Abstract
The presence of a left ventricular (LV) thrombus is considered an absolute contraindication to transcatheter aortic valve implantation (TAVI) because of the very high embolic risk. We report a case of TAVI under complete protection of the cerebral arteries in a patient with a large LV thrombus, severely impaired LV function, and no surgical options. (Level of Difficulty: Beginner.)
Key Words: embolic protection device, left ventricular thrombus, TAVI
Abbreviations and Acronyms: LV, left ventricular; TAVI, transcatheter aortic valve implantation
Graphical abstract
The presence of a left ventricular (LV) thrombus is considered an absolute contraindication to transcatheter aortic valve implantation (TAVI) because of…
Case Presentation
A 56-year-old patient was referred to our hospital for further evaluation of acute severe heart failure secondary to aortic valve stenosis. The patient reported progressive dyspnea (New York Heart Association functional class IV; N-terminal pro–B-type natriuretic peptide, 35,000 ng/l), peripheral edema and fatigue for the past 2 weeks. On presentation, his blood pressure was 90/60 mm Hg, heart rate was 110 beats/min, and central venous saturation was 49%. No significant lactate level (1.6 mmol/l) was measured while the patient was receiving 5 μg/kg/min dobutamine (Interagency Registry for Mechanically Assisted Circulatory Support [INTERMACS] class III). He had pleural effusions on both sides and was not able to lie flat. A list of the patient’s initial laboratory values is given in Supplemental Table 1.
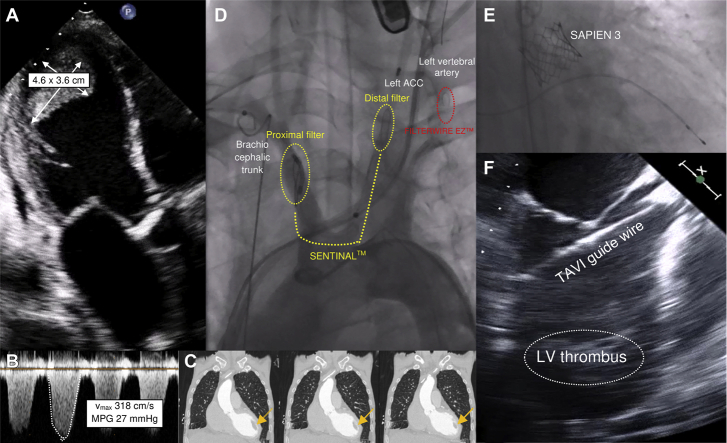
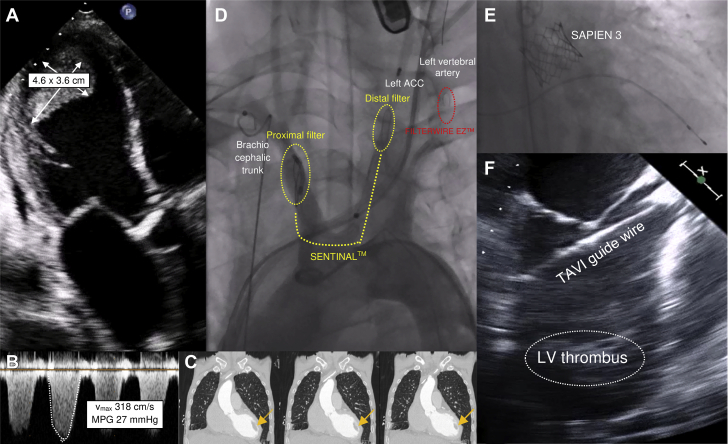
A transthoracic echocardiogram showed a dilated left ventricle (end-diastolic diameter, 59 mm) with severely impaired systolic function (left ventricular [LV] ejection fraction, 22%) and a large apical thrombus (46 mm × 36 mm) (Figures 1A and 1C, Video 1). Right ventricular function was moderately decreased (tricuspid annular plane systolic excursion, 10 mm). Furthermore, the imaging revealed the following: severe low-flow, low-gradient stenosis of a tricuspid aortic valve with a 3-dimensional valve opening area of 0.4 cm2 (maximum aortic velocity, 318 cm/s; mean pressure gradient, 27 mm Hg) (Figures 1A and 1B); a severe tricuspid valve insufficiency (vena contracta, 7 mm; right ventricular end-diastolic ventricular pressure, 30 mm Hg); and moderate mitral valve insufficiency. Repeated 12-lead electrocardiograms and a 24-h Holter electrocardiographic recording could not detect atrial fibrillation or atrial flutter at any time. The coronary arteries showed no relevant alterations.
Figure 1.
TAVI With Large Left Ventricular Thrombus
(A) Severely calcified aortic valve stenosis accompanied by a large left ventricular (LV) thrombus. (B) Aortic valve gradients measured before transcatheter aortic valve implantation (TAVI). (C) Computed tomography scan of the spatial relation of the aorta ascendens, the native aortic valve, the left ventricular outflow tract and the left ventricular thrombus (arrows). (D) Implantation of the protection devices Sentinel (Boston Scientific, Natick, Massachusetts) and FilterWire EZ (Boston Scientific) in the brachiocephalic trunk and the left common carotid artery and left vertebral artery, respectively. (E) Fluoroscopy of the expanded Edwards Sapien 3 prosthesis (Edwards Lifesciences, Irvine, California). (F) Transesophageal visualization of the transcatheter aortic valve implantation guidewire during the procedure. ACC = arteria carotis communis; MPG = mean pressure gradient; Vmax = maximum aortic velocity.
Online Video 1.
Large Left Ventricular Thrombus
Transthoracic echocardiogram, 3-chamber view, showing severe aortic valve stenosis and large a left ventricular thrombus.
In a multidisciplinary heart team consisting of a cardiologist, an interventional cardiologist, and a cardiac surgeon, it was decided to perform transcatheter aortic valve implantation (TAVI) under complete embolic protection of the cerebral arteries because of the patient’s very high surgical risk (European System for Cardiac Operative Risk Evaluation II [EuroSCORE II], 31.60%). First, the SENTINELTM Cerebral Protection System (Boston Scientific Corporation, Marlborough, Massachusetts) was inserted through the right brachial artery and placed in the brachiocephalic trunk and the left common carotid artery. Next, the left common femoral artery was punctured, and the FilterWire EZ Embolic Protection System (Boston Scientific) was placed in front of the left vertebral artery (Figure 1D). The TAVI guidewire (Confida Brecker Guidewire, Medtronic CoreValve, Medtronic, Minneapolis, Minnesota) was inserted through the right common femoral artery and carefully placed within the left ventricle. Transesophageal echocardiography reassured the team that the guidewire had no contact with the thrombus at any time. Afterwards, a Sapien 3 prosthesis (29 mm, Edwards Lifesciences, Irvine, California) (Figures 1E and 1F, Videos 2, 3, and 4) was implanted. A balloon-expandable prosthetic valve was used because of severe aortic valve sclerosis and the patient’s high risk for development of coronary artery disease, including the potential need for cardiac catheterization in the future. A subsequent angiogram showed proper position of the valve without any paravalvular leak. Both protection devices were retrieved and showed minimal embolic debris.
Online Video 2.
TAVI With Large Left Ventricular Thrombus
Modified midesophageal long-axis transesophageal echocardiographic (154°) view showing the transcatheter aortic valve implantation (TAVI) guidewire approaching from the left ventricular outflow tract near the left ventricular thrombus.
Online Video 3.
TAVI With Large Left Ventricular Thrombus
Modified midesophageal long-axis transesophageal echocardiographic (133°) view showing an expanded prosthetic valve during rapid pacing. TAVI = transcatheter aortic valve implantation.
Online Video 4.
TAVI With Large Left Ventricular Thrombus
Modified midesophageal 4-chamber transesophageal echocardiographic (0°) view with visualization of the left ventricular outflow tract, the guidewire for transcatheter aortic valve implantation with an unfolded prosthetic valve, and the left ventricular thrombus. TAVI = transcatheter aortic valve implantation.
In the post-operative intensive care unit setting, neither a cerebrovascular event nor ischemia of the abdominal organs or the lower extremities was noted. At 2-month follow-up, the patient showed significantly improved clinical status (New York Heart Association functional class I to II). The LV ejection fraction increased up to 48%, N-terminal pro–B-type natriuretic peptide fell to 1,451 ng/l, and the LV thrombus decreased more than 75% (Video 5).
Online Video 5.
Post-Operative Imaging
Transthoracic echocardiogram at 2-month follow-up showing an increased left ventricular ejection fraction and a decreased left ventricular thrombus.
This is the first report of complete protection of the cerebral arteries during TAVI in a patient with a large LV thrombus.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
For a supplemental table and videos, please see the online version of this paper.
Appendix
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.