Abstract
Background
Effectively communicating with parents about children’s obesity risk is of critical importance for preventive medicine and public health.
Purpose
The current study investigates the efficacy of communications focused on two primary causes of obesity: genes and environment.
Methods
We compared parental feeding responses to messages focused on (i) genetics alone, (ii) family environment alone, (iii) genetics–family environment interaction (G × FE), and (iv) no causal message. We also examined whether parental guilt mediates the effect of message type on feeding. Our sample consisted of 190 parents, half mothers and half fathers, of children 3–7 years old. After receiving one of the four types of messages, parents chose foods for their child using the Virtual Reality Buffet measure. Parents responded to questionnaires in the lab and at 1-week follow-up.
Results
In the VR Buffet, parents did not feed their children differently in message provision conditions versus control. There were, however, differences among message provision conditions wherein mothers who received any genetic information chose higher-calorie meals in the VR Buffet. At 1-week follow-up, parents who received information about genetics alone reported feeding their child more junk food and fatty meat on self-report food frequency assessments; there were no such differences for sugary beverages, sugary foods, or fast foods. Parental guilt was typically higher for participants who received family environment information alone but did not mediate the relation between information provision and feeding outcomes.
Conclusions
While none of the messages improved feeding above the control condition, GxFE messages were associated with a better overall profile of outcomes. As such, it may be beneficial for messaging for parents about children’s obesity risk to include content that reflects the complexity of genetic and environmental contributions to obesity risk.
Keywords: Genomics, Obesity, Communication, Parents
Risk information highlighting gene-environment interaction had a better profile of outcomes for encouraging parent behavior change than genetics- or family environment-only information.
Introduction
Developing precision medicine approaches for common health conditions continues with large-scale efforts such as the All of Us research program [1], and smaller-scale efforts in research groups around the world. These approaches involve the exploration of polygenic risk scores, gene–environment interactions, and other complex phenomena, with the ultimate goal of leveraging genomic information to prevent and treat disease. Obesity is one disease that poses a great public health burden, and thus is a prized target for precision medicine approaches. Although scientists have yet to identify clinical precision medicine approaches for obesity prevention and treatment, precision medicine-based obesity treatments have garnered much attention and may one day be effective [2, 3]. If we wait until this comes to fruition, we will miss key opportunities for determining how to optimally communicate these topics to facilitate healthy behaviors. It is, therefore, crucial to begin developing effective communication approaches for populations that stand to benefit from such interventions.
Some of the interest and excitement around genomics products in weight management is driven by the fact that there has been little success of any single approach for achieving sustained, long-term weight loss [4, 5]. As such, researchers and clinicians have suggested that targeting public health efforts to parents and families about weight gain among children may be most effective in the long term [6–8]. From a genomics perspective, prevention communications might be especially important for children with a family history of obesity, as they are at high risk [9].
A challenge is that most research examining communication about obesity and genomics has been conducted in the context of adults’ personal behavior following information receipt, rather than on parents’ choices for a child. Research has previously suggested that genetic risk information does not lead to increased health behavior motivation or actual change in dietary behavior among adults [10–12]. However, a focus on children has opened up new avenues for use of genomics in health promotion. Only a limited amount of work has been done in this arena, partially due to open ethical and practical questions about genetic testing for children [13, 14], and also due to a general lack of child-relevant genomics approaches to address weight.
Nonetheless, one study has shown that personalized information about a child’s risk holds promise for changing mothers’ feeding behavior, although the effects are nuanced [15]. In this study, mothers exhibited positive changes in feeding behavior when risk information indicated that the mother alone (rather than together with the biological father) was responsible for transmitting increased obesity risk to her child. However, other research has suggested that such communication can backfire. A different study found that parents of higher weight who perceived their child to also have higher weight reported defensive cognitions and affect in response to a media-based risk message that conveyed information about how a child’s genetic makeup interacts with the family home environment to confer obesity risk in later life [16]. It is now becoming clear that, much like obesity etiology itself, communication with parents about the causes of obesity is likely to have complicated effects.
Communicating about causal risk factors for obesity is challenging for several other reasons. While a primary goal of conveying genomics information is to spur behavior change, theoretical and empirical work over the last several years has shown that other effects can also emerge. Because genes are immutable, genetic information has been considered a potential source of fatalistic attitudes. Outright expression of fatalism is rare, however in some studies, consideration of genetic factors related to diet and weight has been linked to reduced self-efficacy and motivation for health behaviors [17–19]. A key approach to tempering information about genetic factors, and thereby avoiding fatalistic interpretations, may be to highlight how genetics interacts with the environment to confer risk. In this case, parents are alerted to their child’s increased risk on multiple potential fronts (genes and environment), but also given a mechanism to reduce this risk (family environmental changes). As noted above, this approach has shown mixed success in the context of parental feeding behavior [15, 16]. An additional benefit to the communication of gene–environment interaction causal messages is that they are most accurate. Lay conceptions of body weight are typically multifactorial [20, 21], and health communication consistent with preexisting conceptions is less likely to be rejected [22, 23]. As such, further exploration into parents’ reactions to such messages is crucial.
The extant parenting research tends to focus on mothers, leaving us with very little evidence of how fathers react to such information [24, 25]. Gaining this knowledge will be important given the central and increasing role that fathers play in child feeding [26]. Mothers and fathers may have different cognitive and affective responses to information about child feeding [24, 27], likely due in part to differences in gender norms and previous parenting experiences [28, 29]. As such, it is essential to understand the effects of messages on mothers and fathers.
Finally, although there has been some suggestion that the use of genomics-informed approaches may be effective for parental behavior change vis-à-vis child feeding, the pathway to such behavior change is unclear. An intriguing possibility is that feelings of parental guilt might motivate parental behavior change [30]. This could be due to the reparative nature of guilt [31, 32] such that parents feed healthier foods in order to correct the perceived wrongdoing of passing down the risk of obesity. There is, however, counter-evidence suggesting that guilt may instead be linked with maladaptive parent behavior in some cases, including making higher-calorie choices for children, and unrestrained food consumption [33]. In all, the potential association of genomics-informed risk information provision and parental behavior via guilt has not been extensively tested despite having clear theoretical and practical relevance.
The current study investigates the two primary elements of contemporary messages that parents receive regarding obesity risk transmission to their child: genetic contributions and family environment contributions. We therefore compared messages focused on (i) genetics alone, (ii) family environment alone, (iii) genetic–family environment interaction, and (iv) control with no causal message. In so doing, we aim to disentangle the unique and interactive contributions of these message types to parental feeding behavior. In addition, this study explores parental guilt reactions as a potential pathway through which risk information may lead to feeding behavior differences. Importantly, we investigate these aims among both mothers and fathers to assess possible gender differences. Risk information was provided to parents in a lab study using public health-style presentations, and child feeding behavior was measured in the lab with a validated virtual reality-based child feeding assessment [34]. Child diet was measured at a 1-week follow-up via questionnaires. Our hypotheses were as follows. Across all of the proposed hypotheses, we were further interested in whether effects would differ between mothers and fathers.
Hypothesis 1: Information that discusses any route through which parents confer obesity risk to their children will lead to serving fewer calories from the VR buffet and fewer servings of unhealthy foods at a 1-week follow-up than information that does not contain any causal information (i.e., control).
Hypothesis 1a: Among the messages that contain causal information, gene–family environment interaction messages will be associated with the lowest-calorie meal choices and fewest unhealthy feeding choices at follow-up. In contrast, genetics-only messages will be associated with the highest-calorie meal choices and most unhealthy choices at follow-up.
Hypothesis 2: Information that discusses any route through which parents confer obesity risk to their children will lead to more parental guilt than information that does not contain any causal information (i.e., control).
Hypothesis 2a: Gene–family environment interaction messages will be associated with the most parental guilt whereas genetics-only messages will be associated with the least guilt.
Hypothesis 3: Guilt will mediate the relationship between the presentation of obesity risk information and food choices, both in the VR buffet and at a 1-week follow-up.
Methods
Participants
Participants included 190 biological parents of a 3- to 7-year-old child with no major food allergies to VR buffet items, diet-related health conditions, developmental delays, or dietary restrictions that would limit the ability to eat most foods on the VR buffet. Participants were recruited from the metropolitan Washington DC area and were incentivized $90 for completing all parts of the study including the follow-up questionnaire. Inclusion criteria included self-reported overweight status, at least some responsibility for child feeding, and the ability to read and write in English. Exclusion criteria included having a vestibular or seizure disorder, high propensity for motion sickness, known pregnancy, uncorrected poor vision or hearing, past or current eating disorder, or participation of another household member or another biological parent of the relevant child in the study. Recruitment was stratified to ensure an equal representation of mothers and fathers. Recruitment methods included posting advertisements in social and traditional media, posting flyers, and word-of-mouth. This study was approved by the IRB of the National Human Genome Research Institute.
We arrived at the sample size included here using a calculated sample size from McBride et al. [15] of d =.412 between control and genomic information groups. Power calculations were based on a 2 × 2 ANCOVA, with an alpha of .05 and power of .8, resulting in a sample size of 187. This power analysis did not take into account the inclusion of participant gender as an additional factor in analyses as it was a secondary research question.
Design
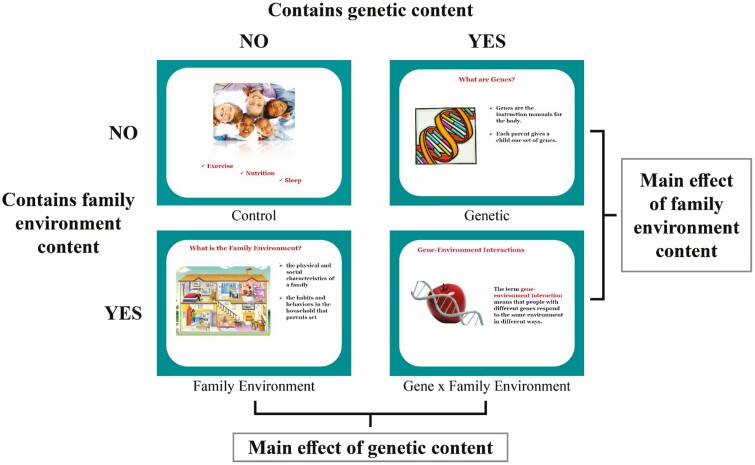
This randomized, experimental study employed a 2 × 2 design. The first factor was the inclusion of genetic information in the message presented to parents—present or absent. The second factor was the inclusion of family environment information in the message presented to parents—present or absent. This resulted in four experimental conditions: no genetics/no family environment (control), genetics only, family environment only, or both genetics and family environment (gene–family environment interaction; G × FE). Analyses also considered parent gender (mother versus father) as an additional factor crossed with the two experimental variables.
Procedure
Participants entered the study through a link to an online screening survey, or through a telephone call if they preferred to be phone screened. For eligible participants, an “index child” was identified who met study inclusion criteria (being between 3 and 7 years old without disqualifying dietary or health conditions as mentioned above). Parents were asked to consider only this child as they completed the study. After providing consent, participants completed the pretest questionnaire online, which included baseline measures of attitudes, beliefs, and demographic characteristics.
Participants were then contacted by phone to schedule an appointment for a lab visit. These visits took place, on average, 19 days after completion of the pretest questionnaire. At the visit, participants were randomized to condition via random number generator and consented again. Parents were also trained on the use of the VR Buffet, in which they were asked to make a meal from the training food and drink for their child.
Participants then received information about their child’s future risk for obesity consistent with their randomized experimental condition. Full materials are available here: https://osf.io/ceakg/?view_only=bba945942a3043f69f8140dae26657e2. Information was presented via pre-recorded PowerPoint presentation with auditory narration (see Fig. 1). All conditions received general information about the importance of nutrition, physical activity, and sleep for children. Those randomized to the genetic condition additionally received information about genetic risk for obesity and its transmission across generations (e.g., through inheriting genes predisposing individuals to higher levels of hunger; [35]). Participants randomized to the family environment condition were given information about obesity risk transmission through features of the environment in which children grow up (e.g., foods kept in the household; [36]). Those randomized to the G × FE condition received the genetic information, the family environment information, and a short module on how genes and the environment interact to influence obesity risk (e.g., children who are hungry more often have the worst outcomes when raised in a home where unhealthy feeding behaviors are commonplace; [37, 38]). All participants who received information about genetic and/or home environment risk factors also received epidemiological information stating that children who have one parent with overweight are at increased risk for obesity in adulthood relative to children with lean parents (28% chance as opposed to 13% chance), and that children with two overweight parents are at even greater risk (58% chance). Although it is atypical to present numeric risk information with family environment-focused educational materials, we did so to hold this factor constant across groups. Materials were gender-matched to refer to either mothers or fathers depending upon the participant’s gender.
Fig. 1.
Study design.
Following information provision, participants filled out a short questionnaire, which included the primary guilt assessment. We assessed guilt in three ways: a global measure, a context-specific item addressing genetics-oriented guilt, and a context-specific item assessing family environment-oriented guilt (see below for details). Participants then used the validated VR Buffet tool [34] to make food and drink choices for their child. Following the VR Buffet measure, participants filled out a final questionnaire in the lab related to their attitudes and beliefs about their child’s health (no data from this questionnaire were used in the current study).
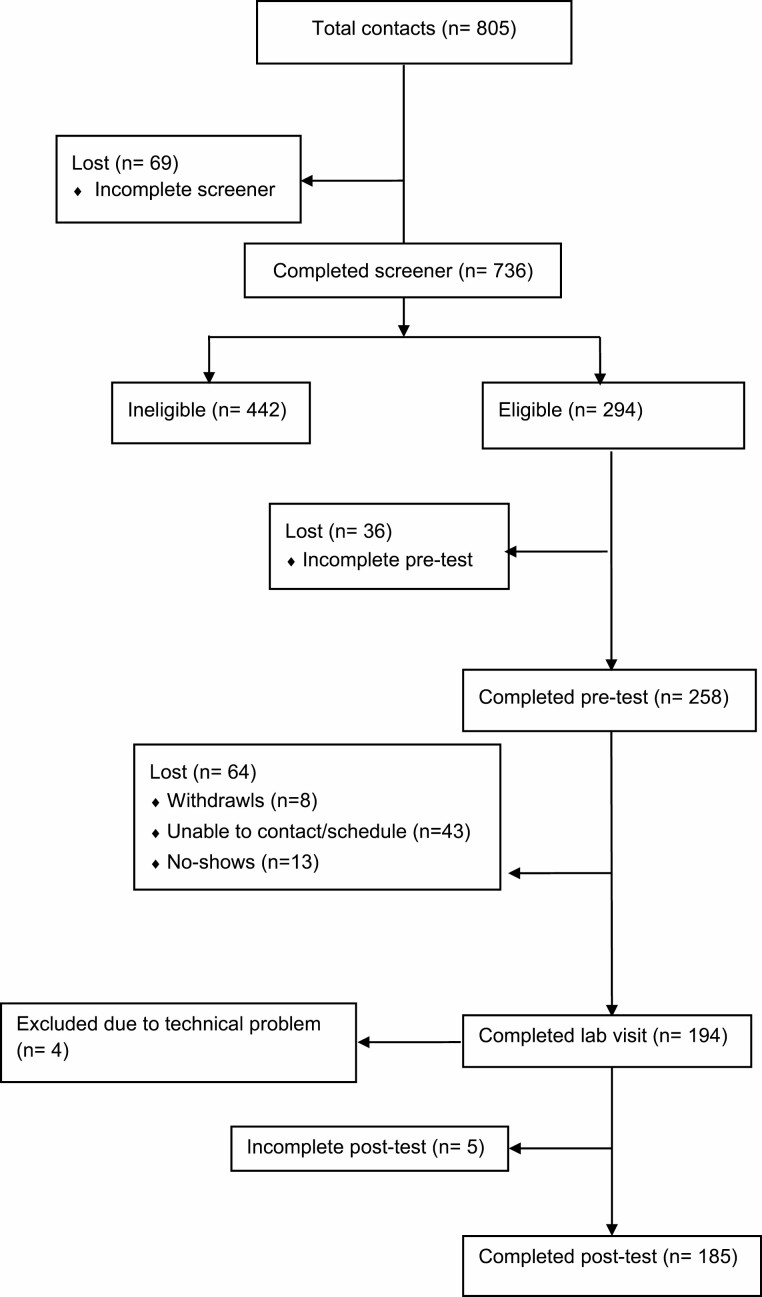
At the conclusion of the lab visit, participants who agreed to be recontacted were sent a link to a follow-up survey 1 week after their lab visit, which was then available for one week. One hundred and eighty-five parents completed the follow-up survey. Included in this follow-up survey was a food frequency assessment (FFA) that addressed several categories of food served to the index child in the past week [39, 40]. Participants who completed the one-week follow-up questionnaire read a debriefing at its conclusion. Those who did not complete the questionnaire were debriefed either in person (if they refused the follow-up survey) or by email or phone (see Fig. 2).
Fig. 2.
Participant flow diagram.
Measures
Behavioral assessment of food chosen for the index child took place using the VR Buffet [34], a validated assessment of parent feeding behavior. Parents were asked to assemble a lunch plate that their child would likely eat (see supplement for specific instructions https://osf.io/ceakg/?view_only=bba945942a3043f69f8140dae26657e2). If parents filled a plate to capacity, research assistants re-started the program so that participants could fill a second plate for the child (this occurred for approximately 10% of the sample). Total calories were calculated using a nutritional database based on the serving size of the virtual foods and drinks [15]. For example, a serving of virtual grapes occupied 7.3 cm3 in the VR Buffet, and thus calorie information associated with that volume of food was added to the calorie count for each serving of grapes a parent selected. Additionally, parent feeding behavior during the VR Buffet training session, which took place prior to receipt of experimental information, was recorded. Amount of training food (pasta with tomato sauce) and drink (apple juice) was analyzed for calorie count and used as a covariate, representing a proxy for general serving tendency prior to receipt of risk information.
Self-report measures included all demographic variables (see Table 1) and guilt assessments. Global guilt was assessed immediately after presentation of the information with an adapted version of the Personal Feelings Questionnaire 2 [41, 42] that refer to state emotions felt “currently” (as opposed to trait-level tendencies to feel guilt). Following factor analysis, the seven items from the guilt subscale that loaded highly onto the same factor were used to index state guilt (mild guilt, worry about injuring/hurting someone, self-consciousness, regret, intense guilt, deserving of criticism, remorse). Responses were collected on a 0–4 scale where 0 = I do not experience the feeling and 4 = I experience the feeling very strongly. Context-specific guilt was assessed at baseline and following the VR Buffet with a single item each for genetics- and family environment-oriented guilt (“I feel guilty about the genetic risk for obesity that I may have passed down to [INDEX CHILD]”; “I feel guilty that our home environment could increase [INDEX CHILD’S] risk for obesity”). These items were assessed with a 1–7 Likert-type scale (1 = strongly disagree; 7 = strongly agree).
Table 1:
Demographics, n (%) or M(SD)
| Demographics by experimental condition | ||||
|---|---|---|---|---|
| Control (n = 46) | Family environment (n = 48) | Genetic only (n = 48) | G × FE (n = 48) | |
| Gender: female | 24 (52%) | 25 (52%) | 24 (50%) | 25 (52%) |
| Age | 40.00 (7.07) | 38.23 (4.86) | 39.67 (6.10) | 39.96 (7.33) |
| Race: White | 30 (65%) | 36 (75%) | 36 (75%) | 32 (67%) |
| Race: Black/AA | 10 (22%) | 5 (10%) | 10 (21%) | 11 (23%) |
| Race: Asian | 7 (15%) | 6 (13%) | 6 (13%) | 6 (13%) |
| College education | 36 (78%) | 41 (85%) | 38 (79%) | 41 (85%) |
| BMI | 31.75 (8.85) | 29.30 (4.46) | 30.57 (5.66) | 30.44 (5.78) |
| Other bio parent: overweight | 25 (54%) | 25 (52%) | 27 (56%) | 25 (52%) |
| Index child gender: female | 21 (46%) | 25 (52%) | 25 (52%) | 19 (40%) |
| Index child age | 4.58 (1.42) | 4.29 (1.27) | 4.51 (1.41) | 4.60 (1.43) |
| Index child: overweight | 6 (13%) | 7 (15%) | 8 (17%) | 2 (4%) |
| Demographics by gender | ||||
| Male (n = 92) | Female (n = 98) | |||
| Age | 40.9 (6.96) | 38.87 (5.80) | ||
| Race: White | 68 (74%) | 66 (67%) | ||
| Race: Black/AA | 12 (13%) | 24 (24%) | ||
| Race: Asian | 14 (15%) | 11 (11%) | ||
| College education | 78 (85%) | 78 (80%) | ||
| BMI | 30.60 (6.39) | 30.42 (6.40) | ||
| Other bio parent: overweight | 54 (59%) | 48 (49%) | ||
| Index child gender: female | 42 (46%) | 48 (49%) | ||
| Index child age | 4.45 (1.28) | 4.57 (1.48) | ||
| Index child: overweight | 7 (8%) | 16 (16%) | ||
Child diet at the 1-week follow-up was assessed using the Food Frequency Assessment (FFA) from the National Cancer Institute FLASHE study [39, 40]. Parents were asked to report on the eating behavior of the index child over the last seven days on a scale from 1 (child did not eat the food in the past 7 days) to 6 (child ate the food three or more times per day). Items from the FFA were assembled into five subscales assessing unhealthy feeding, including junk food (e.g., pizza), sugar-sweetened beverages (e.g., sweetened fruit drinks), fatty meats (e.g., fried chicken), fast or convenience foods (e.g., heat and serve foods), and sugary foods (e.g., candy).
Data Analysis
Demographics were stratified by the experimental group and parent gender. There were no differences in demographic factors by experimental conditions (see Table 1). As reporting Black/African-American race differed by gender, African-American race was entered as a control variable in all analyses. We examined the distribution of all outcome variables; although some variables exhibited skew (see Supplementary Table), transforming variables did not change the pattern of results. Therefore, analyses are reported with untransformed variables to facilitate interpretation. One variable, frequency of serving sugar-sweetened beverages, was dichotomized into “sometimes” versus “never” due to a severe right skew. For the VR buffet calories outcome, there was one major outlier at >2 SD above the mean, which was excluded from analysis (this exclusion did not change the pattern of results). Additionally, although all participants self-reported overweight status at screening, 31 participants later reported being “just about the right weight” (n = 30) or “underweight” (n = 1) in the follow-up questionnaire. Prevalence of such reports did not vary by the experimental group. Because all participants indicated overweight status at screening and were thus eligible for the study, and because the period between the lab visit and follow-up assessment was set at 1–2 weeks, precluding major weight loss during this time frame, all participants were retained in the dataset.
We conducted ANCOVA to assess the relation between the experimental group, gender, and outcome variables including guilt, feeding in the VR Buffet, and feeding at follow-up. The exception was the dichotomized outcome (frequency of serving sugar-sweetened beverages), which was assessed with binary logistic regression. For outcomes where baseline values were available (VR Buffet calories during training, context-specific guilt items) these values were entered as covariates in the model following previous research [15]. We also assessed whether the control group was different from each of the experimental groups using contrasts of estimated marginal means.
We then assessed correlations between variables related to child feeding and guilt. Following these, we conducted mediation analyses using the PROCESS macro Module 8 [43] to explore whether guilt mediated the relations uncovered between the experimental group and child feeding outcomes. Mediation analyses were only performed for the primary hypothesized mediations (global guilt as a mediator of information effects on food choice in the VR Buffet), and for other relations where the necessary prerequisite relations were uncovered in the preceding ANCOVA and correlation analyses. In all mediation models, we controlled for African American race as in other analyses, and we controlled for baseline levels of variables where available. For the VR Buffet feeding outcome, we assessed global guilt as the potential mediator. For the FFA outcomes, we tested both global and context-specific guilt in separate models. The research questions tested here were preregistered, however, the preregistration did not specify hypotheses or specific analytic approaches (https://osf.io/ceakg/?view_only=bba945942a3043f69f8140dae26657e2).
Results
Demographics and Baseline Characteristics
Participant demographic characteristics are presented in Table 1. There were no differences by experimental condition; mothers and fathers differed in their racial composition in that more mothers than fathers reported identifying as Black or African-American, X2 = 4.04, p =.033.
Calories Selected in VR Buffet
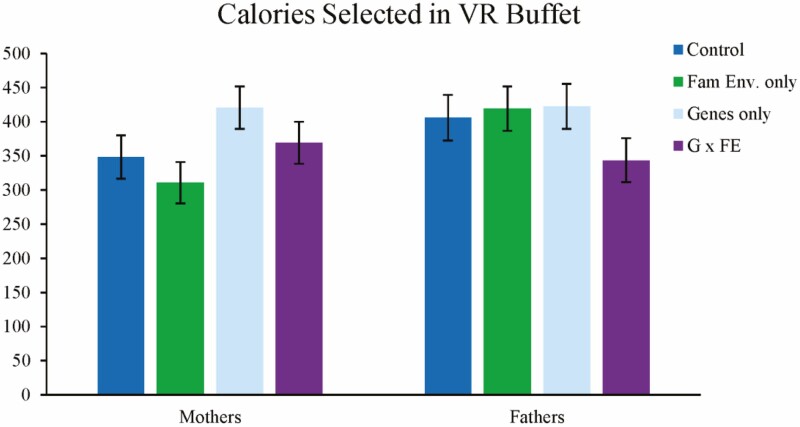
ANCOVA revealed that, controlling for pretest calories, there were no main effects of information type or gender on calories selected. In addition, the interaction between family environment information and gender, and the three-way interaction were not significant. There was a significant interaction between genetic information provision and gender such that among mothers, receiving any genetic content (whether or not it was accompanied by family environment content) was associated with choosing more calories in the buffet compared to mothers who did not receive genetic content, F(1,172) = 4.45, p = .036. In further contrasts of the estimated marginal means following ANCOVA, none of the experimental groups differed significantly from control. Pairwise comparisons also showed that, among parents who received genetic information, mothers and fathers did not differ in the number of calories selected; however in the nongenetic conditions, fathers selected higher calorie meals than mothers, F(1,172) = 6.73, p = .010. See Fig. 3 for a visual representation of these results and Table 2 for descriptive statistics.
Fig. 3:
Calories selected in the VR Buffet. Error bars represent 95% confidence interval.
Table 2:
Descriptive statistics by experimental group; M (SD)
| Control | Fam Env | Genetic | G × FE | |||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | |
| VR Buffet calories | 430.43 (223.01) | 341.95 (137.33) | 407.59 (215.71) | 319.76 (124.13) | 408.75 (108.48) | 428.95 (129.29) | 336.29 (176.79) | 364.98 (145.39) |
| FFA: junk food | 4.52 (2.44) | 3.92 (1.56) | 5.00 (2.91) | 3.75 (2.01) | 5.18 (2.13) | 4.92 (2.06) | 4.13 (2.10) | 3.56 (1.66) |
| FFA: fatty meats | 2.05 (1.32) | 1.71 (1.30) | 2.18 (1.59) | 1.79 (1.06) | 2.68 (1.62) | 2.33 (1.58) | 1.01 (1.16) | 1.52 (1.05) |
| FFA: fast/convenience food | 4.19 (2.16) | 3.25 (1.33) | 4.05 (2.44) | 2.92 (1.56) | 4.32 (2.75) | 3.83 (2.30) | 4.04 (2.06) | 3.20 (1.76) |
| FFA: sugary foods | 3.38 (1.96) | 2.88 (1.23) | 3.73 (2.51) | 2.79 (1.56) | 3.91 (2.02) | 3.75 (2.05) | 3.35 (1.82) | 2.08 (1.82) |
| FFA: sugar-sweetened beverages* | .38 (.50) | .50 (.51) | .36 (.49) | .38 (.49) | .52 (.51) | .46 (.51) | .48 (.51) | .24 (.44) |
| Global guilt | 0.36 (0.56) | 0.26 (0.28) | 0.76 (0.83) | 1.0 (0.91) | 0.75 (0.76) | 0.58 (0.84) | 0.52 (0.63) | 0.73 (0.65) |
| Genetic guilt | 2.32 (1.78) | 2.91 (1.68) | 2.35 (1.58) | 3.20 (1.96) | 2.29 (1.43) | 2.46 (1.93) | 2.52 (1.70) | 3.33 (2.20) |
| Fam Env guilt | 2.59 (1.97) | 3.52 (1.59) | 2.87 (1.89) | 4.0 (1.68) | 2.71 (1.60) | 3.25 (1.94) | 3.26 (1.49) | 3.46 (1.77) |
FFA, food frequency assessment.
*Proportion of parents reporting child consumed any sugar-sweetened beverages.
Food Frequency at 1-Week Follow-Up
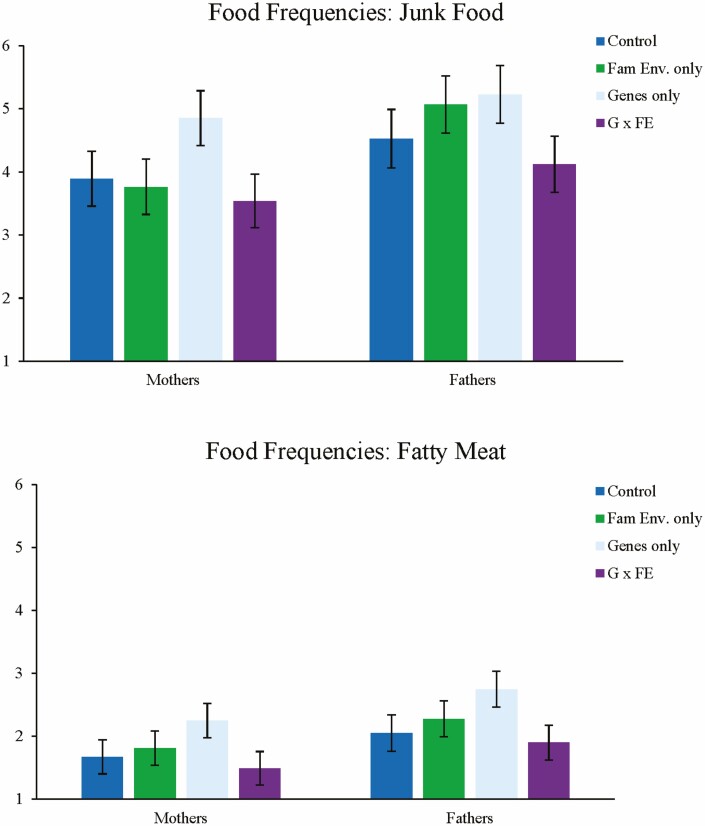
For junk food, there were no main effects of information type, and no interaction between gender and genetics information, or between gender and family environment information. The three-way interaction was also not significant. There was, however, a significant main effect of gender, F(1,176) = 5.23, p = .023, such that fathers reported more servings of junk food than mothers. There was also a two-way interaction between genetic and family environment information provision, F(1,176) = 5.10, p = .025, such that when genetic information was presented without family environment information (i.e., genetics alone), parents reported giving more servings of junk food than when family environment information was present (i.e., G × FE), F(1,176) = 7.61, p = .006. Further pairwise contrasts revealed that none of the experimental groups differed significantly from control.
The same patterns were exhibited for servings of fatty meats, such that there were no main effects of information type, nor any interactions involving gender. Fathers reported more fatty meat servings than mothers F(1,176) = 4.92, p = .028, and there was an analogous interaction between genetic and family environment information provision, F(1,176) = 6.27, p = .013. Parents reported giving more servings of fatty meat when genetic information was presented without family environment information than when the family environment was presented, F(1,176) = 8.54, p = .004. Pairwise contrasts showed that parents in the genetics-only group reported feeding children significantly more fatty meats than control F(1,176) = 5.18, p = .024. No other groups differed significantly from control. See Fig. 4.
Fig. 4.
Responses for food frequency assessment, junk food and fatty meats. Error bars represent 95% confidence interval.
No main or interactive effects emerged for sugar-sweetened beverages or sugary foods. There was a significant main effect of gender for fast and convenience foods such that fathers reported more servings, F(1,176) = 12.32, p = .001, with no other main effects or interactions. See Table 2.
Guilt Outcomes
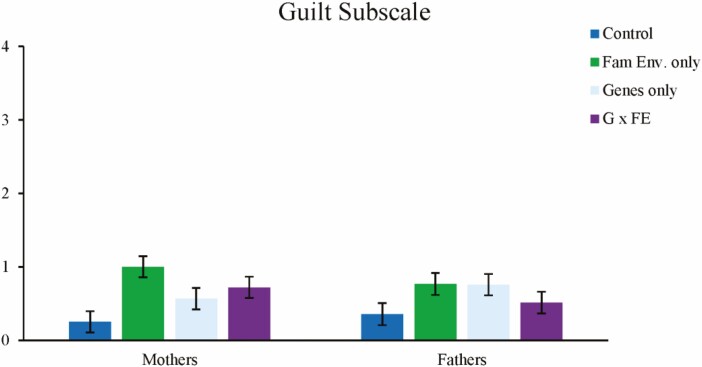
For global guilt, the main effect of family environment information provision emerged such that parents who received family environment information reported higher levels of guilt, F(1,180) = 6.70, p = .010; there were no other main effects. There was also a significant interaction between genetic and family environment information provision, F(1,180) = 9.07, p = .003. Here, parents who received family environment content alone (without genetic content) reported the highest levels of global guilt. There were no other significant interactions. Pairwise contrasts showed that both the genetics-only, F(1,180) = 4.96, p = .016, and the family environment-only conditions, F(1,180) = 15.51, p < .0001, differed significantly from control, while the G × FE group did not differ from control. See Table 2 and Fig. 5.
Fig. 5.
Reported levels of global guilt. Error bars represent 95% confidence interval.
For genetic guilt, pairwise t-tests revealed that only parents in the control condition exhibited a pre-vs-post difference in that they reported less guilt following information provision, t(47) = –2.50, p = .016. No other groups exhibited a difference in genetic guilt from pretest to post-test. For family environment guilt, when family environment information was present, parents exhibited an increase in guilt from baseline. Differences were significant for both the family environment only group, t(47) = 3.71, p = .001, and the G × FE group, t(46) = 2.70, p = .010. Neither the control group nor the genetics-only group exhibited differences from pretest.
ANCOVAs revealed that, for family environment-specific guilt following information provision (controlling for pretest guilt), there was a main effect of family environment information provision, F(1,178) = 5.45, p = .021, such that parents who received this information type reported higher levels of guilt. There was also a main effect of gender, F(1,178) = 4.71, p = .031, wherein mothers reported higher levels of guilt. There were no other main effects or interactions. For genetics-oriented guilt, there were no significant main effects or interactions. Taken together, results suggest that the provision of family environment information was most strongly related to increases in parental guilt.
Correlations and Mediation of Food Choice by Guilt
Bivariate correlations are listed in Table 3. Calories selected in the VR Buffet were uncorrelated with all guilt measures. In a linear regression model that included the covariates listed above, none of the guilt variables significantly predicted calories selected (data not shown). Three of the food frequency outcomes from the 1-week follow-up (sugar-sweetened beverages, fatty meats, and fast/convenience foods) were positively correlated with family environment-oriented guilt.
Table 3:
Bivariate correlations
| Global guilt | Fam Env Guilt | Genetic guilt | VR Buffet calories | |
|---|---|---|---|---|
| VR Buffet calories | –0.043 | –0.002 | –0.051 | – |
| FFA: junk food | 0.047 | 0.091 | –0.078 | 0.43** |
| FFA: sugar-sweetened beverages (yes vs. no) | 0.040 | 0.159 * | 0.072 | 0.31** |
| FFA: sugary foods | 0.010 | 0.019 | -0.098 | 0.41** |
| FFA: fatty meats | 0.014 | 0.156 * | 0.006 | 0.39** |
| FFA: fast/convenience foods | 0.089 | 0.167 * | 0.066 | 0.38** |
FFA, food frequency assessment.
* p < .05; ** p < .001.
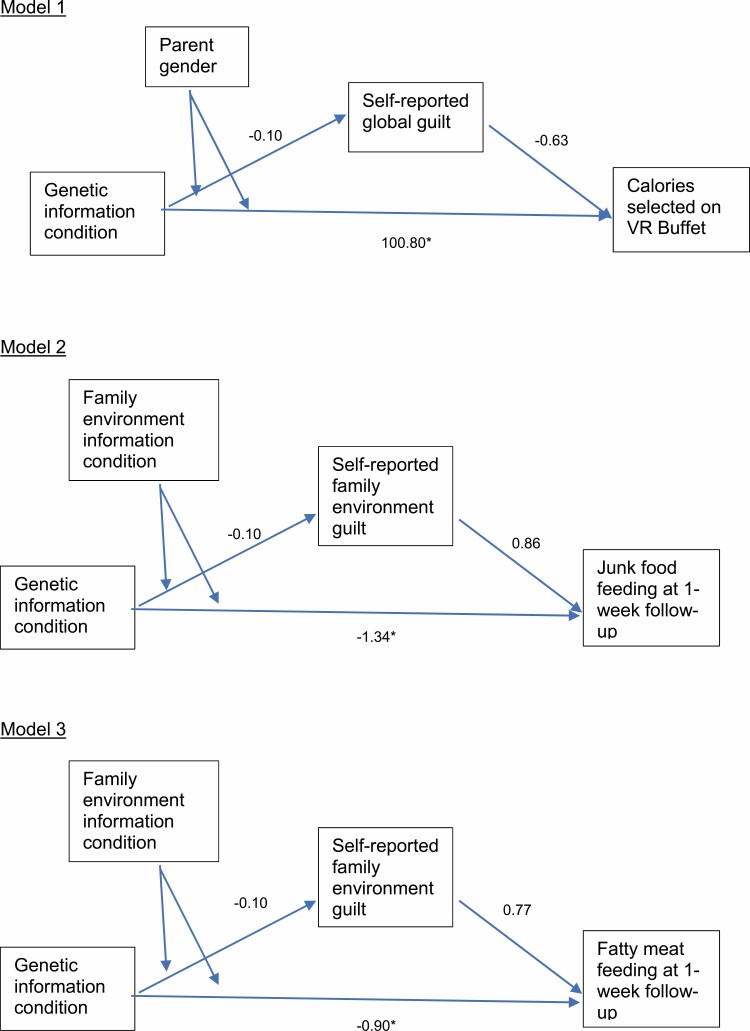
For calories chosen in the VR Buffet, we assessed whether global guilt mediated the previously identified relations: among mothers, receiving genetic content was associated with choosing more calories in the buffet. Although guilt and VR Buffet calories were uncorrelated, we performed this test as it was a primary study hypothesis. Here for Model 1 (Fig. 6), we assessed mediation with receipt of genetic information (yes or no) as the predictor, parent gender as the moderator, global guilt as a mediator, and calories chosen on the VR Buffet as the outcome. In this model, there was a direct effect of the interaction between genetic information and gender on calories (t = 2.19, p = .030), but the confidence interval on the indirect effect crossed zero (95% CIs: [–8.79, 4.44] and [–5.39, 6.45] for fathers and mothers, respectively). Thus, there was no reliable support for the mediation hypothesis.
Fig. 6.
Mediation models tested. *p < .05.
In subsequent models, we assessed mediation for the food frequency relations previously uncovered. Here, genetic content was the independent variable, family environment content was the moderator, and junk food feeding as well as fatty meat feeding were the outcome variables (in two separate models). We ran each model once to test family environment guilt as the mediator (Models 2 and 3; Fig. 6). For junk food with the family environment guilt mediator, we found that the interaction (genetic by family environment information) was related to junk food feeding (t = –2.19, p = .030), however the confidence intervals for the indirect effect crossed zero at both levels of the moderator (95% CIs: [–0.14, 0.083] and [–0.21, 0.067] for with and without environmental content, respectively). Results for fatty meat mirror those for junk food where for family environment guilt the interaction was significantly associated with the outcome (t = –2.29, p = .023), however, the indirect effect confidence interval crossed zero for both levels of the moderator (95% CIs: [–0.098, 0.050] and [–0.14, 0.041] for with and without environmental content, respectively). In sum, there was no evidence of mediation by guilt for any of the relations reported here.
Discussion
In this study, we did not find evidence that providing information about genetics and/or the family environment improved parent feeding. This finding is consistent with the literature in the self-focused domain wherein genetic information provision has not been found to change individuals’ lifestyle behavior [10]. In both the self-focused and child-focused literature, it is well-known that dietary behavior change can be difficult to initiate and sustain [44]; this pattern is once again reflected here. Although the provision of multifactorial genetics and family environment-focused information may not be a silver bullet for parental behavior change, there are, in fact, many situations in which this information may be communicated for other reasons. For example, genetic information regarding health and disease risk is routinely provided in response to DNA testing marketed directly to consumers (often in the absence of behavioral or environmental information) [45, 46]. In addition, health education and public health communications often provide information about the family environment to parents without invoking genetics concepts. The findings of the current study shed light on the relative benefit of communicating about both genes and the family environment as opposed to providing either genetic or family environment information in isolation.
Indeed, the current study indicated the provision of genetic information in isolation may negatively impact parent feeding. Presentation of genetic information alone was associated with higher levels of child feeding for two out of five types of unhealthy foods in the week following information receipt. Specifically, parents reported feeding more junk food and fatty meats to their child when they had received genetic information alone. Among mothers, in the VR Buffet measure provision of genetic information was associated with higher-calorie meals, and this effect appeared to be largely driven by the group who received genetic information alone. Together, these results imply that isolating genetic information can negatively impact parent feeding behaviors, which partially supports hypothesis 1a. These reactions may be indicative of determinism, wherein an emphasis on genetic risk leads parents to assume that their feeding decisions are less impactful. Future work should investigate more proximal assessments of potential determinism such as perceived parental responsibility for child health outcomes. In the larger literature, although determinism was a widely hypothesized reaction to obesity-focused genetic information, it has rarely been observed [47, 48]. The difference here may be related to a focus on one’s child as opposed to the self or may stem from our specific presentations of the message. Unsurprisingly, more research is needed.
Similarly, information about the influence of the family environment alone had negative effects on parental guilt. Our findings indicate that these messages gave rise to increased parental guilt, both global and family environment-specific. This is unsurprising as the family environment is shaped directly by parents. Previous research has shown that discussing child obesity risk factors that are shaped by parent behavior tend to engender parental guilt [49, 50]. Genetics-only information was also associated with some increase in parental guilt, but surprisingly G × FE information was not. The patterns partially support hypothesis 2 in that some types of causal information were associated with increased guilt over control. Hypothesis 2a was not supported; we found no evidence that G × FE information was associated with higher levels of guilt, nor was genetic-only information associated with lower levels.
At the outset of this study, we hypothesized that guilt may be a route through which risk information provision leads to healthier child feeding (hypothesis 3). This hypothesis was unsupported. Although some messages led to increased guilt from baseline, guilt did not go on to improve any healthy feeding-related outcomes. Furthermore, guilt was positively associated with a handful of the unhealthy food frequency assessment outcomes at a 1-week follow-up. Although this diverges from our hypothesis, it is consistent with previous findings that parental guilt can be associated with worse feeding outcomes [33], and can lead to impaired control with respect to food [33, 51]. As such, we can consider the possibility that guilt serves as a negative, undesirable outcome in the child feeding domain. It is important to note, however, that in this study, all information conditions led only to mild feelings of guilt, and in some cases, genetics-specific guilt even decreased from baseline to post-test. It is possible that elicitation of guilt among parents may not be of great concern in this context. To the extent, however, that guilt is elicited, the current results indicate that when family environment-based risk information is provided to parents, combining it with genetic information may have the capacity to reduce guilt response. This would likely happen by the suggestion that there are elements of child risk that lie outside of parental control. Communication approaches which could evoke higher levels of guilt should, however, be directly investigated, as it is unclear whether guilt evoked at higher levels may go on to affect parental feeding practices, either positively or negatively.
These preliminary findings suggest some drawbacks of genetics-only and some drawbacks of family environment-only information. This supports the notion that we should strive for accuracy in our health communication efforts by portraying the complexity of obesity risk transmission processes rather than over-simplifying. Importantly, this more complex approach diverges from how this communication is typically undertaken at present, where messages to the public tend to focus on either genetic or behavioral influences in isolation [52–54]. This suggests potential benefits from shifting existing messages to encompass a wider range of causal factors in obesity etiology.
Notably, differences in outcomes emerged by parent gender. The primary difference was in VR Buffet calories chosen by mothers versus fathers. We observed that fathers chose more calories in general, consistent with studies finding less healthful feeding among fathers than mothers [55], though this is an understudied area. Interestingly, mothers in the genetic condition chose meals with calorie counts similar to fathers’ choices on average. In general, the consistency between mothers and fathers as to the relative superiority of G × FE among the messages suggests that future health communications may not require much tailoring in their content to be effective for mothers versus fathers.
The current study had several limitations. First, a subset of participants screened into the study by indicating they perceived themselves to be overweight or very overweight, and subsequently indicated they perceived themselves to not be overweight at follow-up. Because participants met the screening criteria for the study, we chose not to exclude them, however, this may have weakened our effects if some parents did not truly see themselves as overweight and therefore were less influenced by the child-focused risk message. In addition, the follow-up period for assessing the influence of risk messages was only one week which may not be indicative of long-term change. We limited the follow-up period because the messages were relatively weak and were provided only once; we, therefore, did not expect long-term effects. Additionally, our sample was skewed toward being highly educated. Finally, there were very few parents who reported that their child was overweight. This precluded testing the effect of child weight in these analyses, a variable found to be a crucial moderator of parent responses to G × FE messages in past work [16].
The precise composition of optimal messages remains a target for future research. Indeed, the current study evaluated only one particular framing and presentation of this information. The G × FE information presented in the current study was quite lengthy and more intensive than could routinely be provided in most health or education contexts. In addition, the scientific understanding of gene–environment interaction changes over time, and different message components may become necessary or important. Finally, previous research with media-based G × FE messages had harmful outcomes for some parents [16] which will be important to avoid when crafting future communication approaches.
Taken together, it appears that incorporation of causal routes for obesity risk is unlikely to change parents’ child-feeding behavior above and beyond providing basic child health information. When the provision of information to parents about their child’s obesity risk is needed or otherwise warranted, the current findings suggest that multifactorial G × FE approaches may be preferable to single-factor approaches for optimizing outcomes. This has implications for precision medicine and other contexts where genetic information is frequently shared. Not only might they reduce any unwarranted fatalistic response, but G × FE explanations for obesity risk are the most accurate and convey the complex interplay that underlies children’s obesity risk.
Supplementary Material
Acknowledgments
This study was supported by the Intramural Research Program of the National Human Genome Research Institute Z01-HG200396-05. We thank Sarah Boland for assistance with study material preparation and data collection and Christopher Fortney, Macred Gbenro and Rachel Solonsky for assistance with data collection. Thank you to Emma Schopp and Sydney Telaak for assistance with manuscript preparation, and Corey Miller, Lori Erby, and Michael Dial for contribution to stimulus materials.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards The authors declare that they have no conflict of interest.
Authors’ Contributions SP, MRG, RAF conceived and designed the study; HEY, MKR, BMH collected the data; SP performed the data analysis; SP, HEY, MRG, RAF, MKR and BMH wrote the paper.
Ethical Approval This study was approved by the IRB of the National Human Genome Research Institute.
References
- 1.Denny JC, Rutter JL, Goldstein DB, Philippakis A, Smoller JW, Jenkins G, Dishman E; All of Us Research Program Investigators . The “All of Us” research program. New Engl J Med. 2019;381:668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray MS, Loos RJF, McCaffery JM, et al. NIH working group report—using genomic information to guide weight management: From universal to precision treatment. Obesity. 2016;24:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCaffery JM. Precision behavioral medicine: Implications of genetic and genomic discoveries for behavioral weight loss treatment. Am Psychol. 2018;73:1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long-term weight loss studies in obese adults: Clinical significance and applicability to clinical practice. Int J Obes (Lond). 2005;29:1153–1167. [DOI] [PubMed] [Google Scholar]
- 5.Kraschnewski JL, Boan J, Esposito J, et al. Long-term weight loss maintenance in the United States. Int J Obes (Lond). 2010;34:1644–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wadden TA, Brownell KD, Foster GD. Obesity: Responding to the global epidemic. J Consult Clin Psychol. 2002;70:510–525. [DOI] [PubMed] [Google Scholar]
- 7.Stice E, Shaw H, Marti CN. A meta-analytic review of obesity prevention programs for children and adolescents: The skinny on interventions that work. Psychol Bull. 2006;132:667–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katzmarzyk PT, Barlow S, Bouchard C, et al. An evolving scientific basis for the prevention and treatment of pediatric obesity. Int J Obes (Lond). 2014;38:887–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kipping RR, Jago R, Lawlor DA. Obesity in children. Part 1 Epidemiology, measurement, risk factors, and screening. BMJ. 2008;337:a1824. [DOI] [PubMed] [Google Scholar]
- 10.Hollands GJ, French DP, Griffin SJ, et al. The impact of communicating genetic risks of disease on risk-reducing health behaviour: Systematic review with meta-analysis. BMJ. 2016;352:i1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marteau TM, French DP, Griffin SJ, et al. Effects of communicating DNA‐based disease risk estimates on risk‐reducing behaviours. Cochrane Database of Systematic Reviews. 2010:CD007275. [DOI] [PubMed] [Google Scholar]
- 12.McBride CM, Koehly LM, Sanderson SC, Kaphingst KA. The behavioral response to personalized genetic information: Will genetic risk profiles motivate individuals and families to choose more healthful behaviors? Annu Rev Public Health. 2010;31:89–103. [DOI] [PubMed] [Google Scholar]
- 13.Segal ME, Sankar P, Reed DR. Research issues in genetic testing of adolescents for obesity. Nutr Rev. 2004;62:307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Botkin Jeffrey R, Belmont John W, Berg Jonathan S, et al. Points to consider: Ethical, legal, and psychosocial implications of genetic testing in children and adolescents. Am J Hum Genet. 2015;97:6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McBride CM, Persky S, Wagner LK, Faith MS, Ward DS. Effects of providing personalized feedback of child’s obesity risk on mothers’ food choices using a virtual reality buffet. Int J Obes (Lond). 2013;37:1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Persky S, Goldring MR, El-Toukhy S, Ferrer RA, Hollister B. Parental defensiveness about multifactorial genomic and environmental causes of children’s obesity risk. Child Obes. 2019;15:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Persky S, Ferrer RA, Klein WM. Genomic information may inhibit weight-related behavior change inclinations among individuals in a fear state. Ann Behav Med. 2016;50:452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Persky S, Bouhlal S, Goldring MR, McBride CM. Beliefs about genetic influences on eating behaviors: Characteristics and associations with weight management confidence. Eat Behav. 2017;26:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dar-Nimrod I, Cheung BY, Ruby MB, Heine SJ. Can merely learning about obesity genes affect eating behavior? Appetite. 2014;81:269–276. [DOI] [PubMed] [Google Scholar]
- 20.Persky S, Sanderson SC, Koehly LM. Online communication about genetics and body weight: Implications for health behavior and internet-based education. J Health Commun. 2013;18:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lippa NC, Sanderson SC. Impact of informing overweight individuals about the role of genetics in obesity: An online experimental study. Hum Hered. 2013;75:186–203. [DOI] [PubMed] [Google Scholar]
- 22.Waters EA, Ball L, Gehlert S. “I don’t believe it.” Acceptance and skepticism of genetic health information among African-American and White smokers. Soc Sci Med. 2017;184:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang DS, Kang OS, Kim HH, et al. Pre-existing beliefs and expectations influence judgments of novel health information. J Health Psychol. 2012;17:753–763. [DOI] [PubMed] [Google Scholar]
- 24.Khandpur N, Blaine RE, Fisher JO, Davison KK. Fathers’ child feeding practices: A review of the evidence. Appetite. 2014;78:110–121. [DOI] [PubMed] [Google Scholar]
- 25.Davison KK, Gicevic S, Aftosmes-Tobio A, et al. Fathers’ representation in observational studies on parenting and childhood obesity: A systematic review and content analysis. Am J Public Health. 2016;106:e14–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guerrero AD, Chu L, Franke T, Kuo AA. Father involvement in feeding interactions with their young children. Am J Health Behav. 2016;40:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Persky S, Ferrer RA, Klein WMP, et al. Effects of fruit and vegetable feeding messages on mothers and fathers: Interactions between emotional state and health message framing. Ann Behav Med. 2019;53:789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pratt M, Hoffmann D, Taylor M, Musher-Eizenman D. Structure, coercive control, and autonomy promotion: A comparison of fathers’ and mothers’ food parenting strategies. J Health Psychol. 2019;24:1863–1877. [DOI] [PubMed] [Google Scholar]
- 29.Tan CC, Domoff SE, Pesch MH, Lumeng JC, Miller AL. Coparenting in the feeding context: Perspectives of fathers and mothers of preschoolers. Eat Weight Disord. 2020;25:1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Persky S, McBride CM, Faith MS, Wagner LK, Ward DS. Mothers’ guilt responses to children’s obesity risk feedback. J Health Psychol. 2015;20:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tangney JP. Shame and guilt in interpersonal relationships. In: Tangney JP, Fischer KW, eds. Self-Conscious Emotions: The Psychology of Shame, Guilt, Embarrassment, and Pride. New York, NY: Guilford Press; 1995:114–139. [Google Scholar]
- 32.Tignor SM, Colvin CR. The interpersonal adaptiveness of dispositional guilt and shame: A meta-analytic investigation. J Pers. 2017;85:341–363. [DOI] [PubMed] [Google Scholar]
- 33.Hagerman CJ, Ferrer RA, Klein WMP, Persky S. Association of parental guilt with harmful versus healthful eating and feeding from a virtual reality buffet. Health Psychol. 2020;39:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Persky S, Goldring MR, Turner SA, Cohen RW, Kistler WD. Validity of assessing child feeding with virtual reality. Appetite. 2018;123:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimm ER, Steinle NI. Genetics of eating behavior: Established and emerging concepts. Nutr Rev. 2011;69:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krahnstoever Davison K, Francis LA, Birch LL. Reexamining obesigenic families: Parents’ obesity-related behaviors predict girls’ change in BMI. Obes Res. 2005;13:1980–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faith MS, Berkowitz RI, Stallings VA, Kerns J, Storey M, Stunkard AJ. Parental feeding attitudes and styles and child body mass index: Prospective analysis of a gene-environment interaction. Pediatrics. 2004;114:e429–e436. [DOI] [PubMed] [Google Scholar]
- 38.Silventoinen K, Rokholm B, Kaprio J, Sørensen TI. The genetic and environmental influences on childhood obesity: A systematic review of twin and adoption studies. Int J Obes (Lond). 2010;34:29–40. [DOI] [PubMed] [Google Scholar]
- 39.Nebeling LC, Hennessy E, Oh AY, et al. The FLASHE study: Survey development, dyadic perspectives, and participant characteristics. Am J Prev Med. 2017;52:839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calloway E, Smith T, Pinard C, et al. Method of assessing daily intake-frequencies for major food groups using dietary screener data. J Nutr. 2016;30:43.41–43.41. [Google Scholar]
- 41.Harder DW, Greenwald DF. Further validation of the shame and guilt scales of the Harder Personal Feelings Questionnaire-2. Psychol Rep. 1999;85:271–281. [DOI] [PubMed] [Google Scholar]
- 42.Harder DH, Zalma A. Two promising shame and guilt scales: A construct validity comparison. J Pers Assess. 1990;55:729–745. [DOI] [PubMed] [Google Scholar]
- 43.Hayes AF.Introduction to Mediation , Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY: Guilford publications; 2017. [Google Scholar]
- 44.Dunton GF. Sustaining health-protective behaviors such as physical activity and healthy eating. JAMA. 2018;320:639–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferguson LR, Parslow VR. Chapter 70 - direct-to-consumer testing. In: Caterina RDE, Martinez JA, Kohlmeier M, eds. Principles of Nutrigenetics and Nutrigenomics. Cambridge, MA: Academic Press; 2020:529–537. [Google Scholar]
- 46.Niemiec E, Kalokairinou L, Howard HC. Current ethical and legal issues in health-related direct-to-consumer genetic testing. Per Med. 2017;14:433–445. [DOI] [PubMed] [Google Scholar]
- 47.Collins RE, Wright AJ, Marteau TM. Impact of communicating personalized genetic risk information on perceived control over the risk: A systematic review. Genet Med. 2011;13:273–277. [DOI] [PubMed] [Google Scholar]
- 48.Cheera EK, Klarich DS, Hong MY. Psychological and behavioral effects of genetic risk testing for obesity: A systematic review. Per Med. 2016;13:265–277. [DOI] [PubMed] [Google Scholar]
- 49.Pescud M, Pettigrew S. ‘I know it’s wrong, but.’: A qualitative investigation of low-income parents’ feelings of guilt about their child-feeding practices. Matern Child Nutr. 2014;10:422–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris HA, Jansen E, Rossi T. ‘It’s not worth the fight’: Fathers’ perceptions of family mealtime interactions, feeding practices and child eating behaviours. Appetite. 2020;150:104642. [DOI] [PubMed] [Google Scholar]
- 51.Kuijer RG, Boyce JA, Marshall EM. Associating a prototypical forbidden food item with guilt or celebration: Relationships with indicators of (un)healthy eating and the moderating role of stress and depressive symptoms. Psychol Health. 2015;30:203–217. [DOI] [PubMed] [Google Scholar]
- 52.Yoo JH, Kim J. Obesity in the new media: A content analysis of obesity videos on YouTube. Health Commun. 2012;27:86–97. [DOI] [PubMed] [Google Scholar]
- 53.So J, Prestin A, Lee L, Wang Y, Yen J, Chou WY. What do people like to “share” about obesity? A content analysis of frequent retweets about obesity on Twitter. Health Commun. 2016;31:193–206. [DOI] [PubMed] [Google Scholar]
- 54.Kim SH, Willis LA. Talking about obesity: News framing of who is responsible for causing and fixing the problem. J Health Commun. 2007;12:359–376. [DOI] [PubMed] [Google Scholar]
- 55.Rahill S, Kennedy A, Kearney J. A review of the influence of fathers on children’s eating behaviours and dietary intake. Appetite. 2020;147:104540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.