Abstract
Coronary artery aneurysms are rare conditions with potentially devastating consequences. We describe the case of an athletic 40-year-old woman who received a diagnosis of giant right coronary artery aneurysm while she was undergoing a work-up for lower extremity varicose veins. She underwent successful surgical treatment without any complications. (Level of Difficulty: Intermediate.)
Key Words: atherosclerosis, coronary vessel anomaly, imaging, peripheral edema, vascular disease
Abbreviations and Acronyms: CAA, coronary artery aneurysm; CAD, coronary artery disease; CMR, cardiac magnetic resonance; CT, computed tomography; MTHFR, methylene tetrahydrofolate reductase; RCA, right coronary artery; VV, varicose vein
Graphical abstract
Coronary artery aneurysms are rare conditions with potentially devastating consequences. We describe the case of an athletic 40-year-old woman…
History of Presentation
A 40-year-old marathon running white woman was evaluated as an outpatient for right leg varicosities that first occurred with her third pregnancy and worsened with 2 other subsequent pregnancies. She had mild right leg swelling with dull aching pain and no other signs of inflammation or ulcers. She denied any history of chest pain, dyspnea, dizziness, or syncope. Her cardiovascular risk factor was heart disease in her father, who had coronary artery bypass surgery in his 50s. Blood pressure was 120/70 mm Hg, heart rate was 70 beats/min, respiratory rate was 18/minute, and temperature was 98.6°F. A computed tomography (CT) scan of the abdomen and pelvis performed to rule out intra-abdominal causes of venous compression causing right lower extremity varicosities revealed a cardiac mass with a calcified rim. A referral to cardiology led to cardiac magnetic resonance (CMR) for a possible pericardial cyst, but thus imaging instead demonstrated a giant right coronary artery (RCA) aneurysm. Further coronary anatomy was delineated with coronary CT angiography.
Learning Objectives
-
•
To identify CAA with different imaging modalities.
-
•
To describe the etiology, natural course, and associations of CAA.
-
•
To discuss the management options of CAA.
Past Medical History
Her history was significant for a methylene tetrahydrofolate reductase (MTHFR) gene mutation diagnosed as a part of work-up for infertility before her first pregnancy. Thereafter, she had 5 uneventful pregnancies during which she received enoxaparin therapy. She had no history of chest trauma, childhood Kawasaki disease, or tobacco use.
Differential Diagnosis
The differential diagnosis included coronary aneurysm, pseudoaneurysm, pericardial cyst, dilated coronary sinus, and dilated coronary fistula.
Investigations
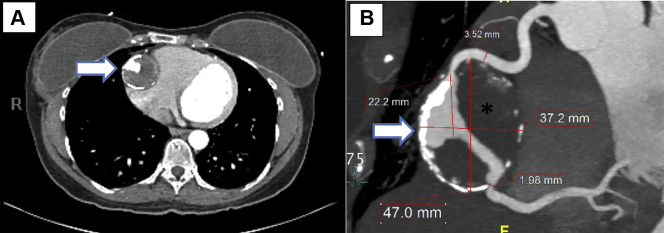
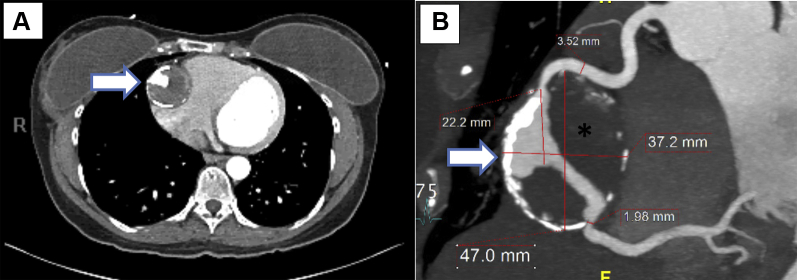
CT imaging revealed a large mid-RCA aneurysm measuring 4.7 × 3.9 cm (in transverse and anteroposterior dimensions, respectively) with surrounding eggshell calcification and good distal outflow (Figures 1A and 1B). Extensive thrombus was noted within the aneurysm. Reference RCA measured 3.5 mm both proximal and distal to the aneurysm. Within the aneurysm, the proximal neck measured 2.6 mm and the distal was 1.9 mm. The RCA aneurysm was noted to cause a mild mass effect with compression of the atrioventricular groove adjacent to the tricuspid annulus, with no clear evidence of significant right-sided heart compression. The RCA was dominant, and the left coronary arteries were normal by CT angiography. On CMR, the left ventricle was normal in size and function, with a calculated left ventricular ejection fraction of 57%. With intravenous gadolinium injection on CMR (Figure 2), there was partial filling of the aneurysm along with a rim of delayed enhancement that represented fibrotic scarring in the wall of the RCA aneurysm. The CT scan of the abdomen and chest did not demonstrate aneurysm formation in other arterial vessels, and additional imaging to exclude arterial aneurysms in other vessels was not performed. The laboratory work-up was unremarkable except for a mildly elevated low-density lipoprotein level of 120 mg/dl; the high-density lipoprotein level was 74 mg/dl.
Figure 1.
Computed Tomography Angiography
(A) Computed tomography coronary angiography demonstrating a giant right coronary artery aneurysm (arrow) in the axial plane. (B) Reconstructed computed tomography coronary angiography demonstrating a right coronary artery with a giant aneurysm in its midsegment (arrow). The aneurysm shows rim calcification and thrombosis (asterisk) in its cavity.
Figure 2.
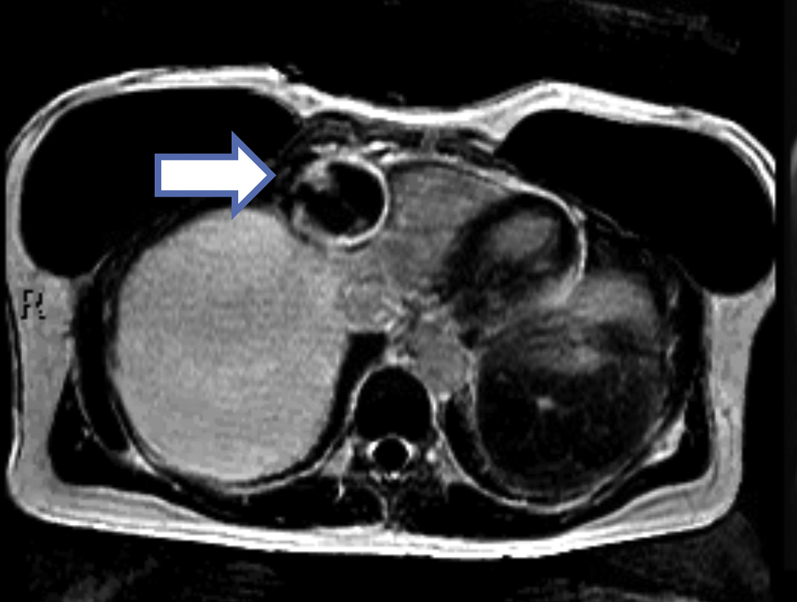
Cardiac Magnetic Resonance Imaging
Axial slice cardiac magnetic resonance with a phase-sensitive inversion recovery sequence showing partial filling of the aneurysm along with a rim of delayed gadolinium enhancement in the periphery of the aneurysm (arrow).
Management
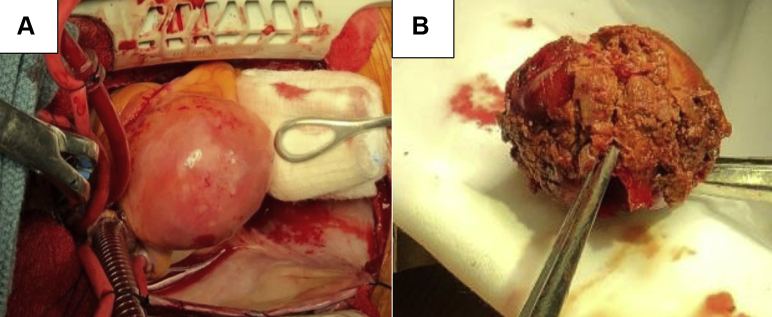
The patient was referred for cardiac surgery, which was preferred over medical therapy because of the possible risks of thrombosis, distal embolization, and rupture of the aneurysm. Percutaneous coronary intervention with a covered stent was not recommended because of the increased risk of thrombosis. The patient underwent elective aneurysm resection with suture ligation of the inflow and outflow and a reverse saphenous vein graft from the aorta to the distal RCA, without any complications (Figures 3A and 3B). A vein graft was selected for coronary artery bypass grafting over an arterial graft to preserve the arterial vessels for future possible bypass grafting, given the patient’s relatively younger age and early onset coronary artery disease (CAD). She made an uneventful recovery. Histopathologic examination of the aneurysm revealed hyalinization and obliteration of the vascular wall, with severe calcific atherosclerotic changes, mild focal chronic inflammation, and fibrin thrombus.
Figure 3.
Surgical Specimens
(A) Intact mid–right coronary artery giant aneurysm. (B) Large thrombus evacuated in toto from the right coronary artery aneurysmal cavity.
Discussion
Coronary artery aneurysms (CAAs) are rare, with an incidence between 0.3% and 5% (1,2). Giant CAAs are even rarer, with an incidence of 0.02% (3). CAA is defined as localized irreversible dilation of a coronary artery 1.5 times the adjacent normal coronary artery diameter. If similar dilation involves >50% of the arterial length, it is more appropriately termed coronary ectasia. Giant CAAs comprise a rare subset of CAAs in which the diameter exceeds >2 cm in adults and >8 mm in children (3). CAAs are more common in boys and men (1,4). These aneurysms most commonly involve the proximal and middle RCA. It is proposed that the RCA is more prone to aneurysmal formation because of the relatively low pressures around the right atrium and right atrioventricular sulcus compared with other intrapericardial regions (4).
Etiology and pathophysiology
In adults, atherosclerosis accounts for approximately 50% of CAA cases. Kawasaki disease is the second most common cause of CAA in adults and the most common cause in children (1). CAAs are true aneurysms. Histologically, in CAAs related to atherosclerosis, inflammation involves all 3 layers of the arterial wall, but most prominent is the destruction of the medial elastic fibers leading to weakening and remodeling of the vascular wall (5). Immunohistochemical studies have revealed increased matrix metalloproteinase activity within the vessel wall (5).
CAAs can be associated with other vessel wall disorders. Interestingly, primary varicose veins (VVs) of the lower extremities are known to occur more commonly in patients with CAAs (5,6). In a study by Androulakis and Katsaros (5), of their 181 patients with CAA, VVs were found in 40%, compared with 17% in patients with CAD without CAAs and 23% in randomly selected control subjects. Histologically, VVs also show loss of medial elastic tissue. In another study, by Yetkin et al. (7), a significant association between varicocele (dilated and tortuous spermatic veins within the pampiniform plexus of the testes) and CAA was noted.
CAA is also associated with other arterial aneurysms, most commonly in the aorta (8,9). One study reported that the incidence of CAA in patients with ascending or abdominal aortic aneurysms was 16% to 20% (8). Matrix metalloproteinase gene polymorphism has been proposed as a possible factor for different vascular aneurysms (9); whether these vascular disorders share common pathogenic mechanisms is not fully understood and needs more thorough investigation.
Natural course
The natural history of CAA is not fully established. CAAs are generally asymptomatic and found incidentally on imaging studies. Symptoms can be caused by myocardial ischemia or infarction from stenosis or thromboembolism of the involved artery. Microvascular dysfunction in CAA can cause ischemic symptoms. Potential complications include local mass effect of the aneurysm compressing nearby structures, spontaneous rupture into the cardiac chambers or into the pericardium causing cardiac tamponade, and sudden death.
In adults, CAA is considered a variant of coronary atherosclerotic disease and shares the same risk factors and prognosis as CAD (1,2,4). Data from some of the largest studies reviewing patients with CAA (1,2) suggest better prognoses in patients with isolated CAA without CAD. Interestingly, our patient had an MTHFR gene mutation that encodes the enzyme 5,10-MTHFR, which is involved in the methylation of the amino acid homocysteine to methionine in a multistep process. Homozygous mutation of this gene causes hyperhomocysteinemia, which is known to be a risk factor for premature CAD. Although high homocysteine levels could be contributory to the premature CAD seen in our patient, the homocysteine level was not measured in our patient.
Management
Given the paucity of publications on CAAs, there are no guidelines for its management. Management options include medical treatment with antiplatelet and anticoagulant agents, percutaneous treatment with stents or coil embolization, or surgical treatment. Because giant CAAs are more complex, surgical treatment is the preferred approach.
Follow-Up
At 3 months post-bypass surgery, the patient underwent VV stripping and ligation on the right lower extremity. She is currently doing well and recently ran a half marathon.
Conclusions
Traditionally, CAAs have been thought to be on a spectrum of atherosclerotic CAD. However, reviewing the associations of CAAs with VVs and other arterial aneurysms highlights the possibility of a more systemic vessel wall disorder. Management of these lesions can be complex, with little guidance from publications. Further studies are needed to characterize the disease and optimal treatment.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Swaye P.S., Fisher L.D., Litwin P. Aneurysmal coronary artery disease. Circulation. 1983;67:134–138. doi: 10.1161/01.cir.67.1.134. [DOI] [PubMed] [Google Scholar]
- 2.Demopoulos V.P., Olympios C.D., Fakiolas C.N. The natural history of aneurysmal coronary artery disease. Heart. 1997;78:136–141. doi: 10.1136/hrt.78.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marla R., Ebel R., Crosby M., Almassi G.H. Multiple giant coronary artery aneurysms. Tex Heart Inst J. 2009;36:244–246. [PMC free article] [PubMed] [Google Scholar]
- 4.Keyser A., Hilker M.K., Husser O., Diez C., Schmid C. Giant coronary aneurysms exceeding 5 cm in size. Interact Cardiovasc Thorac Surg. 2012;15:33–36. doi: 10.1093/icvts/ivs111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Androulakis A.E., Katsaros A.A. Varicose veins are common in patients with coronary artery ectasia. Just a coincidence or a systemic deficit of the vascular wall? Eur J Vasc Endovasc Surg. 2004;27:519–524. doi: 10.1016/j.ejvs.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Triantafillidi H., Rizos I., Androulakis A., Stratos C., Arvaniti C., Toutouzas P. Coronary artery ectasia, aneurysm of the basilar artery and varicose veins. Common presentation or generalized defect of the vessel wall? Angiology. 2001;52:287–291. doi: 10.1177/000331970105200410. [DOI] [PubMed] [Google Scholar]
- 7.Yetkin E., Kilic S. Increased prevalence of varicocele in patients with coronary artery ectasia. Coron Artery Dis. 2005;16:261–264. doi: 10.1097/00019501-200508000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Balderston J.R., Giri J., Kolansky D.M., Bavaria J.E., Gertz Z.M. Coronary artery aneurysms associated with ascending aortic aneurysms and abdominal aortic aneurysms: pathophysiologic implications. Catheter Cardiovasc Interv. 2015;85:961–967. doi: 10.1002/ccd.25726. [DOI] [PubMed] [Google Scholar]
- 9.Lamblin N., Bauters C., Hermant X., Lablanche J.M., Helbecque N., Amouyel P. Polymorphisms in the promoter regions of MMP-2, MMP-3, MMP-9 and MMP-12 genes as determinants of aneurysmal coronary artery disease. J Am Coll Cardiol. 2002;40:43–48. doi: 10.1016/s0735-1097(02)01909-5. [DOI] [PubMed] [Google Scholar]