Abstract
A 54-year-old male with history of end-stage renal disease secondary to hypertension on hemodialysis with moderate aortic valve insufficiency presented with progressive exertional dyspnea and lower extremity edema over several weeks. Relevant history included hospitalization for Staphylococcus epidermidis bacteremia secondary to dialysis catheter line infection 6 months prior. (Level of Difficulty: Advanced.)
Key Words: aortic valve, endocarditis, mitral valve
Central Illustration
A 54-year-old male with history of end-stage renal disease secondary to hypertension on hemodialysis with moderate aortic valve insufficiency…
History of Presentation
A 54-year-old male presented to the outpatient cardiology clinic for echocardiogram and scheduled follow-up of his moderate-to-severe aortic regurgitation with moderately dilated aortic root and hypertension. The patient presented with severe progressive exertional dyspnea and lower extremity edema over several weeks. Relevant history included hospitalization for Staphylococcus epidermidis bacteremia secondary to dialysis catheter line infection 6 months prior. He completed a course of vancomycin and his dialysis catheter was removed during that hospitalization. All symptoms resolved and he returned to work as a carpenter.
Learning Objectives
-
•
To understand that de novo left atrial dissection is rare, but treatable.
-
•
To gain knowledge that native paravalvular mitral regurgitation due to dehiscence of the valve is possible and can be repaired by resuspending the mitral annulus.
Transthoracic echocardiogram on the day of admission appeared to show rupture of the left sinus of Valsalva resulting in a left to left shunt. The patient was sent to the emergency department and his workup escalated quickly. The patient was admitted to the cardiac intensive care unit in stable condition and the cardiothoracic surgery department was consulted. The patient's vital signs on admission were temperature 36.3°C, heart rate 79 beats/min, blood pressure 116/72 mm Hg, respiratory rate 19, and oxygen saturation 95% on room air.
Medical History
The patient’s history included end-stage renal disease secondary to hypertension, hemodialysis 3 days a week via left upper extremity arteriovenous fistula, chronic pain, gout, and gastroesophageal reflux.
Differential Diagnosis
Given the history of recent acute bacterial native valve endocarditis, the initial concern was for multivalvular endocarditis. It is also possible that the patient developed a second primary degenerative valve problem resulting in severe mitral regurgitation.
Investigations
Blood cultures were obtained to assess for active endocarditis. Computed tomography angiogram revealed no obstructive coronary disease with sinuses of Valsalva measured at 35 mm, and the sinotubular junction was moderately effaced with an ascending aorta measuring at 42 mm (Figure 1, Central Illustration). Transesophageal echocardiogram showed disruption of the aorticomitral continuity causing a large dissection plane with disruption of the adventitia at the base of the aorta near the aortic valve annulus extending approximately 6 cm along the left atrial wall. Systolic flow was noted within this space with an exit point approximately 5 cm from the origin and flow continuing into the left atrium resulting in severe paravalvular native valve mitral regurgitation (Videos 1, 2, 3, 4, 5, and 6).
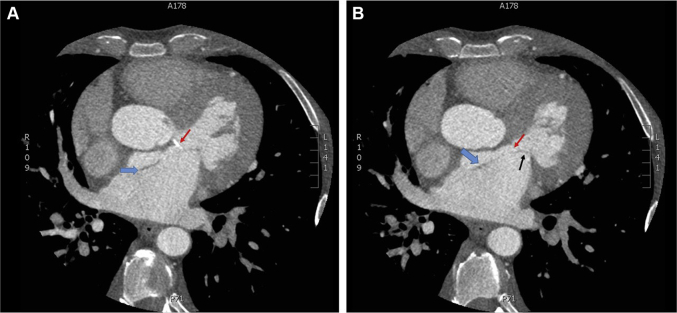
Figure 1.
Coronary Computed Tomography Angiogram Demonstrating the Left Atrial Dissection
(A) Single-axial image from a coronary computed tomography angiogram (CTA) showing calcification of the aorticomitral curtain (red arrow) that is secondary to a chronic aortic insufficiency jet. This lesion served as the nidus for the dissection (blue arrow). (B) Single-axial image from a coronary CTA showing the left atrial dissection flap (blue arrow) and native perivalvular mitral regurgitation due to anterior mitral leaflet (black arrow) dehiscence.
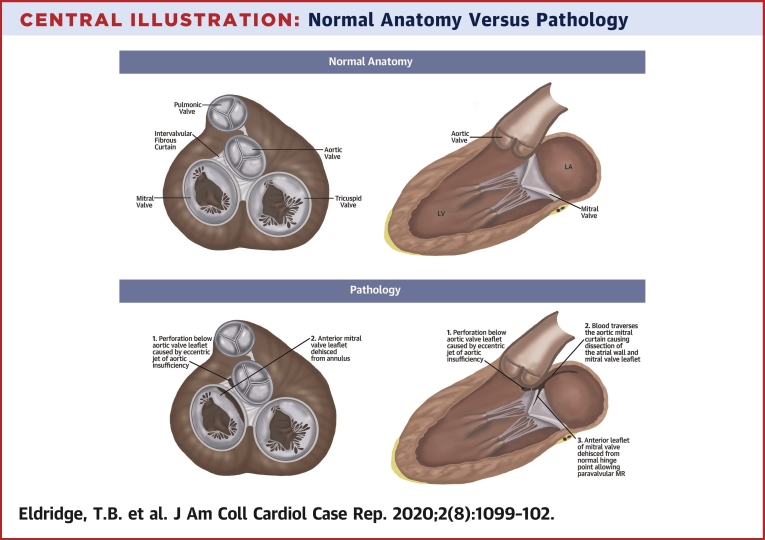
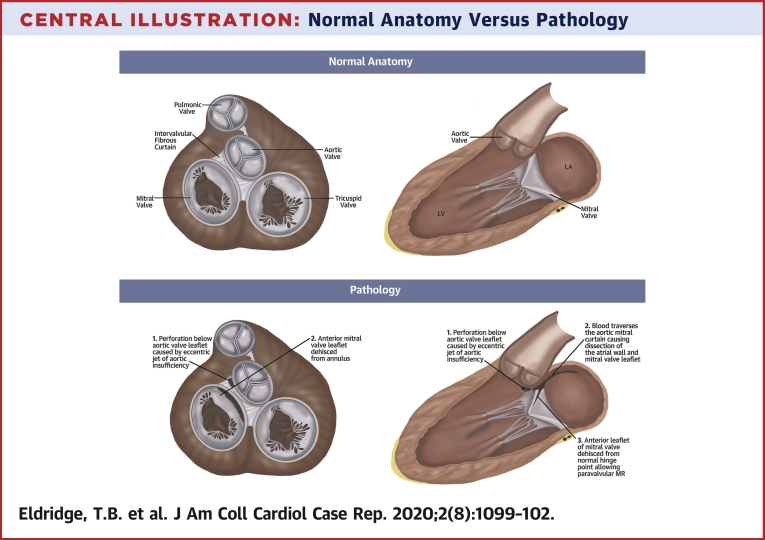
Central Illustration.
Normal Anatomy Versus Pathology
Disruption of the aorto-mitral continuity causing a large dissection plane with disruption of the adventitia at the base of the aorta near the aortic valve annulus extending approximately 6 cm along the left atrial wall. Systolic flow was noted within this space with an exit point approximately 5 cm from the origin and flow continuing into the left atrium (LA) resulting in severe paravalvular native valve mitral regurgitation. The perforation below the aortic leaflet (1) induced blood flow across the aorto-mitral curtain causing dissection of the atrial wall and mitral leaflet (2). Subsequently, the anterior mitral leaflet dehisced allowing significant paravalvular leak (3). LV = left ventricle.
Online Video 1.
TEE ME3D color. 3D color imaging of the mitral valve in the surgeon's view shows flow in the dissection flap.
Online Video 2.
TEE ME AV LAX B W. Mid esophageal long axis aortic valve without color shows aortic leaflet calcification and minimal coaptation. There is a dissection flap in the left atrium which is contiguous with the anterior mitral leaflet.
Online Video 3.
TEE ME AV LAX color. Mid esophageal long axis aortic valve with color shows eccentric aortic insufficiency and native mitral paravalvular leak into the false lumen of the left atrial dissection.
Online Video 4.
|TEE ME AV SAX B W. Mid esophageal short axis without color of aortic valve and left atrium demonstrating a dissection flap in the left atrium with a fenestration.
Online Video 5.
TEE ME Av SAX color. Mid esophageal short axis with color shows flow in the left atrial false lumen with flow across the fenestration into the left atrium.
Online Video 6.
TEE ME LA MV 3D. 3D imaging of the mitral valve in the surgeon's view demonstrates the dissection flap.
Management
The patient was sent to the hospital from clinic after routine outpatient echocardiogram for direct intensive care unit admission. Expedited workup and surgical planning were completed. The patient underwent sternotomy, ascending aortic replacement, aortic valve replacement with a mechanical prosthesis, autologous pericardial patch repair of the aorticomitrial continuity, unroofing and exclusion of the left atrial dissection, and mitral and tricuspid valve repairs (Figures 2 and 3). A perforation in the anterior leaflet of the mitral valve was primarily repaired with a 5-0 Prolene suture in a figure-8 fashion, the valve resuspended and a 30-mm annuloplasty ring was placed (Figures 4 and 5). The tricuspid valve was structurally normal with a dilated annulus and repaired with a 32-mm annuloplasty ring. It appeared that the chronic aortic regurgitation jet lesion on the aorticomitrial continuity became seeded during the initial infection leading to the perforation and left atrial dissection. The dissection allowed for the dehiscence of the anterior leaflet of the mitral valve which led to the severe paravalvular mitral regurgitation and patient’s significant symptoms. The mitral apparatus was otherwise normal and standard repair techniques resulted in a competent valve.
Figure 2.
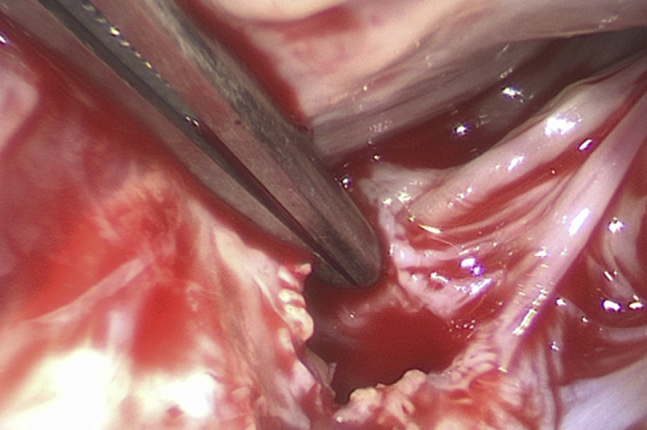
A Subannular Perforation of the Aorticomitral Curtain at the Base of the Anterior Mitral Leaflet
Figure 3.
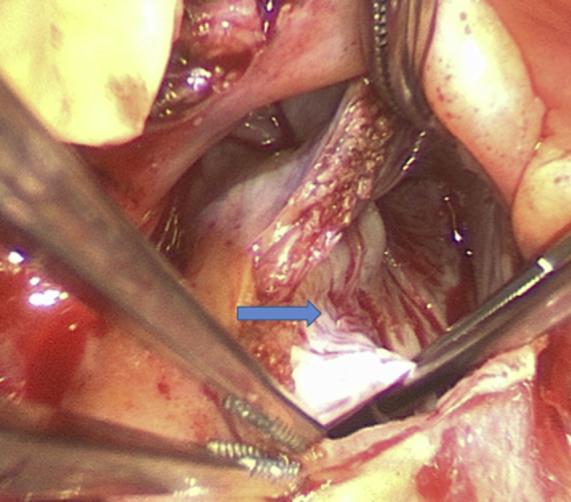
The Dome of the Left Atrium
The arrow indicates the aorticomitral curtain left atrial dissection flap.
Figure 4.
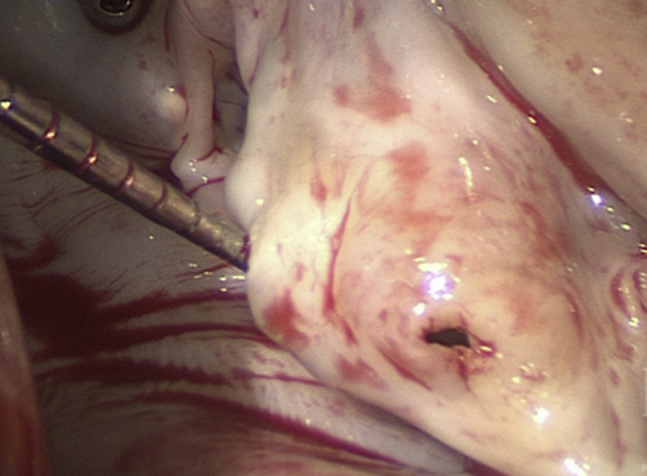
Perforation of the Anterior Mitral Leaflet
Figure 5.
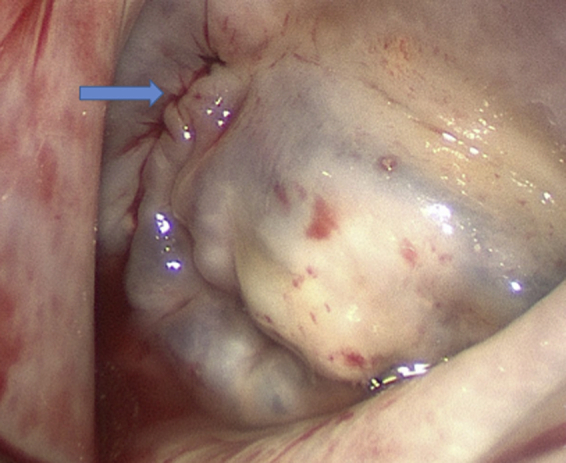
Mitral Valve Saline Test After Leaflet Repair
The arrow indicates the suture line from the aorticomitral curtain patch.
Discussion
From a technical surgical standpoint, there were many considerations in this case. There was no evidence of active endocarditis on the aortic valve leaflets, annulus, or along the aorticomitral continuity. There was calcification on the aorticomitral continuity to suggest healed endocarditis and a possible nidus to allow the left atrial dissection to start. Repair of the aortic valve was considered; however, the right coronary cusp and annulus were calcified and there was concern for the potential durability of repair in this situation. We believe the etiology of left atrial dissection was from the discrete perforation of the aorticomitral continuity just below the left coronary cusp of the aortic valve secondary to chronic aortic insufficiency due to healed endocarditis. The adventitial disruption of the aortic root did not result in a true aortic dissection. The disruption was limited enough to allow for root repair, and root replacement was not necessary. The sinuses of Valsalva were 35 mm, the sinotubular junction was moderately effaced, the ascending aorta was 42 mm and thin-walled, thus we believed that replacement of the ascending aorta was warranted.
There was significant discussion related to the appropriate extent of an operation that would be reasonable for this patient. Despite the fact that the patient has end-stage renal disease and is on hemodialysis, he is a young and vibrant man. We believed that the patient could tolerate addressing all of the cardiac defects. We did consider a simple patch closure of the left atrial dissection that likely would have been reasonable. However, we had concern that a patch on the entry point would create a closed cavity that may have been a risk for becoming infected and that was the reason for the left atrial dissection unroofing. The tricuspid regurgitation was mild in the operating room, but the annulus was 45 mm in diameter and we believed that it should be repaired.
Left atrial dissection is a rare condition usually described as complication of mitral valve surgery, but has been reported as a complication of many cardiac interventions (1). Left atrial dissection has been quoted as a complication of 0.16% of mitral valve operations, most commonly valve replacements and 0.02% of isolated coronary artery bypass grafting operations (2). It is unusual for left atrial dissection to present spontaneously, and in this case it was managed surgically with standard workup and surgical techniques.
Follow-Up
The patient is now 20 months out from surgery. The operation was April 2018, and the last echocardiogram was July 2019 (16 months). This showed a well-seated mechanical aortic valve with mitral annuloplasty ring present. Mean mitral valve gradient 6 mm Hg. There was trace mitral regurgitation. The tricuspid annuloplasty ring was intact with no tricuspid regurgitation and a mean valve gradient of 3 mm Hg. Clinically, he was last seen October 2019, he continues to work, and is doing well.
Conclusions
Native paravalvular mitral regurgitation due to dehiscence of the valve is possible and can be repaired by resuspending the mitral annulus.
Footnotes
The authors have reported that they have no relationships relative to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
References
- 1.Fukuhara S., Dimitrova K.R., Geller C.M., Hoffman D.M., Ko W., Tranbaugh R.F. Left atrial dissection: etiology and treatment. Ann Thorac Surg. 2013;95:1557–1562. doi: 10.1016/j.athoracsur.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 2.Tsukui H., Iwasa S., Yamazaki K. Left atrial dissection. Gen Thorac Cardiovasc Surg. 2015;63:434–445. doi: 10.1007/s11748-015-0562-7. [DOI] [PubMed] [Google Scholar]