]
- 88.Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review--part III: clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36(3):400–413. doi: 10.1016/j.joen.2009.09.009. DOI: [DOI] [PubMed] [Google Scholar]
- 89.Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review--part I: chemical, physical, and antibacterial properties. J Endod. 2010;36(1):16–27. doi: 10.1016/j.joen.2009.09.006. DOI: [DOI] [PubMed] [Google Scholar]
- 90.Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review--part II: leakage and biocompatibility investigations. J Endod. 2010;36(2):190–202. doi: 10.1016/j.joen.2009.09.010. DOI: [DOI] [PubMed] [Google Scholar]


