Abstract
Background and Purpose.
Women are twice as likely as men to develop post-traumatic stress disorder (PTSD) making the search for biological mechanisms underlying these gender disparities especially crucial. One of the hallmark symptoms of PTSD is an alteration in the ability to extinguish fear responses to trauma-associated cues. In male rodents, the endocannabinoid system can modulate fear extinction and has been suggested as a therapeutic target for PTSD. However, whether and how the endocannabinoid system may modulate fear expression and extinction in females remains unknown.
Experimental Approach.
To answer this question, we pharmacologically manipulated endocannabinoid signalling in male and female rats prior to extinction of auditory conditioned fear, and measured both passive (freezing) and active (darting) conditioned responses.
Key Results.
Surprisingly, we found that acute systemic inhibition of the endocannabinoid anandamide (AEA) or 2-arachidonoyl glycerol (2-AG) hydrolysis did not significantly alter fear expression or extinction in males. However, the same manipulations in females produced diverging effects. Increased AEA signalling at vanilloid TRPV1 receptors impaired fear memory extinction. In contrast, inhibition of 2-AG hydrolysis promoted active over passive fear responses acutely via activation of cannabinoid type-1 receptors. Measurement of AEA and 2-AG levels after extinction training revealed sex- and brain region-specific changes.
Conclusion and Implications.
We provide the first evidence that AEA and 2-AG signalling affect fear expression and extinction in females in opposite directions. These findings are relevant to future research on sex differences in mechanisms of fear extinction and may help develop sex-specific therapeutics to treat trauma-related disorders.
Keywords: endocannabinoid system, fear extinction, TRPV1 receptors, CB1 receptors, sex differences
Introduction
Impaired fear extinction contributes to the development and persistence of post-traumatic stress disorder (PTSD) (Milad et al., 2009; Jovanovic and Norrholm, 2011). While only a small proportion of trauma-exposed individuals develop PTSD, women have a two-fold greater risk, prevalence, and duration of PTSD than men (Breslau, 2009). The biological mechanisms underlying these gender disparities remain unclear and controversial. Yet, most preclinical studies on fear memory processes are exclusively performed in males and studies comparing the sexes are few and inconsistent (Shansky, 2015). In rodents, learned fear responses are traditionally assessed by quantifying freezing behaviour, a passive fear response defined as the absence of movements except for respiration (Fanselow, 1980), predominately expressed by males. In contrast, females generally exhibit lower freezing and express darting behaviour, a rapid, forward movement that resembles an active and escape-like fear response (Gruene et al., 2015a; Colom-Lapetina et al., 2019). A better understanding of the mechanisms that mediate these sex differences in fear responding may inform sex-specific pharmacological approaches to the management of PTSD (Velasco et al., 2019).
Compelling evidence from studies in males demonstrates the importance of the endocannabinoid system in modulating fear responses and memory for aversive experiences (Lutz et al., 2015; Morena et al., 2016b). The endocannabinoid system consists of the cannabinoid type-1/2 receptors (CB1R and CB2R), two main endogenous ligands anandamide (AEA) and 2-arachidonoylglycerol (2-AG), and their respective degrading enzymes fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) (Blankman and Cravatt, 2013). In addition to binding to cannabinoid receptors, AEA is also an endogenous ligand for the non-selective cation channel, transient potential receptor of vanilloid type-1 channel (TRPV1R) (Zygmunt et al., 1999). Both CB1R and TRPV1R are widely expressed in brain areas involved in anxiety and fear (Tsou et al., 1999; Mezey et al., 2000; Cristino et al., 2008) and like CB1R, AEA activation of TRPV1R can regulate synaptic plasticity (Chávez et al., 2010; Grueter et al., 2010). While CB1R activation has overall inhibitory effects through reduction of neurotransmitter release (Katona and Freund, 2012; Yasmin et al., 2020), activation of TRPV1R promotes membrane depolarization, increases neuronal firing rate and facilitates neurotransmitter release (Marinelli et al., 2003; Xing and Li, 2007; Musella et al., 2009; Bialecki et al., 2020). Behaviourally, activation of CB1R or TRPV1R in male rodents has also been shown to induce opposing responses. Specifically, CB1R stimulation reduces anxiety and facilitates fear extinction, while TRPV1R activation promotes anxiety-like behaviour and increases fear expression (Moreira et al., 2012).
Interestingly, compelling preclinical evidence has shown sex differences in endocannabinoid content and CB1R binding and affinity in different stress- and fear-related brain areas (de Fonseca et al., 1994; Bradshaw et al., 2006; Riebe et al., 2010; Castelli et al., 2014; Cooper and Craft, 2018). In humans, sex differences have been reported as well, showing higher CB1R binding in the limbic system and cortico-striato-thalamic-cortical circuit in males compared to females (Van Laere et al., 2008), but higher AEA levels in females compared to males (Neumeister et al., 2013). In parallel, cannabinoid compounds have been reported to have sex-divergent effects both in animal and human studies, due to direct gonadal hormone influence on the endocannabinoid system, and pharmacokinetic and pharmacodynamic differences in drug metabolism (Wiley and Burston, 2014) and potency (Craft et al., 2012), reviewed in (Cooper and Craft, 2018).
We recently reported that repeatedly enhancing AEA signalling accelerated extinction learning in male rats (Morena et al., 2018). Specifically, the amygdala represents an important brain region for AEA regulation of fear extinction (Gunduz-Cinar et al., 2013). Recent evidence has shown that the endocannabinoid signaling regulates plasticity within the amygdala-prefrontal cortex (PFC) circuit under stressful experiences (Marcus et al., 2020), and fundamental sex differences have been identified within this amygdala-PFC fear circuit, underlying differences in fear expression (Gruene et al., 2015b). Together with the amygdala and PFC, the periaqueductal grey (PAG) represents an important fear-related brain area (Maren, 2001), wherein the dorsal subregion (dPAG) primarily regulates innate and active fear responses (Bandler et al., 2000; Watson et al., 2016), more prominent in females (Gruene et al., 2015a), while the ventral PAG (vPAG) seems to be more involved in the regulation of freezing behavior (Watson et al., 2016), more prominent in males (Gruene et al., 2015a). To date, little is known about whether endocannabinoids regulate conditioned fear extinction in females. To answer this question, we employed systemic pharmacological inhibition of either AEA or 2-AG in male and female rats and determined the role of CB1R or TRPV1R in mediating any potential behavioural changes observed. Finally, we measured post-extinction AEA and 2-AG levels in the amygdala, PFC, dPAG and vPAG, to identify potential sex differences in these extinction-related brain regions (Maren, 2001). Results from this study may inform future research aiming at investigating sex-differences in endocannabinoid regulation of fear memory dynamics within specific brain regions and neuronal circuits.
Materials and Methods
Animals
Male and female Sprague Dawley rats (10–11 weeks old at the time of testing; Charles River, Montreal, QB, Canada; RRID:RGD_10395233) were pair housed in clear plastic cages (47 × 25 × 20 cm) in separate temperature-controlled (20 ± 1°C) rooms and maintained under a 12 h/12 h light/dark cycle (8:00 A.M. to 8:00 P.M. lights on) with ad libitum access to food and water and environmental enrichment (i.e. polycarbonate play tunnels and sizzle-nest). This animal model was chosen because processing of emotional information, including memory of aversive experiences and expression of fear, rely on the activation of an evolutionary primitive subcortical and cortical circuit, highly conserved across species, including humans, and pattern of fear response expressed by rats parallels that observed in humans (Lang et al., 2000). All tests were performed during the light phase of the cycle between 10:00 A.M. and 5:00 P.M. Animals were randomly assigned to the experimental groups. Male and female rats were tested separately, in different cohorts and different days. All experimental procedures were in compliance with protocols approved by the University of Calgary Animal Care Committee, guidelines from the Canadian Council on Animal Care. Animal studies are reported in compliance with the ARRIVE guidelines (Percie du Sert et al., 2020) and with the recommendations made by the British Journal of Pharmacology (Lilley et al., 2020). All efforts were made to minimize animal suffering and to reduce the number of animals used. Except for the animals used for brain endocannabinoid measurements, all rats were killed at the end of the behavioural experiments with CO2. Recommendations set out in the BJP editorials, where relevant, were followed by the authors.
Experimental procedures
Auditory fear conditioning and extinction paradigm.
Rats underwent auditory fear conditioning, extinction, and extinction retrieval with a slightly different procedure as the ones previously described (Gruene et al., 2015a; Morena et al., 2019) (Fig. 1A). Behavioural testing occurred in two different contexts (A and B). Context A consisted of a chamber with a grid floor, back and side metal walls, clear Plexiglas front door and ceiling, and white light. Context A was cleaned with 70% ethanol between rats. Context B consisted of a white opaque plastic floor and curved walls, and was cleaned with Virkon solution between rats. To habituate the animals to the behavioural testing room, rats were transferred to the behavioural room and their home cages were placed in sound attenuating, ventilated, and lighted cabinets for at least 30 minutes before and after the handling on day 1, 2, and 3, and for at least 90 min before and after testing, the following days. Fear conditioning chambers and cabinets were cleaned thoroughly with soapy water and ethanol at the end of each experimental run, in between male and female experimental cohorts. Rats were handled for 1 min each. On day 2 and 3, immediately after the handling procedure animals were habituated to context A and B for 10 minutes. Auditory fear conditioning (day 4), was performed in context A. After a 5-minute acclimation period, all rats were exposed to seven conditioning trials. Each conditioning trial involved presentation of the conditioned stimulus (CS; 80dB, 4Hz tone) for 30 s, co-terminating with a 1 s unconditioned stimulus (US; 0.65 mA shock). Inter-trial interval (ITI) between two consecutive CS-US pairings was 3 min. After conditioning, each rat was returned to its home cage. On day 5, rats underwent the extinction training consisting of 20 CS presentations with an ITI between CSs of 2 min, in context B. On day 6, rats received an extinction retrieval session in context B. After a 2-min acclimation period, rats were presented with five CSs (2-min ITI). Behaviour was video-recorded, scored, and analyzed for freezing (i.e. absence of any movement except for those necessary for respiration) using Video Freeze software (Med Associates Inc., St. Albans, VT, USA; RRID:SCR_014574). Darting behaviour (i.e. rapid, forward movement across the chamber that resembles an escape-like response) was scored manually as number of discrete darting events and expressed as darting rate (dart/min), by two trained observers blinded to the experimental conditions.
Figure 1. Sexually divergent expression of fear responses during the auditory fear conditioning paradigm.
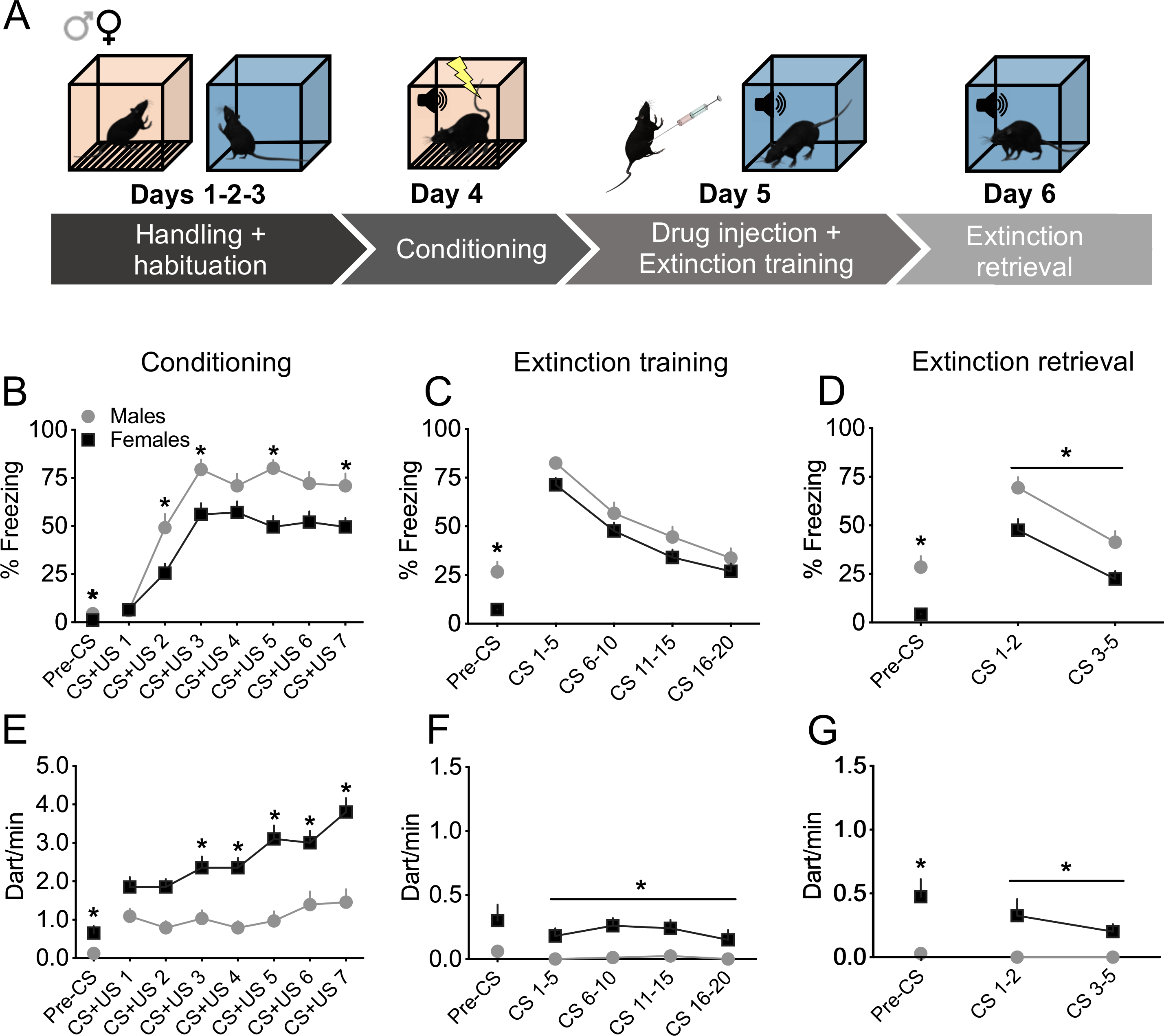
(A) Schematic representation of the experimental design. (B-D) Except during CS presentations at extinction training (C), male rats consistently showed higher freezing behaviour than females during the auditory fear conditioning paradigm. Percentage of freezing during auditory fear conditioning (B), extinction training (C) and extinction retrieval (D). (E-G) Female rats consistently showed higher darting behaviour than males throughout the auditory fear conditioning paradigm. Number of darting events/min (dart/min) during auditory fear conditioning (E), extinction training (F) and extinction retrieval (G). Data are expressed as mean ± SEM. * P < 0.05, males vs females, the horizontal line below the star indicates main effect of sex; (males, n = 33; females, n = 40).
To test the effects of the AEA or 2-AG hydrolysis inhibitors on fear extinction, rats were injected intraperitoneally (i.p.) with URB597 or MJN110 (respectively), or their vehicle, 60 min prior to the extinction training. The CB1R antagonist AM251, or its vehicle, was injected i.p. 30 min before URB597 or MJN110 administration (i.e. 90 min before extinction training). A separate group of rats was injected with AM251 in combination with the TRPV1R antagonist Capsazepine (CPZ), 30 min before URB597 injection.
Endocannabinoid extraction and analysis
To assess whether learned fear expression and extinction learning elicits sex-specific patterns of endocannabinoid release, male and female rats were randomly assigned to either extinction [Ext] or no-extinction [No-Ext] groups. Ext groups underwent fear conditioning and extinction training as described above. No-Ext groups underwent fear conditioning but were exposed to the extinction context for an equivalent amount of time without CS presentations. Immediately after the extinction training, rats underwent rapid decapitation, and the brain regions of interest (amygdala, PFC, dPAG and vPAG) were dissected, frozen on dry ice and stored at −80°C until endocannabinoid level determination. Lipid extraction to determine AEA and 2-AG levels was performed as described previously (Morena et al., 2015; Qi et al., 2015).
Data and Statistical analysis
The manuscript complies with BJP’s recommendations and requirements on experimental design and analysis (Curtis et al., 2018). All data were analyzed using GraphPad Prism 6 (RRID:SCR_002798) and are expressed in all Figures as mean ± standard error of the mean (SEM). Statistical analysis was run using independent values and outliers were included in data analysis and presentation. To better evaluate any difference in drug effects in the early or late phases of the behavioral sessions, percentage of freezing or darting rate during extinction training sessions were averaged in four blocks of five consecutive CSs each (CS1–5, CS6–10, CS11–15, CS16–20); behavioural measures for extinction retrieval were averaged in two blocks (CS1–2 and CS3–5). CS-US- or CS-evoked freezing and darting were analyzed with repeated measures (RM) ANOVA. Freezing and darting during the pre-CS period were analyzed with Student’s t test or one-way ANOVA, when appropriate. Student’s t test was used to analyze brain endocannabinoid levels. Adjusted Bonferroni’s multiple comparison post-hoc tests were run when F achieved P < 0.05 and there was no significant variance in homogeneity. The correlation analyses were performed with the Pearson correlation test. A probability level of < 0.05 was accepted as statistically significant. Group size, shown in the figure legends, is the number of independent values (i.e. number of rats). To achieve a power of 0.80–0.95, a sample size of at least 10 (for behavioural experiments), or 8 (for biochemical experiments) animals per group was calculated. Studies were designed to generate groups of equal size, using randomisation and blinded analysis. However, sizes for the Vehicle, URB597 and MJN110 groups are higher than those of the remaining groups, as they were combined from separate sets of experiments which were originally run separately to generate pilot data and then replicated when the remaining groups were added to the study. Statistical analysis was undertaken only for studies where each group size was at least n = 5. All experiments and data analyses were carried out by operators blinded to the experimental conditions.
Materials
The AEA hydrolysis inhibitor URB597 (0.3 mg/kg; Cayman Chemical, Cedarlane®, Burlington, ON, Canada), the 2-AG hydrolysis inhibitor MJN110 (10 mg/kg; provided by B.F. Cravatt), the CB1R antagonist/inverse agonist AM251 (1 mg/kg; Tocris, Cedarlane®, Burlington, ON, Canada), the TRPV1R antagonist Capsazepine (CPZ; 5 mg/kg; Cayman Chemical, Cedarlane®, Burlington, ON, Canada) or their vehicle (5% polyethylene glycol, 5% Tween-80, 90% saline) were injected i.p. at a volume of 1 ml/kg. URB597, MJN110 or their vehicle were injected 60 min before the extinction training session, AM251, CPZ or their vehicle were injected 90 min before the extinction training session.
Doses and timing were chosen based on previously published papers (Kathuria et al., 2003; Colangeli et al., 2017; Ratano et al., 2017; Morena et al., 2018; Sticht et al., 2019) and pilot experiments performed in our laboratory. All drug solutions were freshly prepared before each experiment.
Nomenclature of Targets and Ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019a, 2019b, 2019c).
Results
Sexually divergent expression of fear responses during auditory fear conditioning and extinction
We first examined whether sex-specific conditioned fear strategies emerged across the different sessions of the auditory fear conditioning paradigm by assessing CS-US- and CS-evoked freezing and darting behaviour of all rats that received an i.p. injection of vehicle used in the subsequent experiments, pooled together (Fig. 1). As shown in Fig. 1B–D and Table S1, we found significant main effects of CS trial across all three test days, and significant main effect of sex at conditioning and extinction retrieval indicating higher freezing in males. We also observed significant sex x trial interaction for fear conditioning training. Post-hoc comparisons indicated that male rats showed significant higher freezing levels as compared to females during presentations of CS-US 2, 3, 5 and 7. Student’s t tests for freezing before CS presentations (pre-CS period) at conditioning, extinction training and retrieval indicated that male rats showed significant higher freezing levels as compared to females, potentially suggesting higher innate fear and context generalization in males. Figure 1E–G shows CS-US-evoked darting during conditioning, CS-evoked darting during extinction training, and extinction retrieval. Analysis of darting behaviour during fear conditioning revealed significant main effects of trial and sex, and a significant trial x sex interaction. Post-hoc comparisons revealed that females darted more than males at CS-US 3, 4, 5, 6 and 7. During both extinction training and retrieval we found a main effect of sex. Student’s t tests for darting during the pre-CS period indicated higher darting in females as compared to males at conditioning and extinction retrieval. These results indicate that, as we have shown previously (Gruene et al., 2015a), males and females engage different fear responses; males consistently show greater freezing than females, while females consistently show higher darting than males.
AEA hydrolysis inhibition does not significantly affect auditory fear memory expression and extinction in males
Figure 2 shows behavioural data for pre-extinction administration of AEA hydrolysis inhibitor URB597 alone, CB1R antagonist AM251 alone and URB597+AM251 (A-F) in males; statistics are in Table S2. In freezing measures (Fig. 2A–C), we observed significant main effects of trial for all three days of testing, suggesting successful fear conditioning and extinction learning. We found a main effect of drug at extinction training and a significant trial x drug interaction for extinction retrieval (Fig. 2B–C). However, although URB597 treatment trended to decrease freezing, post-hoc analyses did not reveal a significant difference compared to the vehicle group, but did show a significant difference compared to the URB597+AM251 group at CS3–5 at extinction retrieval (Fig. 2C). In darting measures (Fig. 2D–F), we found a significant main effect of trial during fear conditioning only, and observed very little or no darting at all during extinction or extinction retrieval. Importantly, there was no main effect of drug during fear conditioning, suggesting that there were no pre-existing differences in these cohorts (Fig. 2A,D). One-way ANOVAs for freezing or darting during the pre-CS period did not show significant effects in any of the testing sessions.
Figure 2. Increased AEA signalling did not significantly alter auditory fear memory expression and extinction in males.
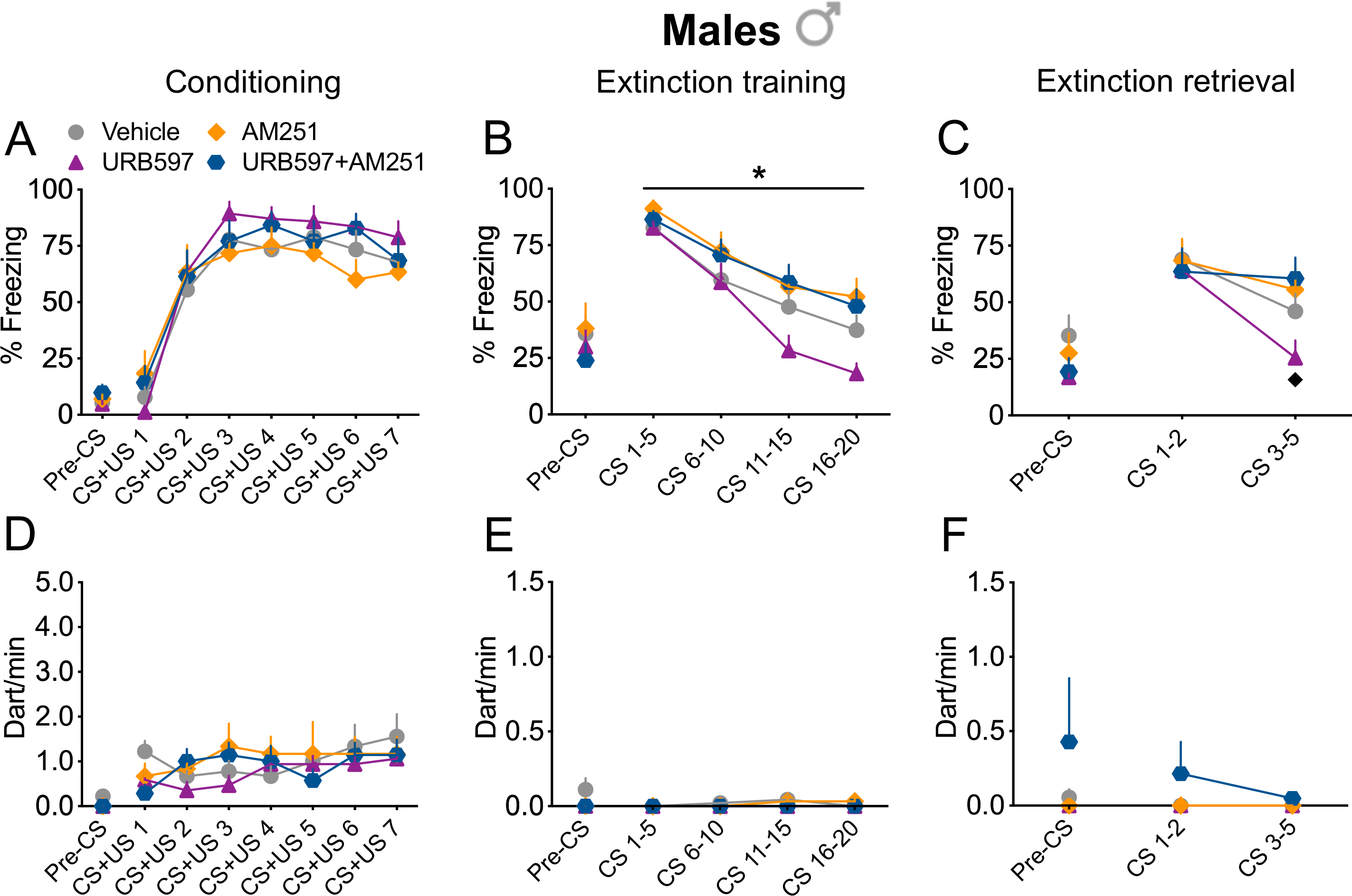
(A-F) Treatment with the AEA hydrolysis inhibitor URB597, the CB1R antagonist AM251 or their combination (URB597+AM251) did not significantly alter freezing or darting behaviour in male rats, although URB597 showed a trend toward reducing conditioned freezing as compared to the vehicle group during the late phases of extinction training and retrieval and significantly reduced freezing compared to URB597+AM251 (at extinction retrieval). Percentage of freezing during auditory fear conditioning (A), extinction training (B) and extinction retrieval (C). Number of darting events/min (dart/min) during auditory fear conditioning (D), extinction training (E) and extinction retrieval (F). Vehicle, n = 18; URB597, n = 17; AM251, n = 12; URB597+AM251, n = 14. Data are expressed as mean ± SEM. * P < 0.05 main effect of drug; ◆ P < 0.05 vs URB597+AM251.
2-AG hydrolysis inhibition does not affect auditory fear memory expression and extinction in males
Figure 3 shows behavioural data for pre-extinction administration of 2-AG hydrolysis inhibitor MJN110 alone, AM251 alone and MJN110+AM251 (A-F) in males; statistics are in Table S3. We only found significant main effects of trial for freezing at conditioning, extinction training and retrieval, indicating successful fear conditioning and extinction learning. We did not observe any main effects of drug or drug x trial interactions for freezing at conditioning, extinction training or retrieval (Fig. 3A–C). No statistically significant effects were observed in darting measures (Fig. 3D–F) or during the pre-CS period for freezing or darting in all the three testing sessions.
Figure 3. Increased 2-AG signalling did not alter auditory fear memory expression and extinction in males.
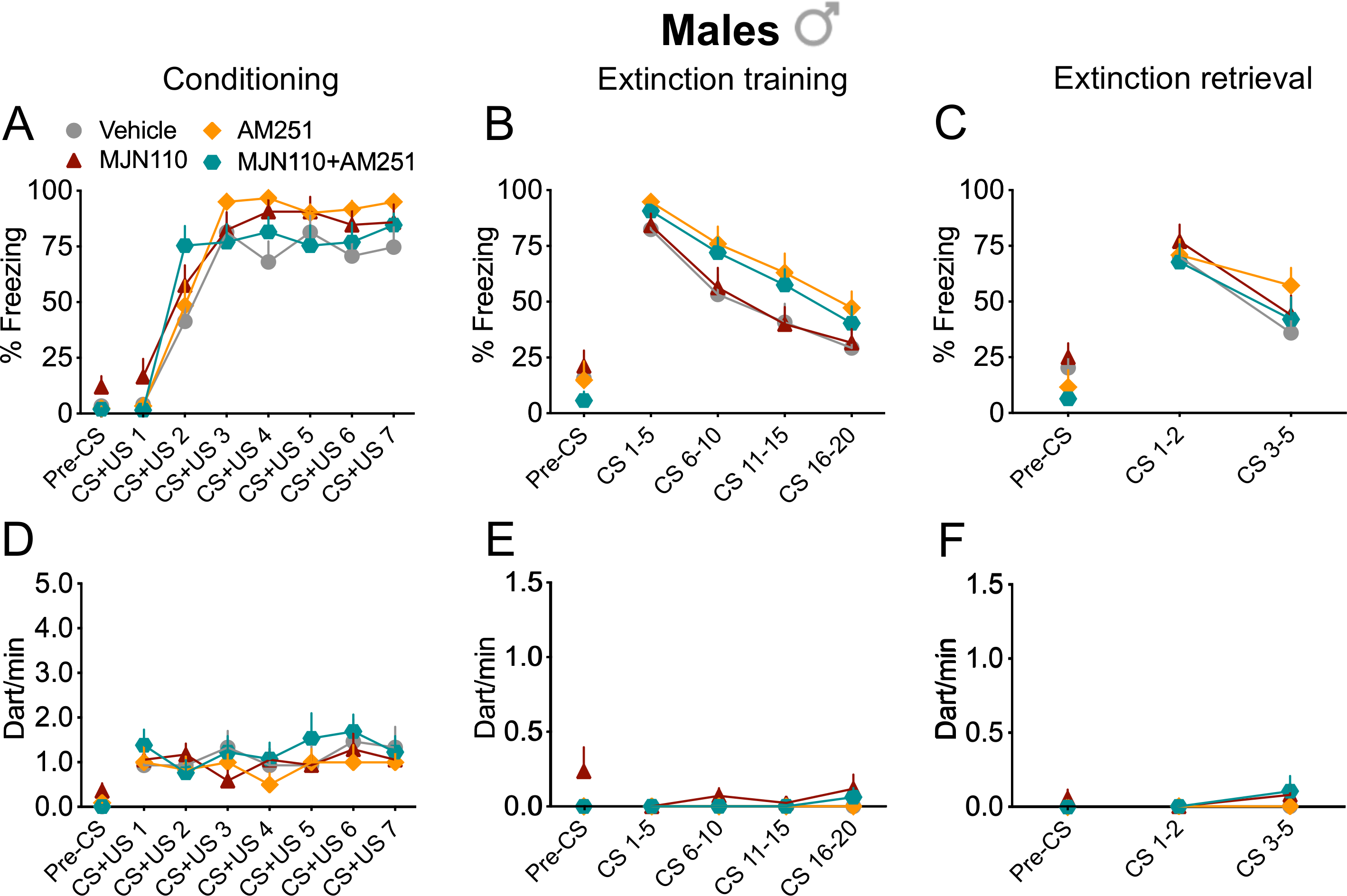
(A-F) Treatment with the 2-AG hydrolysis inhibitor MJN110, the CB1R antagonist AM251 or their combination (MJN110+AM251) did not alter freezing or darting behaviour in male rats. Percentage of freezing during auditory fear conditioning (A), extinction training (B) and extinction retrieval (C). Number of darting events/min (dart/min) during auditory fear conditioning (D), extinction training (E) and extinction retrieval (F). Vehicle, n = 15; MJN110, n = 17; AM251, n = 12; MJN110+AM251, n = 13. Data are expressed as mean ± SEM.
Increased AEA signalling at TRPV1R augments freezing behaviour at extinction training and retrieval in females
Figure 4 shows the effects of systemic pre-extinction administration of URB597 alone, AM251 alone, URB597+AM251 and URB597+AM251 together with the TRPV1R antagonist CPZ on freezing (Fig. 4A–C) and darting (Fig. 4D–F) behaviour during the auditory fear conditioning paradigm. Detailed statistics is reported in Table S4. Analysis of freezing during fear conditioning revealed a significant main effect of trial, but no significant drug treatment or drug x trial interaction (Fig. 4A), or differences during the pre-CS period. This confirms no pre-existing differences between groups before drug treatment and shows that all groups exhibited fear learning. Analysis of freezing behaviour during extinction (Fig. 4B) revealed significant main effects of trial, drug, and a trial x drug interaction. Post-hoc comparisons showed that rats treated with URB597+AM251 exhibited higher freezing at later time blocks compared to vehicle (CS6–10, CS11–15, CS16–20), URB597 alone (CS11–15 and CS16–20), AM251 alone (CS16–20) and URB597+AM251+CPZ groups (CS11–15 and CS16–20; Fig. 4B). The same group also showed higher freezing levels during the pre-CS period than vehicle-, URB597- and AM251-treated rats, suggesting higher context generalization. During extinction retrieval (Fig. 4C), we found significant main effects of trial and drug, but no interaction. Post-hoc comparisons showed that URB597+AM251 rats exhibited overall higher freezing levels than vehicle-treated rats (Fig. 4C). No differences were observed for freezing during the pre-CS period at extinction retrieval. Analysis of darting behaviour during conditioning, extinction training, and retrieval (Fig. 4D–F) revealed a significant main effect of trial for conditioning, and a significant main effect of drug at extinction training, but no other significant effects. Post-hoc comparisons revealed that URB597-treated rats exhibited overall lower darting than the vehicle group did, across all the CS trials presented during the extinction training (Fig. 4E). One-way ANOVAs for darting during the pre-CS period did not reveal any significant effects for conditioning, extinction training and retrieval.
Figure 4. Increased AEA signalling at TRPV1Rs augmented freezing behaviour at extinction training and retrieval in females.
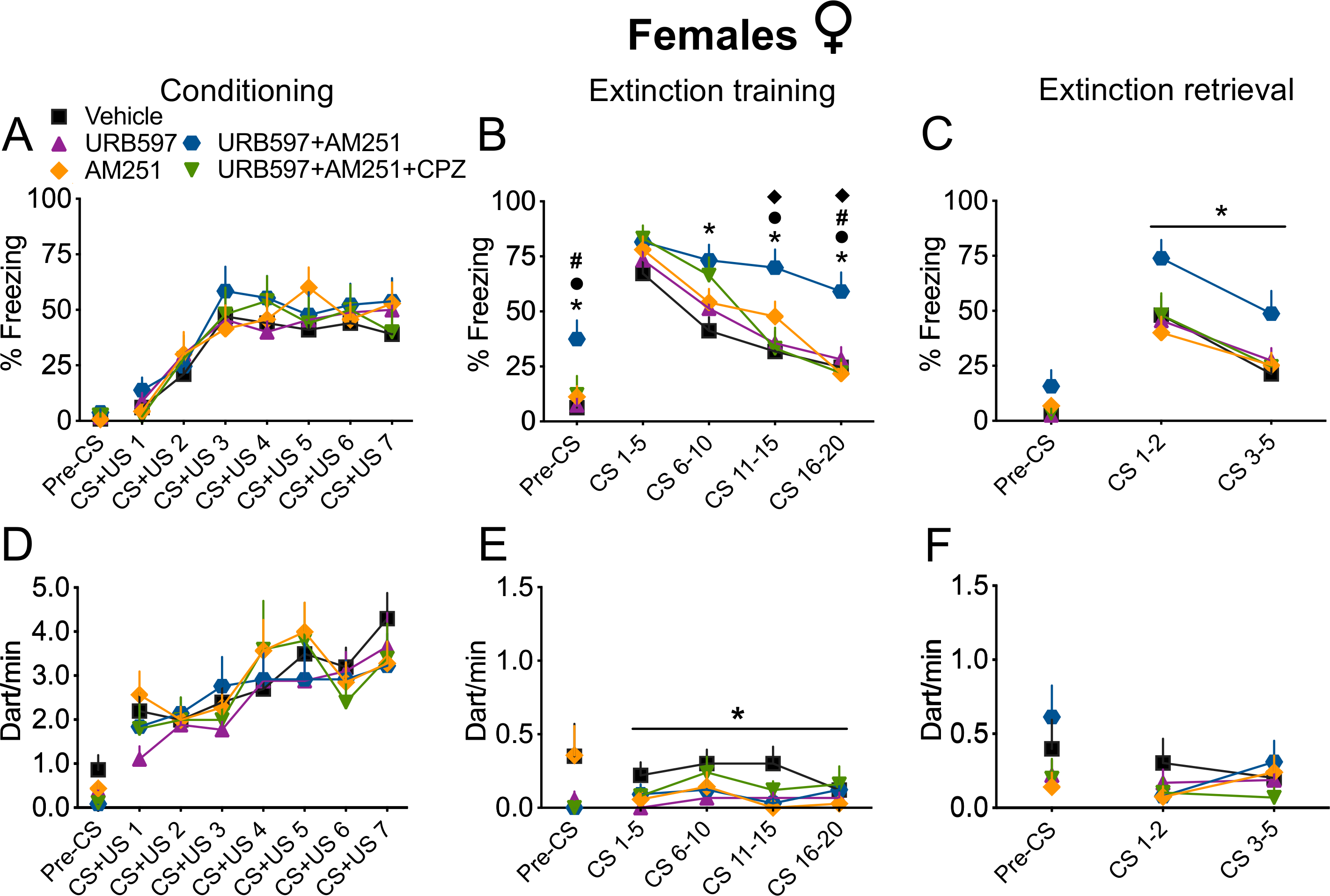
(A-F) Treatment with the AEA hydrolysis inhibitor URB597 with concurrent blockade of CB1R with the antagonist AM251 (URB597+AM251) induced fear generalization, impaired within-session extinction and extinction retrieval. These effects were mediated by AEA signalling at TRPV1Rs, as they were completely blocked by concomitant injection with the TRPV1R antagonist CPZ (URB597+AM251+CPZ). Furthermore, treatment with URB597 alone induced an overall reduction of darting behaviour during CS presentation at extinction training compared to the vehicle group. Percentage of freezing during auditory fear conditioning (A), extinction training (B) and extinction retrieval (C). Number of darting events/min (dart/min) during auditory fear conditioning (D), extinction training (E) and extinction retrieval (F). Vehicle, n = 20; URB597, n = 18; AM251, n = 14; URB597+AM251, n = 13; URB597+AM251+CPZ, n = 10. Data are expressed as mean ± SEM. * P < 0.05 vs Vehicle; • P < 0.05 vs URB597; # P < 0.05 vs AM251; ◆ P < 0.05 vs URB597+AM251+CPZ. Horizontal line below the star indicates a main effect of drug: P < 0.05, URB597+AM251 group vs Vehicle group (C) and URB597 group vs Vehicle group (E).
Increased 2-AG signalling at CB1R reduces freezing and enhances darting behaviour at extinction training in females
Figure 5 shows the effects of systemic pre-extinction administration of MJN110 alone, AM251 alone and MJN110+AM251 on freezing (Fig. 5A–C) and darting (Fig. 5D–F) behaviour during the auditory fear conditioning paradigm. Detailed statistics is reported in Table S5. Analysis of freezing during conditioning (Fig. 5A) revealed a significant main effect of trial, but no significant drug treatment effect or significant trial x drug interaction, or differences during the pre-CS period, thus confirming no pre-existing differences between groups before drug treatment. Analysis of freezing during extinction training (Fig. 5B) revealed significant main effects of both trial and drug, but no interaction. Post-hoc comparisons showed that the MJN110 alone group exhibited overall less freezing than vehicle-treated rats did across all the CS presentations, and there were no significant differences between the vehicle group and the MJN110+AM251 group, suggesting that MJN110 reduces freezing behaviour through a CB1R-mediated mechanism. Analysis of freezing during extinction retrieval (Fig. 5C) showed only a significant main effect of trial. One-way ANOVAs for freezing during the pre-CS period at extinction training and retrieval did not reveal any significant effects. Analysis of darting (Fig. 5D–F) revealed a significant main effect of trial during conditioning (Fig. 5D), significant effects of trial, and trial x drug interaction at extinction training (Fig. 5E), but no significant effects at extinction retrieval (Fig. 5F). Post-hoc analyses for extinction training showed that treatment with MJN110 alone significantly increased darting during CS11–15 as compared with both the vehicle and the AM251 groups (Fig. 5E). One-way ANOVAs for darting during the pre-CS periods for all the three testing days revealed a significant effect only for extinction training. Post-hoc analysis showed that the MJN110 group presented higher darting than the vehicle group (Fig. 5E).
Figure 5. Increased 2-AG signalling at CB1Rs reduced freezing and augmented darting behaviour at extinction training in females.

(A-F) The 2-AG hydrolysis inhibitor MJN110 decreased freezing and increased darting behaviour at extinction training. These effects were mediated by activation of CB1Rs, as they were blocked by concurrent injection with the CB1R antagonist AM251 (MJN110+AM251). Percentage of freezing during auditory fear conditioning (A), extinction training (B) and extinction retrieval (C). Number of darting events/min (dart/min) during auditory fear conditioning (D), extinction training (E) and extinction retrieval (F). Vehicle, n = 20; MJN110, n = 18; AM251, n = 15; MJN110+AM251, n = 11. Data are expressed as mean ± SEM. * P < 0.05 vs Vehicle; # P < 0.05 vs AM251. Horizontal line below the star indicates a main effect of drug: P < 0.05, MJN110 group vs Vehicle group (B).
Sex-dependent effects of auditory fear extinction training on endocannabinoid brain levels
To assess how extinction training may differentially alter endocannabinoid levels in males and females, we fear conditioned new cohorts of animals and measured AEA and 2-AG in the amygdala, PFC, dPAG, and vPAG immediately after extinction training (Ext) or a no-CS control session (No-Ext). All data are shown in Fig. 6 and statistics are in Table S6. Surprisingly, we observed effects of extinction on endocannabinoid levels in males only. In the amygdala, we found that male Ext rats had significantly higher AEA levels than male No-Ext rats, while no differences in AEA levels between the No-Ext and Ext groups in females were detected (Fig. 6A). No significant differences were found for amygdala 2-AG levels in either sex (Fig. 6B). In the PFC, we did found no significant differences in AEA or 2-AG levels between No-Ext and Ext groups in males or females (Fig. 6C,D). In the dPAG, we found a reduction in AEA levels in Ext males compared to No-Ext males, but no significant differences for AEA levels in females (Fig. 6E). No significant effects were observed for 2-AG levels in the dPAG (Fig. 6F) or for AEA or 2-AG in the vPAG (Fig. 6G,H). We also found significant positive correlations between amygdala 2-AG levels and darting rate (r = 0.72, P < 0.05) and between vPAG AEA levels and freezing behaviour (r = 0.74, P < 0.05) and a negative correlation between PFC AEA levels and darting rate (r = −0.67, P < 0.05) shown during CS presentations at the extinction session in females (Table S7). Freezing and darting behaviour for rats in the No-Ext and Ext groups is shown in Fig. S1.
Figure 6. Sex-dependent effects of auditory fear extinction training on endocannabinoid brain levels.

AEA and 2-AG brain levels (pmol/g tissue and nmol/g tissue, respectively) in the amygdala (AMY; A, B; for AEA and 2-AG: males No-Ext, n = 10, males Ext, n = 11, females No-Ext, n = 10, females Ext, n = 10), prefrontal cortex (PFC; C, D; for AEA and 2-AG: males No-Ext, n = 10, males Ext, n = 11, females No-Ext, n = 10, females Ext, n = 10), dorsal periaqueductal grey (dPAG; E, F; for AEA and 2-AG: males No-Ext, n = 8, males Ext, n = 9, females No-Ext, n = 9, females Ext, n = 9) and ventral periaqueductal grey (vPAG; G, H; for AEA and 2-AG: males No-Ext, n = 8, males Ext, n = 11, females No-Ext, n = 8, females Ext, n = 10) in male and female rats immediately after the extinction training session (Ext group) or in control groups only exposed to the extinction context without the CS presentations (No-Ext group). Data are expressed as mean ± SEM. * P < 0.05.
Discussion
Although the effects of endocannabinoid system manipulation on fear memory in males have been well investigated, our study provides the first systematic pharmacologic examination of endocannabinoid regulation of fear extinction in both sexes and reveals for the first time a strong sex-dependent effect of endocannabinoids in the acute modulation of fear extinction. Quite surprisingly, we found that acutely elevating AEA or 2-AG signalling at extinction training did not significantly alter fear expression or extinction in males, although increased AEA tended to facilitate fear extinction. Experiments in females revealed an opposite picture to what has been previously reported for males. We observed divergent effects of AEA versus 2-AG signalling manipulations, each mediated by distinct mechanisms.
Consistent with previous findings (Gruene et al., 2015a), we show a robust sexual dimorphism in behavioural expression of fear. While males predominately expressed freezing behaviour, females exhibited both freezing and darting. Although darting increased over time with CS-US presentations at conditioning, it remained mostly unvaried with progression of CS presentations during both extinction training and retrieval, thus, under our experimental conditions, darting did not seem to strictly reflect a learned fear response. Moreover, accordingly with previous studies comparing conditioned freezing behaviour between sexes (Maren et al., 1994; Pryce et al., 1999; Gupta et al., 2001), we found that males showed significantly higher freezing than females at conditioning and extinction retrieval.
The endocannabinoid system has been consistently reported to modulate fear memory extinction and stress/fear coping strategies, in male rodents (Marsicano et al., 2002; Metna-Laurent et al., 2012; Gunduz-Cinar et al., 2013; Llorente-Berzal et al., 2015; Morena et al., 2016a, 2018, 2019; Heinz et al., 2017; Colangeli et al., 2020). Increased AEA has been shown to promote fear extinction by reducing expression of freezing (Marsicano et al., 2002; Chhatwal et al., 2005; Bitencourt et al., 2008; Pamplona et al., 2008; Gunduz-Cinar et al., 2013), via activation of CB1Rs on forebrain glutamatergic neurons (Llorente-Berzal et al., 2015); elevated 2-AG signalling, however, has been reported to impair within-session extinction (Hartley et al., 2016) and increase freezing, via activation of CB1Rs on forebrain GABAergic neurons (Llorente-Berzal et al., 2015), in male rodents. Surprisingly, our pharmacological manipulations did not significantly alter fear responses in males. However, consistent with previously published findings using somewhat different approaches (Chhatwal et al., 2005; Bitencourt et al., 2008; Pamplona et al., 2008; Gunduz-Cinar et al., 2013), elevating AEA signalling did tend to facilitate fear extinction. Differences in species, experimental protocol, type, doses and administration regimen of drugs used, likely contributed to these discrepancies. Indeed, in the work by Gunduz-Cinar et al. (2013) it was used an inbred strain of mice with impaired fear extinction learning and retrieval. Furthermore, in all the above mentioned studies in rats by Chhatwal et al. (2005), Bitencourt et al., (2008), and Pamplona et al. (2008), to explore the effects of increased AEA levels was used AM404, which, in addition to inhibit AEA uptake, has been shown to increase 2-AG signaling and act on many other different sites including TRPV1R and sodium channels (Zygmunt et al., 2000; Nicholson et al., 2003; Hájos et al., 2004; Wiskerke et al., 2012). Consistent with our results, however, pre-extinction injection of URB597, at the same dose we used in the present study, has been reported to not affect fear extinction in male rats under basal conditions, but to only prevent the impairment in fear extinction induced by stress (Zer-Aviv and Akirav, 2016). It is also possible that doses different from the ones used in the present study or repeated dosing are necessary to produce consistent effects as repeated FAAH inhibition enhanced fear extinction in both male rats (Morena et al., 2018) and a mixed sample of males and females in humans (Mayo et al., 2020).
Interestingly, in females, elevated AEA signalling at TRPV1Rs increased freezing behaviour both acutely during the extinction training and the following day during extinction retrieval, unveiling an impairment of within-session extinction and recall of extinction memory. Furthermore, the same manipulation induced a strong fear generalization as indicated by elevated freezing shown before CS presentations in the extinction context, which was never associated to the aversive experience. Specifically, although it reduced darting across all CS trials at extinction training, we did not find that inhibition of AEA hydrolysis per se increased freezing behaviour. Surprisingly, concurrent blockade at CB1R while elevating AEA signalling robustly increased freezing response. Additional treatment with CPZ revealed that this effect was mediated by activation of TRPV1R. Since inhibition of AEA hydrolysis together with CB1R blockade did not influence fear responses and memory in males, the TRPV1R antagonism experiment was carried out exclusively in females. These data indicate, for the first time, that AEA signalling at TRPV1Rs might be biased toward facilitating freezing in female rats, thus, unveiling sex differences in the affinity, expression and/or functionality of TRPV1Rs and endocannabinoid system components. An alternate possibility is that females could exhibit an upregulation of TRPV1Rs in response to the noxious footshocks delivered during fear conditioning itself, which then favors AEA signalling at these receptors. Future work is required to understand this relationship in more depth, exploring the effects of direct TRPV1R agonism in discrete brain regions, as it would be challenging to examine this mechanism with a systemic manipulation and avoid confounding pain-related effects due to peripheral TRPV1R activation.
Elevated 2-AG signalling at CB1Rs in females modulated learned fear expression in the opposite direction. Pre-extinction treatment with MJN110 acutely reduced freezing at the last CS presentations, thus accelerating within-session extinction. This effect was CB1R-mediated as it was blocked by CB1R antagonism. Interestingly, MJN110 affected darting behaviour in females in the opposite direction. Therefore, increased 2-AG signalling promoted active over passive fear responses acutely, without affecting the consolidation of extinction, as treatment with MJN110 did not affect rats’ behaviour at extinction retrieval. This shift from passive to active forms of acute fear coping is consistent with an established role of CB1Rs on glutamatergic neurons (Metna-Laurent et al., 2012), suggesting that in females elevated 2-AG signaling may preferentially engage this receptor population to promote this behavioral transition. These collective findings in females are very reminiscent of a study in a line of male mice, bred to exhibit a high degree of anxiety, where elevated AEA signalling increased passive fear responses whereas inhibition of 2-AG hydrolysis increased active responses (Heinz et al., 2017). While it is not immediately apparent as to why pharmacological manipulations of endocannabinoids in these anxious male mice parallel our results with female rats, it does indicate that bidirectional effects of manipulating endocannabinoid signalling on fear behaviours can occur across species and sexes.
Sex differences were also observed in AEA levels in several brain regions involved in the regulation of fear memory and fear responses. Corroborating previous findings in mice (Marsicano et al., 2002; Gunduz-Cinar et al., 2013), male rats undergone fear extinction exhibited higher amygdala AEA levels than males never exposed to CS extinction. Furthermore, among males, we found decreased dPAG AEA levels in rats undergone fear extinction. Interestingly, a previous study showed increased dPAG AEA levels following a 3 min re-exposure to a context previously associated with a footshock (Olango et al., 2012), thus potentially indicating an opposing role in the regulation of early fear expression/extinction vs late extinction phases of fear memory. Fear extinction did not affect AEA levels in females nor 2-AG levels in either sex in the brain regions examined. However, correlational analyses in females revealed that rats presenting higher amygdala 2-AG levels showed increased darting during extinction training, paralleling our behavioural findings with MJN110 treatment. Interestingly, within the PFC, AEA levels negatively correlated with darting, which paralleled our finding that treatment with URB597 decreased darting across all CS presentations at extinction training in females. Furthermore, accordingly to our behavioural results in females showing increased freezing following AEA-mediated activation of TRPV1Rs, a positive correlation was also detected between freezing during extinction training and AEA levels in the vPAG, a brain region strongly involved in freezing and learned fear responses (Watson et al., 2016). Future work will employ site-specific pharmacological manipulations to establish the sites of action of AEA and 2-AG and to further identify subregion-specific endocannabinoid changes in the amygdala and PFC, also known to play important roles in different phases of fear memory.
Previous studies have shown that, although darting is not affected by estrous cycle (Gruene et al., 2015a), freezing behaviour at extinction varies with estrous phases (Zeidan et al., 2011; Gruene et al., 2015b). Moreover, the estrous cycle has been reported to modulate CB1R density and affinity (de Fonseca et al., 1994), and AEA and 2-AG levels across different brain regions (González et al., 2000; Bradshaw et al., 2006). However, in the present study estrous cycle was not monitored, thus future investigations are warranted to examine the influence of estrous phases on endocannabinoid modulation of fear memory expression and extinction.
The opposing effects of enhanced AEA versus 2-AG signalling in the modulation of fear responses and the biphasic effects of cannabinoid drugs have been largely documented (Moreira et al., 2012; Morena and Campolongo, 2014). Beside the involvement of CB1Rs at different neuronal subpopulations (Rey et al., 2012; Metna-Laurent et al., 2012; Llorente-Berzal et al., 2015; Lutz et al., 2015; Heinz et al., 2017), these opposing effects have also been ascribed to the recruitment of receptors other than CB1R (Casarotto et al., 2012; Moreira et al., 2012; Patel et al., 2017; Colangeli et al., 2019; Di Maio et al., 2019), such as TRPV1Rs, which can be activated by high AEA levels (Zygmunt et al., 1999; Di Marzo, 2008; Bialecki et al., 2020). Both CB1Rs and TRPV1Rs are widely expressed in brain areas involved in anxiety and fear, including the PFC, hippocampus, amygdala and PAG (Tsou et al., 1999; Mezey et al., 2000; Cristino et al., 2008; Bialecki et al., 2020). Consistent with our results, compelling evidence has reported opposing roles for CB1Rs and TRPV1Rs in the modulation of fear and anxiety-related responses, where activation of TRPV1R has been shown to increase fear and anxiety-like behaviour, whereas CB1R activation attenuates these behavioural responses, in male rodents (Rubino et al., 2008; Campos and Guimarães, 2009; Moreira et al., 2012). In agreement with our results, Laricchiuta et al. (2013) found that a systemic-induced augmentation of AEA signalling at TRPV1R increased freezing and impaired extinction in a contextual fear conditioning paradigm in mice (Laricchiuta et al., 2013). Moreover, TRPV1 knock-out (male) mice show low anxiety-like behaviour and conditioned fear responses compared to their wild-type controls (Marsch et al., 2007). Further corroborating our findings, it has been recently reported in males that both antagonism of CB1Rs or activation of TRPV1Rs in the dorsolateral PAG increased fear response, through a mechanism that seemed to involve increased glutamatergic transmission induced by either manipulations (Uliana et al., 2016).
Further supporting our sex-divergent results, a number of preclinical studies have reported sex-differences in the expression and functionality of endocannabinoid system components in fear-related brain regions in both baseline conditions and in models for stress/trauma-related disorders (Reich et al., 2009; Fattore and Fratta, 2010; Xing et al., 2014; Zer-Aviv and Akirav, 2016; Cooper and Craft, 2018).
In conclusion, our data provide the first evidence supporting fundamental sex differences of the endocannabinoid system in the modulation of fear expression and extinction. Augmenting AEA or 2-AG signalling did not significantly alter fear expression in male rats, whereas it did affect fear expression and extinction in females in opposite directions. While increased 2-AG signalling acutely reduced conditioned freezing, facilitated within-session extinction and enhanced darting via activation of CB1Rs, elevated AEA signalling at TRPV1Rs increased conditioned freezing, fear generalization and impaired fear extinction. Processes of fear extinction are profoundly altered in PTSD, and clinical literature provides evidence that the prevalence of PTSD is twice as high in women compared to men (Breslau, 2009), with documented sex differences found in both disease severity and treatment efficacy. Moreover, human studies have reported sex-related changes of endocannabinoid system components in patients suffering from PTSD, showing a more pronounced upregulation of CB1R in the amygdala-hippocampal-cortico-striatal neural circuit in women than men and a decrease in peripheral AEA levels in both sexes (Neumeister et al., 2013). Therefore, understanding how endocannabinoids modulate fear responses and processes of extinction in both sexes will provide new insights into the sex dimorphism documented in the pathophysiology of PTSD and possibly help facilitate the development of sex-specific therapeutic interventions.
Supplementary Material
Bullet points.
What is already known
In male rodents, endocannabinoids modulate fear extinction and have been suggested to treat PTSD.
Increased anandamide signalling at cannabinoid type-1 receptors promotes fear extinction in male rodents.
What this study adds
Increased anandamide signalling impairs fear extinction in female rats via activation of vanilloid TRPV1 receptors.
Increased 2-arachidonoylglycerol signalling at cannabinoid type-1 receptors promotes darting over freezing in female rats.
Clinical significance
Our results provide new insights into the sex dimorphism documented in PTSD.
This study facilitates the development of endocannabinoid-based sex-specific approaches to treat PTSD.
Acknowledgements
We thank Min Qiao for technical assistance. This study was supported by operating funds from the Canadian Institutes of Health Research (CIHR FDN-143329; MNH) and Fondation Brain Canada (MNH) and NIMH R56 MH11493 grant to RMS and MNH. MM received salary support from Alberta Innovates Health Solutions (AIHS) and CIHR postdoctoral fellowships, ASN was the recipient of a studentship from the Branch Out Neurological Foundation (BONF), AS received salary support from the Mathison Centre for Mental Health Research & Education. All funding agencies had no influence on the design, execution, or publishing of this work.
Abbreviations
- 2-AG
2-arachidonoyl glycerol
- AEA
anandamide
- CB1R
cannabinoid type 1 receptor
- CB2R
cannabinoid type 2 receptor
- CS
conditioned stimulus
- US
unconditioned stimulus
- dPAG
dorsal periaqueductal grey
- FAAH
fatty acid amide hydrolase
- MAGL
monoacylglycerol lipase
- PFC
prefrontal cortex
- PTSD
post-traumatic stress disorder
- TRPV1R
transient potential receptor of vanilloid type 1 channel
- vPAG
ventral periaqueductal grey
Footnotes
Conflict of Interest Statement
MNH is a member of the scientific advisory board for Sophren Therapeutics and Lundbeck.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design and Analysis, and Animal Experimentation, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Availability of Data
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Mathie A, Peters JA, Veale EL, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, Davies JA, CGTP Collaborators (2019a). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein-coupled receptors. Br J Pharmacol 176: S57. [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Mathie A, Peters JA, Veale EL, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, Davies JA, CGTP Collaborators (2019b). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Enzymes. Br J Pharmacol 176: S336, S337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA, Veale EL, Striessnig J, Kelly E, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, Davies JA, CGTP Collaborators (2019c). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Ion channels. Br J Pharmacol 176: S190. [Google Scholar]
- Bandler R, Keay KA, Floyd N, and Price J (2000). Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull 53: 95–104. [DOI] [PubMed] [Google Scholar]
- Bialecki J, Werner A, Weilinger NL, Tucker CM, Vecchiarelli HA, Egaña J, et al. (2020). Suppression of Presynaptic Glutamate Release by Postsynaptic Metabotropic NMDA Receptor Signalling to Pannexin-1. J Neurosci 40: 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitencourt RM, Pamplona FA, and Takahashi RN (2008). Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur Neuropsychopharmacol 18: 849–859. [DOI] [PubMed] [Google Scholar]
- Blankman JL, and Cravatt BF (2013). Chemical Probes of Endocannabinoid Metabolism. Pharmacol Rev 65: 849–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw HB, Rimmerman N, Krey JF, and Walker JM (2006). Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. Am J Physiol - Regul Integr Comp Physiol 291: R349–58. [DOI] [PubMed] [Google Scholar]
- Breslau N (2009). The Epidemiology of Trauma, PTSD, and Other Posttrauma Disorders. Trauma, Violence, Abus 10: 198–210. [DOI] [PubMed] [Google Scholar]
- Campos AC, and Guimarães FS (2009). Evidence for a potential role for TRPV1 receptors in the dorsolateral periaqueductal gray in the attenuation of the anxiolytic effects of cannabinoids. Prog Neuro-Psychopharmacology Biol Psychiatry 33: 1517–1521. [DOI] [PubMed] [Google Scholar]
- Casarotto PC, Terzian ALB, Aguiar DC, Zangrossi H, Guimarães FS, Wotjak CT, et al. (2012). Opposing Roles for Cannabinoid Receptor Type-1 (CB1) and Transient Receptor Potential Vanilloid Type-1 Channel (TRPV1) on the Modulation of Panic-Like Responses in Rats. Neuropsychopharmacology 37: 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli M, Fadda P, Casu A, Spano M, Casti A, Fratta W, et al. (2014). Male and Female Rats Differ in Brain Cannabinoid CB1 Receptor Density and Function and in Behavioural Traits Predisposing to Drug Addiction: Effect of Ovarian Hormones. Curr Pharm Des 20: 2100–2113. [DOI] [PubMed] [Google Scholar]
- Chávez AE, Chiu CQ, and Castillo PE (2010). TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci 13: 1511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, and Ressler KJ (2005). Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology 30: 516–524. [DOI] [PubMed] [Google Scholar]
- Colangeli R, Di Maio R, Pierucci M, Deidda G, Casarrubea M, and Di Giovanni G (2019). Synergistic action of CB1 and 5-HT2B receptors in preventing pilocarpine-induced status epilepticus in rats. Neurobiol Dis 125: 135–145. [DOI] [PubMed] [Google Scholar]
- Colangeli R, Morena M, Pittman QJ, Hill MN, and Teskey GC (2020). Anandamide signaling augmentation rescues amygdala synaptic function and comorbid emotional alterations in a model of epilepsy. J Neurosci 40: 6068–6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colangeli R, Pierucci M, Benigno A, Campiani G, Butini S, and Di Giovanni G (2017). The FAAH inhibitor URB597 suppresses hippocampal maximal dentate afterdischarges and restores seizure-induced impairment of short and long-term synaptic plasticity. Sci Rep 7: 11152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom-Lapetina J, Li AJ, Pelegrina-Perez TC, and Shansky RM (2019). Behavioral Diversity Across Classic Rodent Models Is Sex-Dependent. Front Behav Neurosci 13: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, and Craft RM (2018). Sex-Dependent Effects of Cannabis and Cannabinoids: A Translational Perspective. Neuropsychopharmacology 43: 34–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Wakley AA, Tsutsui KT, and Laggart JD (2012). Sex differences in cannabinoid 1 vs. cannabinoid 2 receptor-selective antagonism of antinociception produced by Δ 9- tetrahydrocannabinol and CP55,940 in the rat. J Pharmacol Exp Ther 340: 787–800. [DOI] [PubMed] [Google Scholar]
- Cristino L, Starowicz K, Petrocellis L. De, Morishita J, Ueda N, Guglielmotti V, et al. (2008). Immunohistochemical localization of anabolic and catabolic enzymes for anandamide and other putative endovanilloids in the hippocampus and cerebellar cortex of the mouse brain. Neuroscience 151: 955–68. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA, et al. (2018). Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Br J Pharmacol 175: 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maio R, Colangeli R, and Di Giovanni G (2019). WIN 55,212–2 Reverted Pilocarpine-Induced Status Epilepticus Early Changes of the Interaction among 5-HT2C/NMDA/CB1 Receptors in the Rat Hippocampus. ACS Chem Neurosci 10: 3296–3306. [DOI] [PubMed] [Google Scholar]
- Di Marzo V (2008). Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov 7: 438–455. [DOI] [PubMed] [Google Scholar]
- Fanselow MS (1980). Conditional and unconditional components of post-shock freezing. Pavlov J Biol Sci Off J Pavlov 15: 177–182. [DOI] [PubMed] [Google Scholar]
- Fattore L, and Fratta W (2010). How important are sex differences in cannabinoid action. Br J Pharmacol 160: 544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca F.R. de, Cebeira M, Ramos JA, Martín M, and Fernández-Ruiz JJ (1994). Cannabinoid receptors in rat brain areas: Sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci 54: 159–170. [DOI] [PubMed] [Google Scholar]
- González S, Bisogno T, Wenger T, Manzanares J, Milone A, Berrendero F, et al. (2000). Sex steroid influence on cannabinoid CB1 receptor mRNA and endocannabinoid levels in the anterior pituitary gland. Biochem Biophys Res Commun 270: 260–266. [DOI] [PubMed] [Google Scholar]
- Gruene TM, Flick K, Stefano A, Shea SD, and Shansky RM (2015a). Sexually divergent expression of active and passive conditioned fear responses in rats. Elife 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene TM, Roberts E, Thomas V, Ronzio A, and Shansky RM (2015b). Sex-specific neuroanatomical correlates of fear expression in prefrontal-amygdala circuits. Biol Psychiatry 78: 186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Brasnjo G, and Malenka RC (2010). Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci 13: 1519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O, MacPherson KP, Cinar R, Gamble-George J, Sugden K, Williams B, et al. (2013). Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psychiatry 18: 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, and Maren S (2001). Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats. Brain Res 888: 356–365. [DOI] [PubMed] [Google Scholar]
- Hájos N, Kathuria S, Dinh T, Piomelli D, and Freund TF (2004). Endocannabinoid transport tightly controls 2-arachidonoyl glycerol actions in the hippocampus: Effects of low temperature and the transport inhibitor AM404. Eur J Neurosci 19: 2991–2996. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, Gray AJG, Bruce L, Alexander SPH, Anderton S, Bryant C, Davenport AP, Doerig C, Fabbro D, Levi-Schaffer F, Spedding M, Davies JA, NC-IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 46, D1091–1106. doi: 10.1093/nar/gkx1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley ND, Gunduz-Cinar O, Halladay L, Bukalo O, Holmes A, and Patel S (2016). 2-arachidonoylglycerol signaling impairs short-term fear extinction. Transl Psychiatry 6: e749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz DE, Genewsky A, and Wotjak CT (2017). Enhanced anandamide signaling reduces flight behavior elicited by an approaching robo-beetle. Neuropharmacology 126: 233–241. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, and Norrholm SD (2011). Neural mechanisms of impaired fear inhibition in posttraumatic stress disorder. Front Behav Neurosci 5: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, et al. (2003). Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med 9: 76–81. [DOI] [PubMed] [Google Scholar]
- Katona I, and Freund TF (2012). Multiple Functions of Endocannabinoid Signaling in the Brain. Annu Rev Neurosci 35: 529–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Davis M, and Öhman A (2000). Fear and anxiety: Animal models and human cognitive psychophysiology. In Journal of Affective Disorders, pp 137–159. [DOI] [PubMed] [Google Scholar]
- Laricchiuta D, Centonze D, and Petrosini L (2013). Effects of endocannabinoid and endovanilloid systems on aversive memory extinction. Behav Brain Res 256: 101–7. [DOI] [PubMed] [Google Scholar]
- Lilley E, Stanford SC, Kendall DE, Alexander SP, Cirino G, Docherty JR, … Ahluwalia A (2020). ARRIVE 2.0 and the British Journal of Pharmacology: Updated guidance for 2020. British Journal of Pharmacology. 10.1111/bph.15178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente-Berzal A, Terzian ALB, Di Marzo V, Micale V, Viveros MP, and Wotjak CT (2015). 2-AG promotes the expression of conditioned fear via cannabinoid receptor type 1 on GABAergic neurons. Psychopharmacology (Berl) 232: 2811–2825. [DOI] [PubMed] [Google Scholar]
- Lutz B, Marsicano G, Maldonado R, and Hillard CJ (2015). The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci 16: 705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DJ, Bedse G, Gaulden AD, Ryan JD, Kondev V, Winters ND, et al. (2020). Endocannabinoid Signaling Collapse Mediates Stress-Induced Amygdalo-Cortical Strengthening. Neuron 105: 1062–1076.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S (2001). Neurobiology of Pavlovian Fear Conditioning. Annu Rev Neurosci 24: 897–931. [DOI] [PubMed] [Google Scholar]
- Maren S, Oca B. De, and Fanselow MS (1994). Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res 661: 25–34. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Di Marzo V, Berretta N, Matias I, Maccarrone M, Bernardi G, et al. (2003). Presynaptic facilitation of glutamatergic synapses to dopaminergic neurons of the rat substantia nigra by endogenous stimulation of vanilloid receptors. J Neurosci 23: 3136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch R, Foeller E, Rammes G, Bunck M, Kössl M, Holsboer F, et al. (2007). Reduced anxiety, conditioned fear, and hippocampal long-term potentiation in transient receptor potential vanilloid type 1 receptor-deficient mice. J Neurosci 27: 832–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascioll MG, et al. (2002). The endogenous cannabinoid system controls extinction of aversive memories. Nature 418: 530–534. [DOI] [PubMed] [Google Scholar]
- Mayo LM, Asratian A, Lindé J, Morena M, Haataja R, Hammar V, et al. (2020). Elevated Anandamide, Enhanced Recall of Fear Extinction, and Attenuated Stress Responses Following Inhibition of Fatty Acid Amide Hydrolase: A Randomized, Controlled Experimental Medicine Trial. Biol Psychiatry 87: 538–547. [DOI] [PubMed] [Google Scholar]
- Metna-Laurent M, Soria-Gómez E, Verrier D, Conforzi M, Jégo P, Lafenêtre P, et al. (2012). Bimodal control of fear-coping strategies by CB₁ cannabinoid receptors. J Neurosci 32: 7109–7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezey E, Tóth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, et al. (2000). Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci 97: 3655–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. (2009). Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biol Psychiatry 66: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Aguiar DC, Terzian ALB, Guimarães FS, and Wotjak CT (2012). Cannabinoid type 1 receptors and transient receptor potential vanilloid type 1 channels in fear and anxiety-two sides of one coin? Neuroscience 204: 186–92. [DOI] [PubMed] [Google Scholar]
- Morena M, Aukema RJ, Leitl KD, Rashid AJ, Vecchiarelli HA, Josselyn SA, et al. (2019). Upregulation of Anandamide Hydrolysis in the Basolateral Complex of Amygdala Reduces Fear Memory Expression and Indices of Stress and Anxiety. J Neurosci 39: 1275–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M, Berardi A, Colucci P, Palmery M, Trezza V, Hill MN, et al. (2018). Enhancing Endocannabinoid Neurotransmission Augments the Efficacy of Extinction Training and Ameliorates Traumatic Stress-Induced Behavioral Alterations in Rats. Neuropsychopharmacology 43: 1284–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M, and Campolongo P (2014). The endocannabinoid system: An emotional buffer in the modulation of memory function. Neurobiol Learn Mem 112: 30–43. [DOI] [PubMed] [Google Scholar]
- Morena M, Castro V. De, Gray JM, Palmery M, Trezza V, Roozendaal B, et al. (2015). Training-Associated Emotional Arousal Shapes Endocannabinoid Modulation of Spatial Memory Retrieval in Rats. J Neurosci 35: 13962–13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M, Leitl KD, Vecchiarelli HA, Gray JM, Campolongo P, and Hill MN (2016a). Emotional arousal state influences the ability of amygdalar endocannabinoid signaling to modulate anxiety. Neuropharmacology 111: 59–69. [DOI] [PubMed] [Google Scholar]
- Morena M, Patel S, Bains JS, and Hill MN (2016b). Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology 41: 80–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musella A, Chiara V. De, Rossi S, Prosperetti C, Bernardi G, Maccarrone M, et al. (2009). TRPV1 channels facilitate glutamate transmission in the striatum. Mol Cell Neurosci 40: 89–97. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Normandin MD, Pietrzak RH, Piomelli D, Zheng MQ, Gujarro-Anton A, et al. (2013). Elevated brain cannabinoid CB 1 receptor availability in post-traumatic stress disorder: A positron emission tomography study. Mol Psychiatry 18: 1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson RA, Liao C, Zheng J, David LS, Coyne L, Errington AC, et al. (2003). Sodium channel inhibition by anandamide and synthetic cannabimimetics in brain. Brain Res 978: 194–204. [DOI] [PubMed] [Google Scholar]
- Olango WM, Roche M, Ford GK, Harhen B, and Finn DP (2012). The endocannabinoid system in the rat dorsolateral periaqueductal grey mediates fear-conditioned analgesia and controls fear expression in the presence of nociceptive tone. Br J Pharmacol 165: 2549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona FA, Bitencourt RM, and Takahashi RN (2008). Short- and long-term effects of cannabinoids on the extinction of contextual fear memory in rats. Neurobiol Learn Mem 90: 290–293. [DOI] [PubMed] [Google Scholar]
- Patel S, Hill MN, Cheer JF, Wotjak CT, and Holmes A (2017). The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci Biobehav Rev 76: 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, ... Würbel H (2020). The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biology, 18(7), e3000410. 10.1371/journal.pbio.3000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Lehmann J, and Feldon J (1999). Effect of sex on fear conditioning is similar for context and discrete CS in Wistar, Lewis and Fischer rat strains. Pharmacol Biochem Behav 64: 753–759. [DOI] [PubMed] [Google Scholar]
- Qi M, Morena M, Vecchiarelli HA, Hill MN, and Schriemer DC (2015). A robust capillary liquid chromatography/tandem mass spectrometry method for quantitation of neuromodulatory endocannabinoids. Rapid Commun Mass Spectrom 29: 1889–1897. [DOI] [PubMed] [Google Scholar]
- Ratano P, Palmery M, Trezza V, and Campolongo P (2017). Cannabinoid Modulation of Memory Consolidation in Rats: Beyond the Role of Cannabinoid Receptor Subtype 1. Front Pharmacol 8: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich CG, Taylor ME, and McCarthy MM (2009). Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav Brain Res 203: 264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey AA, Purrio M, Viveros MP, and Lutz B (2012). Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA B receptors in the balance of gabaergic and glutamatergic neurotransmission. Neuropsychopharmacology 37: 2624–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebe CJN, Hill MN, Lee TTY, Hillard CJ, and Gorzalka BB (2010). Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology 35: 1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Realini N, Castiglioni C, Guidali C, Viganó D, Marras E, et al. (2008). Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb Cortex 18: 1292–1301. [DOI] [PubMed] [Google Scholar]
- Shansky RM (2015). Sex differences in PTSD resilience and susceptibility: Challenges for animal models of fear learning. Neurobiol Stress 1: 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sticht MA, Lau DJ, Keenan CM, Cavin J-B, Morena M, Vemuri VK, et al. (2019). Endocannabinoid regulation of homeostatic feeding and stress-induced alterations in food intake in male rats. Br J Pharmacol 176: 1524–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Mackie K, Sañudo-Peña MC, and Walker JM (1999). Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience 93: 969–75. [DOI] [PubMed] [Google Scholar]
- Uliana DL, Hott SC, Lisboa SF, and Resstel LBM (2016). Dorsolateral periaqueductal gray matter CB1 and TRPV1 receptors exert opposite modulation on expression of contextual fear conditioning. Neuropharmacology 103: 257–269. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Goffin K, Casteels C, Dupont P, Mortelmans L, Hoon J. de, et al. (2008). Gender-dependent increases with healthy aging of the human cerebral cannabinoid-type 1 receptor binding using [18F]MK-9470 PET. Neuroimage 39: 1533–1541. [DOI] [PubMed] [Google Scholar]
- Velasco ER, Florido A, Milad MR, and Andero R (2019). Sex differences in fear extinction. Neurosci Biobehav Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson TC, Cerminara NL, Lumb BM, and Apps R (2016). Neural Correlates of Fear in the Periaqueductal Gray. J Neurosci 36: 12707–12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, and Burston JJ (2014). Sex differences in δ9-tetrahydrocannabinol metabolism and in vivo pharmacology following acute and repeated dosing in adolescent rats. Neurosci Lett 576: 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskerke J, Irimia C, Cravatt BF, Vries T.J. De, Schoffelmeer ANM, Pattij T, et al. (2012). Characterization of the effects of reuptake and hydrolysis inhibition on interstitial endocannabinoid levels in the brain: An in vivo microdialysis study. In ACS Chemical Neuroscience, pp 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing G, Carlton J, Jiang X, Wen J, Jia M, and Li H (2014). Differential expression of brain cannabinoid receptors between repeatedly stressed males and females may play a role in age and gender-related difference in traumatic brain injury: Implications from animal studies. Front Neurol 5: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, and Li J (2007). TRPV1 receptor mediates glutamatergic synaptic input to dorsolateral periaqueductal gray (dl-PAG) neurons. J Neurophysiol 97: 503–11. [DOI] [PubMed] [Google Scholar]
- Yasmin F, Colangeli R, Morena M, Filipski S, Stelt M. van der, Pittman QJ, et al. (2020). Stress-induced modulation of endocannabinoid signaling leads to delayed strengthening of synaptic connectivity in the amygdala. Proc Natl Acad Sci U S A 117: 650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, et al. (2011). Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry 70: 920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zer-Aviv TM, and Akirav I (2016). Sex differences in hippocampal response to endocannabinoids after exposure to severe stress. Hippocampus 26: 947–57. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Chuang HH, Movahed P, Julius D, and Högestätt ED (2000). The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur J Pharmacol 396: 39–42. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, et al. (1999). Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400: 452–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


