Abstract
Transcatheter mitral valve-in-valve replacement (TMVR) offers a less invasive strategy for managing bioprosthetic mitral valve dysfunction. TMVR positioning is challenging in the setting of a radiolucent bioprosthetic sewing ring. We present 2 cases demonstrating the roles of fluoroscopy and echocardiography in guiding TMVR placement within bioprostheses with radiolucent sewing rings. (Level of Difficulty: Intermediate.)
Key Words: bioprosthetic valve dysfunction, Medtronic Mosaic, mitral valve, transcatheter valve replacement
Abbreviations and Acronyms: LV, left ventricle; LVOT, left-ventricular outflow tract; MPR, multiplanar reconstruction; MR, mitral regurgitation; MV, mitral valve; RV, right ventricle; S3, Edwards SAPIEN 3 valve; TEE, transesophageal echocardiography; TMVR, transcatheter mitral valve-in-valve replacement; TTE, transthoracic echocardiography
Graphical abstract
Transcatheter mitral valve-in-valve replacement (TMVR) offers a less invasive strategy for managing bioprosthetic mitral valve dysfunction…
There is a trend toward increasing use of bioprosthetic over mechanical valve replacements in contemporary surgical practice. As the incidence of bioprosthetic valve degeneration is 20% to 30% at 10 years (1), a growing number of patients will experience downstream degeneration, requiring intervention. Reoperative mortality remains high at 23%, and individuals who are older with comorbidities or worse functional class are at particularly elevated risk (1). Transcatheter mitral valve-in-valve replacement (TMVR) is an emerging treatment option for these patients and is increasingly used. A recent registry showed a 94% technical success rate and 14% 1-year mortality (2). However, challenges remain, including the risks of left-ventricular outflow tract (LVOT) obstruction, valve embolization, or need for second valve implantation (2) owing to valve malposition. The TMVR can be positioned relative to the sewing ring or ventricular edge of the bioprosthesis, primarily using fluoroscopic landmarks (1,3), but bioprosthetic valves with radiolucent components present a challenge. We present 2 cases of TMVR in the presence of a radiolucent sewing ring and highlight the imaging considerations in obtaining satisfactory valve position.
Learning Objectives
-
•
TMVR is a less invasive option for patients with bioprosthetic valve dysfunction who are at prohibitive risk of surgical reoperation.
-
•
There is growing experience with TMVR using the Edwards SAPIEN 3 valve. However, bioprostheses with radiolucent sewing rings lack the fluoroscopic landmarks that usually guide valve placement.
-
•
TMVR in a radiolucent bioprosthesis with a radiolucent sewing ring requires thorough understanding of the components of the bioprosthesis and their projections on fluoroscopy. Valve positioning requires combined assessment using fluoroscopy and transesophageal echocardiography to prevent valve malposition: in particular, 2D biplane and 3D multiplanar reconstruction imaging.
Patient #1
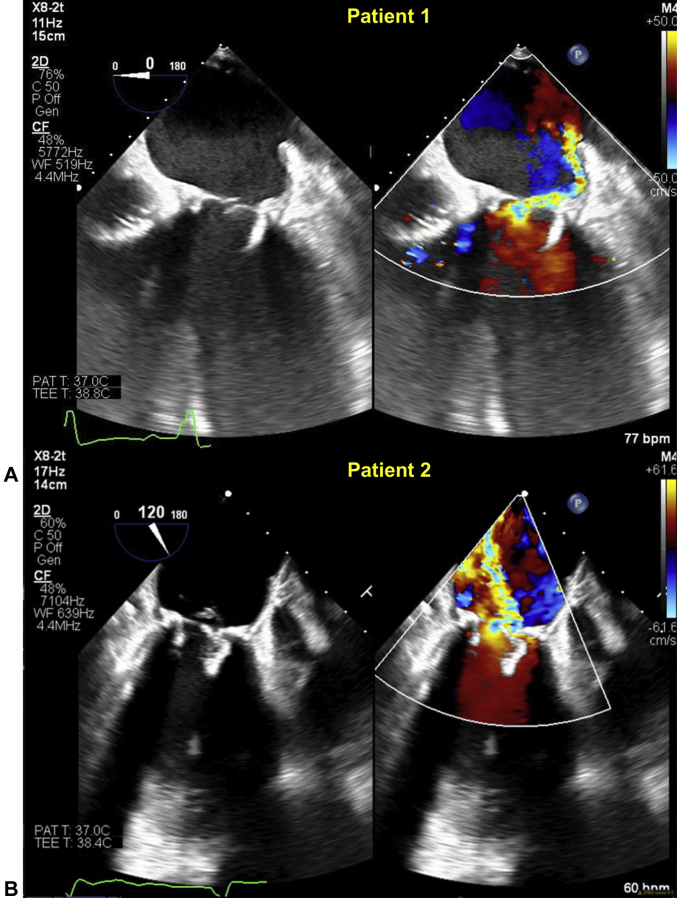
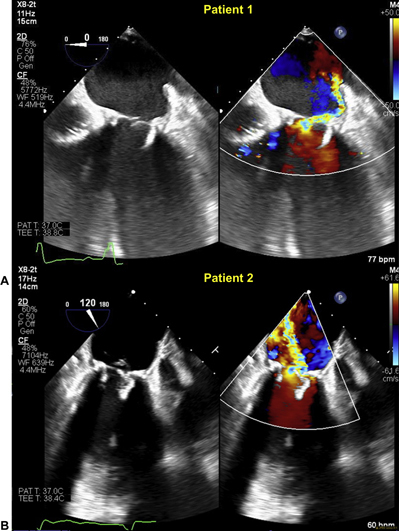
A 61-year-old man presented with 12 months of dyspnea on exertion. He had a history of bioprosthetic aortic and mitral valve (MV) replacements 4 years ago, with a complicated postoperative course. His transthoracic (TTE) and transesophageal (TEE) echocardiogram (Figure 1A) showed severe mitral regurgitation (MR) due to a flail leaflet of a 29 mm Mosaic bioprosthetic valve (Medtronic, Minneapolis, Minnesota), mildly reduced left-ventricular (LV) systolic function, moderately impaired right-ventricular (RV) function, and severe pulmonary hypertension. The heart team deemed him at high risk for reoperation and recommended TMVR.
Figure 1.
Intraprocedural Transesophageal Echocardiography of 2 Patients With Medtronic Mosaic Valves
(A) Patient #1 has a flail bioprosthetic leaflet with severe eccentric mitral regurgitation. (B) Patient #2 has a prolapse and flail bioprosthetic leaflet with severe mitral regurgitation.
Patient #2
A 74-year-old woman presented with 12 months of recurrent heart failure hospitalizations despite optimal medical therapy. She had a history of rheumatic heart disease with a mechanical aortic valve replacement 46 years ago, followed by coronary artery bypass grafting and bioprosthetic aortic and MV replacements 10 years ago, previous stroke, atrial fibrillation, and defibrillator implantation. TTE and TEE demonstrated severe MR due to prolapse and flail leaflets of a 25-mm Mosaic bioprosthetic valve (Figure 1B) with preserved LV and RV function and severe pulmonary hypertension. Bypass grafts were patent. The heart team deemed her at high risk for a third sternotomy and recommended TMVR.
Transcatheter Mitral Valve-in-Valve Replacement
The initial sequence of both procedures was the same. The procedure was performed under general anesthesia. A transvenous pacemaker was placed into the RV. Transseptal puncture was performed under fluoroscopic and TEE guidance (X8-2T, EPIQ CVx, Philips Healthcare, Amsterdam, the Netherlands). A sheath was introduced into the left atrium over a wire, and a pigtail catheter was advanced through the sheath to cross the MV. The transseptal site was balloon dilated and then an Edwards SAPIEN 3 valve (Edwards Lifesciences, Irvine, California) (S3) was advanced into the MV and positioned.
Patient #1
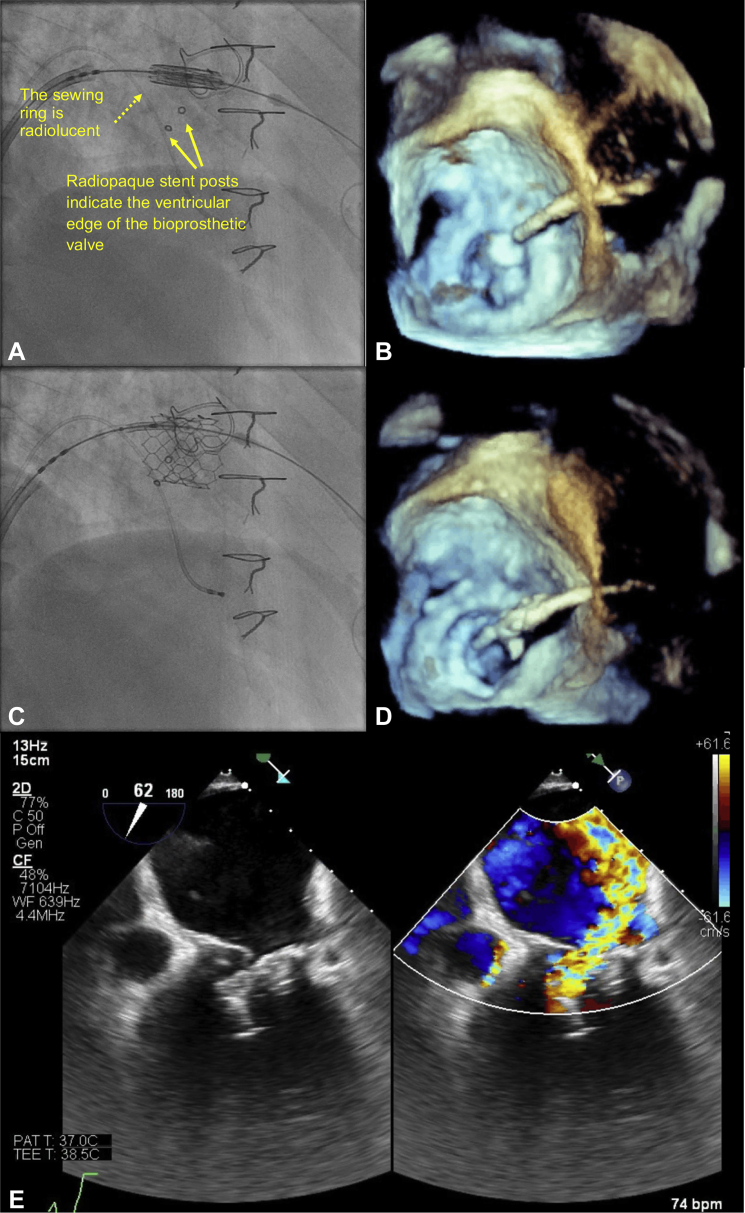
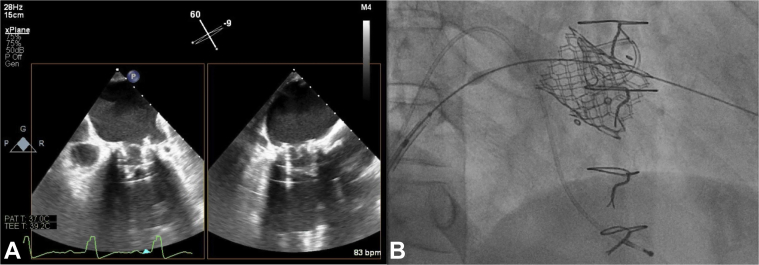
Valve positioning in the mitral bioprosthesis was primarily performed using fluoroscopy: a 26-mm S3 valve was positioned, and the ventricular edge fell just below the ventricular edge of the 3 radiopaque markers pre-deployment (Figure 2A). Pre-deployment TEE, using the 3-dimensional (3D) MV en face view was unable to show that the atrial edge of the S3 valve was not adequately above the sewing ring or that the ventricular edge was below the stent posts (Figure 2B). The S3 valve was deployed under rapid pacing at 180 beats per minute (bpm) with 2 ml of additional contrast (Video 1). Despite slow inflation to allow for adjustment in valve position the valve slipped more ventricularly. Post-deployment fluoroscopy (Figure 2C) and 3D TEE (Figure 2D) suggested possible malposition, but 2-dimensional (2D) biplane imaging confirmed ventricular displacement with noncoaxial alignment (Figure 2E). The patient remained hemodynamically stable, and a second 26-mm S3 valve was immediately implanted atrially to the first S3 valve (Figure 3). After deployment, there was trivial MR, and, despite ventricular displacement, there was no LVOT obstruction. The patient’s post-procedure recovery was uncomplicated, and he remains well at 4-month follow-up, with stable hemodynamics.
Figure 2.
First Valve-in-Valve Implantation in Patient #1
(A) Pre-deployment fluoroscopy shows the ventricular edge of the S3 valve sitting just beyond the radiopaque markers. (B) Pre-deployment transesophageal echocardiography (TEE) 3-dimensional mitral valve en face view is unable to define the location of the atrial edge of the Edwards SAPIEN 3 (S3) valve in relation to the bioprosthetic sewing ring. (C) Post-deployment fluoroscopy shows ventricular displacement of the S3 valve. (D) Post-deployment TEE suggests oblique angulation of the S3 valve with the medial edge not seen. (E) Two-dimensional color-compare imaging confirms malposition with severe mitral regurgitation.
Online Video 1.
Patient 1: deployment of the first 26mm Edwards SAPIEN 3 valve on fluoroscopy.
Figure 3.
Second Valve-in-Valve Implantation in Patient #1
(A) After insertion of a second Edwards SAPIEN 3 (S3) valve, 2 S3 valves in series are seen on transesophageal echocardiography and (B) fluoroscopy.
Patient #2
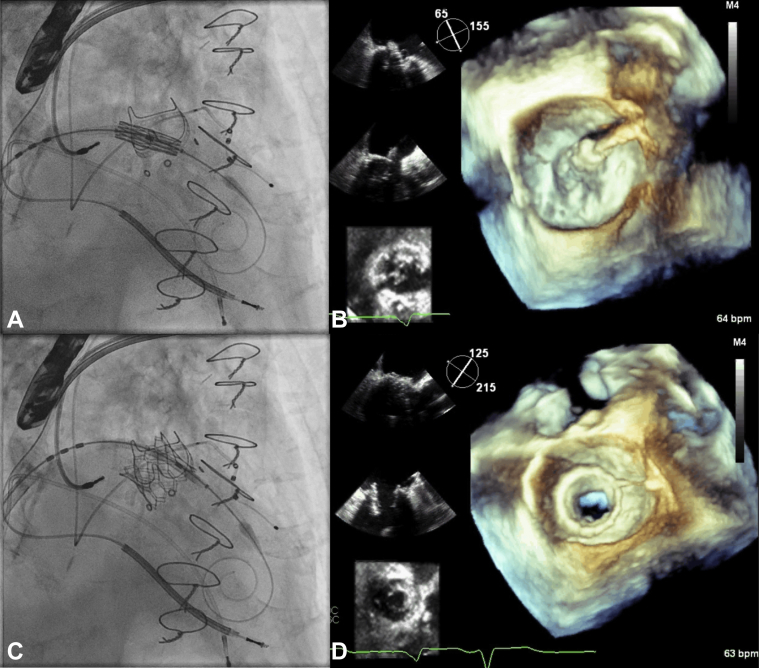
Valve positioning in the mitral bioprosthesis was by both fluoroscopy and TEE: a 23-mm S3 valve was positioned, using fluoroscopy with the ventricular edge of the valve at the atrial edge of the 3 radiopaque markers (Figure 4A). TEE confirmed placement of the atrial edge of the S3 valve 3 mm above the sewing ring using 3D multiplanar reconstruction (MPR) (Figure 4B), allowing inspection of the valve using both 3D and 2D imaging. The valve was deployed under rapid pacing with nominal inflation volume and slow inflation, with good results (Figures 4C and 4D, Video 2). After deployment, there was no MR or LVOT obstruction. The patient recovered well with improvement in functional status.
Figure 4.
Valve-in-Valve Implantation in Patient #2
(A) Pre-deployment the ventricular edge of the Edwards SAPIEN 3 (S3) valve sits at the radiopaque markers. (B) Pre-deployment transesophageal echocardiography (TEE) 3-dimensional imaging with multiplanar reconstruction shows 2-dimensional biplane and mitral valve en face views confirming the atrial edge of the S3 valve to be 3 mm above the sewing ring. (C) Post-deployment the S3 valve is in good position by fluoroscopy and (D) TEE.
Online Video 2.
Patient 2: deployment of the 23mm Edwards SAPIEN 3 valve on fluoroscopy.
Discussion
These 2 cases illustrate the added complexity of TMVR in the presence of bioprosthetic valves with radiolucent sewing rings. Although TEE is routinely used to guide TMVR, fluoroscopy is often sufficient to guide valve placement within a radiopaque bioprosthesis. Some recommend that, in TMVR with an S3 valve, the valve should be placed 3 to 5 mm atrially from the sewing ring of the bioprosthesis (1,4), whereas others suggest that the ventricular edge of the S3 valve should be positioned at the ventricular edge of the bioprosthesis (3,5). The Medtronic Mosaic valve has a radiolucent sewing ring, making the former strategy challenging by fluoroscopy alone and—even for the latter strategy, in which the ventricular edge is denoted by 3 radiopaque markers—focused TEE assistance has proven crucial to confirm optimal positioning. These strategies are generally for valve alignment with nominal volume, and more atrial or ventricular alignment may be required if more or less volume is added (5). However, as in Patient #1, it is also not unusual for implanters to add 2 to 3 ml to plan for flaring of the ventricular aspect of the valve to prevent atrial embolization (3,5).
S3 valve positioning for Patient #2 was optimized by 2 changes that were made after Patient #1. The first was the positioning of the ventricular aspect of the pre-expanded S3 valve in line with the atrial edge of the 3 radiopaque markers on the Mosaic valve. The radiopaque markers define the stent posts and the radiolucent sewing ring is a certain distance (published by Medtronic) atrially above those markers (6). In addition, the atrial edge of the markers denotes the ventricular edge of the Mosaic valve leaflets. For Patient #1, the ventricular edge of the S3 valve was more ventricular than those markers and continued to extend ventricularly with balloon inflation. The second change made for Patient #2 was an emphasis on using 2D and 3D TEE to confirm the position of the atrial edge of the S3 valve relative to the radiolucent sewing ring and not rely solely on the radiopaque markers on fluoroscopy. For Patient #1, the 3D en face view alone was unable define the vertical position of the S3 valve relative to the sewing ring, but for Patient #2, MPR incorporating both 2D biplane and 3D en face views demonstrated this clearly. Thus, by using both fluoroscopy and TEE, both strategies described here for positioning an S3 valve within a Mosaic valve can be concurrently applied to prevent malposition. An additional 4 cases of TMVR in Mosaic valves have been successfully performed using 3D MPR configuration.
Conclusions
TMVR in bioprosthetic valves with radiolucent sewing rings present new challenges and require a comprehensive evaluation of valve position before deployment. In the setting of a radiolucent bioprosthetic sewing ring, both fluoroscopy and TEE are concurrently required for valve positioning. Aligning the ventricular margins of the S3 valve with the atrial edge of the radiopaque stent posts will provide a good approximation of appropriate valve position. This should then be confirmed using TEE imaging, and we suggest that the 3D MPR configuration be used for this purpose.
Footnotes
Dr. Malaisrie is a consultant for Edwards Lifesciences and Medtronic. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
References
- 1.Paradis J.M., Del Trigo M., Puri R., Rodes-Cabau J. Transcatheter valve-in-valve and valve-in-ring for treating aortic and mitral surgical prosthetic dysfunction. J Am Coll Cardiol. 2015;66:2019–2037. doi: 10.1016/j.jacc.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Yoon S.H., Whisenant B.K., Bleiziffer S. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur Heart J. 2019;40:441–451. doi: 10.1093/eurheartj/ehy590. [DOI] [PubMed] [Google Scholar]
- 3.Guerrero M., Salinger M., Pursnani A. Transseptal transcatheter mitral valve-in-valve: A step by step guide from preprocedural planning to postprocedural care. Catheter Cardiovasc Interv. 2018;92:e185–e196. doi: 10.1002/ccd.27128. [DOI] [PubMed] [Google Scholar]
- 4.Cheung A., Webb J.G., Barbanti M. 5-year experience with transcatheter transapical mitral valve-in-valve implantation for bioprosthetic valve dysfunction. J Am Coll Cardiol. 2013;61:1759–1766. doi: 10.1016/j.jacc.2013.01.058. [DOI] [PubMed] [Google Scholar]
- 5.Shivaraju A., Michel J., Frangieh A.H. Transcatheter aortic and mitral valve-in-valve implantation using the edwards sapien 3 heart valve. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007767. e007767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coylewright M., Cabalka A.K., Malouf J.A. Percutaneous mitral valve replacement using a transvenous, transseptal approach: transvenous mitral valve replacement. J Am Coll Cardiol Intv. 2015;8:850–857. doi: 10.1016/j.jcin.2015.01.028. [DOI] [PubMed] [Google Scholar]