Abstract
Background
Dental caries is one of the most common chronic diseases and is influenced by a complex interplay of genetic and environmental factors. Most previous genetic studies of caries have focused on identifying genes that contribute to dental caries in specific ethnic groups, usually of European descent.
Methods
The aim of this study is to conduct a genome-wide association study (GWAS) to identify associations affecting susceptibility to caries in a large multiethnic population from Argentina, the Philippines, Guatemala, Hungary, and the USA, originally recruited for studies of orofacial clefts (POFC, N = 3686). Ages of the participants ranged from 2 to 12 years for analysis of the primary dentition, and 18–60 years for analysis of the permanent dentition. For each participant, dental caries was assessed by counts of decayed and filled teeth (dft/DFT) and genetic variants (single nucleotide polymorphisms, SNPs) were genotyped or imputed across the entire genome. Caries was analyzed separately for the primary and permanent dentitions, with age, gender, and presence/absence of any type of OFC treated as covariates. Efficient Mixed-Model Association eXpedited (EMMAX) was used to test genetic association, while simultaneously accounting for relatedness and stratification.
Results
We identified several suggestive loci (5 × 10−8 < P < 5 × 10−6) within or near genes with plausible biological roles for dental caries, including a cluster of taste receptor genes (TAS2R38, TAS2R3, TAS2R4, TASR25) on chromosome 7 for the permanent dentition analysis, and DLX3 and DLX4 on chromosome 17 for the primary dentition analysis. Genome-wide significant results were seen with SNPs in the primary dentition only; however, none of the identified genes near these variants have known roles in cariogenesis.
Conclusion
The results of this study warrant further investigation and may lead to a better understanding of cariogenesis in diverse populations, and help to improve dental caries prediction, prevention, and/or treatment in future.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-021-01670-5.
Keywords: Genomics, Genetics, Dental caries, Ethnicity
Background
Dental caries is a common multifactorial disease, in which environment and genetics each play important roles. Dental caries is a global public health problem and if left untreated, can lead to serious complications including pain, infection, abscess and loss of teeth [1] There are at least 2.3 billion people globally affected with caries of the permanent dentition, and more than 530 million children with caries of the primary dentition [2]. The 2010 Global Burden of Disease, Injury and Risk Factors (GBD) study estimated that oral diseases, such as untreated dental caries and severe periodontitis, accounted for 15 million disability-adjusted life-years (DALYs) globally among people older than 60 years old; and untreated dental caries in permanent dentition was the most common condition in the entire 2010 GBD study (global prevalence for all ages is 35%) [3].
The role of environmental risk factors in causing dental caries is very well established, and includes factors such as lack of fluoride, poor oral hygiene habits, and consuming a diet high in sucrose [4, 5]. In addition, disparities in terms of income, educational level and access to dental services in areas such as Appalachia in the United States could be associated with an increased risk of oral diseases, especially dental caries [6]. Moreover, a systematic review study indicated that socioeconomic factors, such as educational level, occupation and income, are highly associated with dental caries among adults [7].
Over the last decade, human genetic studies have made significant progress identifying genetic risk factors associated with dental caries. Previous genome-wide association studies (GWAS) of dental caries, summarized in Table 1, have reported numerous associations. However, these prior studies were limited to a single ethnic group and/or in a single dentition [8–10]. Therefore, the aim of the current GWAS study is to investigate both primary and permanent dentitions in a multiethnic cohort of children and adults.
Table 1.
Genome-wide studies of dental caries
| Study | Sample size | Dental caries phenotype | Genes | Genome-wide significant associations |
|---|---|---|---|---|
| Shaffer et al. 2011 | 1305 white children, age 3–12 | Binary affection status in primary dentation | ACTN2, EDARADD, MPPED2, MTR, and LPO | No |
| Wang et al. 2012 |
7443 whites Adult, age 17–89 |
Permanent decayed, missing, filled surfaces index (DMFS) | RPS6KA2, ISL1, TLR2 RHOU, FZD1, PTK2B, and ADMTS3, | No |
| Shaffer et al. 2013 |
920 whites Adult, ages 18–75 |
Permanent cluster- based partial DMFS | LYZL2, AJAP1 | Yes |
| ABCG2, PKD2, the dentin/bone SCPP sub-family, EDNRA, TJFBR1, NKX2-3, IFT88, TWSG1, IL17D, and SMAD7 | No | |||
| Zeng et al. 2013 |
1017 whites Adult, age 14–56 |
Permanent decayed and filled teeth (dft) stratified to generate df-pitt and fissures (dfPF)and df-smooth surface (dfSM) | BCOR, BCORL1, INHBA, CXCR1 and CXCR2 | No |
| Zeng et al. 2014 | 1006 white children, age 3–14 | Primary decayed and filled teeth (dft) stratified to generate df-pitt and fissures (dfPF)and df-smooth surface (dfSM) | KPNA4, ITGAL, PLUNC family genes | Yes |
| MPPED2, AJAP, and PRS6KA2 | No | |||
| Haworth et al. 2018 |
19,003 Primary analysis; 13,353 Permanent analysis European ancestry meta-analysis age 2.5–18 |
Presence or absence of treated or untreated caries | ALLC, NEDD9 for primary and permanent dentition respectively | Yes |
| Shungin et al. 2019 | GLIDE and UKB (n = 26,792) | DMFS, DFSS, Nteeth | C5orf66, CA12 | Yes |
| KRTCAP2, WNT10A, FGF10, HLA, FOXL1, PBX3, MAMSTR | No |
Methods
Sample
The study sample (N = 3686) comprised individuals recruited as part of a large international study of orofacial clefting. Individuals recruited for this study included affected cases, their unaffected family members, and unrelated healthy controls from the United States (Colorado, Iowa, Pennsylvania, Texas, and Puerto Rico) and internationally (Argentina, the Philippines, and Hungary). All participant provided written informed consent for themselves and for children younger than 18 years, informed consent was obtained from their parents or their legally authorized representative. Local ethical approval was obtained at each site and all methods in this study were performed in accordance with the Institutional Review Board policies and guidelines of the University of Pittsburgh and all of the other sites.
The total sample for the primary dentition analysis included 1116 children (age 2–12 years) and 2570 adults for the permanent dentition analysis (ages 18–60 years). Table 2 summarizes the sex and cleft status breakdown for each analysis. Since our participants came from multiple sites, Table 3 summarizes the distribution of participants for the different recruitment sites.
Table 2.
Basic characteristics of the study cohorts
| Male, n (%) | Female, n (%) | Age, range Mean ± SD |
No cleft | With cleft | dft/DFT, range Mean ± SD |
Total |
|---|---|---|---|---|---|---|
| Primary | ||||||
| 619 (55.5%) | 497 (44.5%) |
2–12 6.9 ± 2.6 |
687 | 429 |
0–20 2.6 ± 3.6 |
1116 |
| Permanent | ||||||
| 1001 (38.9%) | 1569 (61.1%) |
18–60 34.4 ± 9.9 |
2408 | 162 |
0–25 5 ± 4.3 |
2570 |
Table 3.
Distribution of participants across different sites
| Site | Primary |
dft Mean ± SD |
Permanent | DFT Mean ± SD |
|---|---|---|---|---|
| US sites | ||||
| Colorado | 26 | 1.42 ± 1.55 | 32 | 8.12 ± 3.87 |
| Iowa | 225 | 0.65 ± 1.48 | 358 | 3.51 ± 3.56 |
| Pittsburgh | 78 | 1.16 ± 2.54 | 147 | 7.48 ± 5.10 |
| Texas | 130 | 2.55 ± 3.21 | 196 | 4.74 ± 4.69 |
| Puerto Rico | 55 | 2.09 ± 2.80 | 121 | 8.53 ± 4.06 |
| Total | 514 (46.1%) | 1.40 + 2.47 | 854 (33.2%) | 5.36 ± 4.66 |
| Non-U.S sites | ||||
| Hungary | 213 | 2.31 ± 2.94 | 565 | 6.45 ± 4.48 |
| Argentina | 106 | 2.46 ± 3.09 | 246 | 4.32 ± 3.95 |
| Philippines | 159 | 6.35 ± 4.86 | 563 | 4.20 ± 3.71 |
| Guatemala | 124 | 3.76 ± 3.34 | 342 | 3.26 ± 3.67 |
| Total | 478 (53.9%) | 3.70 ± 4.00 | 1374 (66.8%) | 4.78 ± 4.19 |
| Total across all sites | 1116 | 2.6 ± 3.6 | 2570 | 5 ± 4.3 |
Dental caries assessment
The same data collection protocols were used for every site. Dental caries scores (dft/DFT) were assessed by trained dentists or dental hygienists, with data collected either through in-person dental examinations and/or intraoral photos (at least 5 per participant, maxillary and mandibular occlusal, right and left lateral, anterior biting) to appropriately cover the entire oral cavity, as previously reported [11]. The dft/DFT index was calculated as the total number of teeth with decayed, and/or filled/restored surfaces. In addition, questionnaires recording dental history were collected for all participants in the study. We used the dft/DFT index instead of dmft/DMFT due to incomplete information regarding missing teeth for the majority of the participants. Note that for the children, primary dentition caries scores (dft) were obtained only from the primary teeth, any permanent teeth in these children were not included. Table 3 summarizes the dental caries mean scores for the different recruitment sites in analyses of both the primary and permanent dentitions.
All of the training and calibration for the intra-oral photos and the in-person dental exam was completed prior to the start of data collection. Each photo rater (BJH, LMU, and ARV) rated randomly selected participants (n = 15) for calibration twice. Results from ratings by LMU and ARV were calibrated against the gold-standard rater, BJH (note BJH’s intra-rater reliability (kappa) = 0.95)). The Inter-rater reliability (kappa) between all the 3 raters = 0.91–0.93. Data from 158 participants who had both intra-oral photos and in-person dental exams were used to measure the consistency between in-person dental exams and the ratings from intra-oral photos; the results showed excellent agreement (kappa > 90%) between the two methods [11].
Genotyping
Participants were genotyped on the Illumina Human610-Quadv1_B BeadChip (Illumina, Inc., San Diego, CA, USA) and Illumina Infinium II assay protocol by the Center for Inherited Disease Research (CIDR) at Johns Hopkins University. Data cleaning and quality assurance procedures were conducted in conjunction with the CIDR data cleaning center at the University of Washington. 529,285 SNPs (single nucleotide polymorphism) were genotyped, and additional SNPs were imputed based on the 1000 Genomes Project reference panel resulting in 35,153,445 SNPs [12]. Of those, 8,859,951 SNPs passed quality control and analysis filters (participant call rates > 90%; SNP call rates > 99%; Hardy–Weinberg p values > 0.0001; MAF > 2%) and were therefore analyzed in this study.
Statistical analysis
Genetic analyses
Caries scores in the primary dentition (dft) were analyzed separately from caries scores in the permanent dentition (DFT), both as quantitative traits. The analysis of dft in the primary dentition included participants of aged 2–12 years, and the analysis of DFT in the permanent dentition included participants aged 18–60 years. The statistical software used, namely, the Efficient Mixed-Model Association eXpedited (EMMAX) [13] uses a variance component-based method for testing the SNP main effects along with a genetic relationship matrix (GRM) and covariates (in this case, sex, recruitment site, age, age2, and cleft status). Results of modeling of those covariates to determine if they are needed to be included to adjust our primary dft and permanent DFT analyses are shown in Table 4. Adjustment for those covariates were included in the final analyses. The genomic inflation factor (λ), and Manhattan plots were generated using the R v3.4.1 statistical analysis environment (R Core Team, 2019). Given the issue of multiple comparisons, conservative p-value thresholds for genome-wide statistical significance were set to P < 5 × 10−8. The p-value thresholds for suggestive significance were set equal to 5 × 10−8 < P < 1 × 10−6.
Table 4.
Regression results for the covariates
| Phenotype | Covariate | Beta | SE | P |
|---|---|---|---|---|
| Primary dft | Age | 1.69641 | 0.19039 | < 2e−16*** |
| Age2 | − 0.12954 | 0.01357 | < 2e−16*** | |
| Sex | − 0.10053 | 0.19735 | 0.611 | |
| Site | 2.27537 | 0.19744 | < 2e−16*** | |
| Cleft status | 0.80861 | 0.20120 | 6.24e−05*** | |
| Permanent DFT | Age | 0.3832854 | 0.0531329 | 7.14e−13*** |
| Age2 | − 0.0039532 | 0.0007238 | 5.17e−08*** | |
| Sex | 0.6350039 | 0.1723444 | 0.000234*** | |
| Site | 0.3680415 | 0.1865084 | 0.048566* | |
| Cleft status | 1.4312329 | 0.3496957 | 4.39e−05*** |
Significant P values codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05
Bioinformatic approaches for the genetic results
Different bioinformatics and visualization tools were used in this study to interpret the GWAS results at both the SNP and gene level. Loci/genes of interest were identified and reported based on physical proximity of ± 500 kb windows from the lead SNP at each of the loci associated with dental caries. We used LocusZoom [14], to visualize the associations results in the regions around the lead SNPs at each locus. To gain more information regarding the biological consequences of the lead SNPs in the risk loci, variant annotation tools, such as HaploReg, were used to annotate regulatory information, such as enhancer/promoter regions, expression quantitative trait loci (eQTLs), and transcription factor binding sites [15]. Other regulatory databases, such as the Genotype-Tissue Expression (GTEx) [16], and Encyclopedia of DNA Elements [17] were used, too, to help in interpreting the associations results.
Results
Cohort characteristics and covariate modeling
The total study sample comprised 3686 genotyped and phenotyped individuals. The primary dentition cohort (n = 1116) included 616 males (55.5%), and 497 females (44.5%) with an age range of 2–12 years and a mean age of 6.9 years (Table 2), 46.1% of the participants were from U.S recruitment sites, and 53.1% from international sites. The permanent dentition cohort (n = 2570) included 1001 males (38.9%), and 1569 females (61.1%) with an age range of 18–60 years and a mean age of 34.4 years (Table 2), with 33.2% of the participants from U.S recruitment sites, and 66.8% of participants from international sites. General linear regression analysis (details in Table 4) showed that age, recruitment site, cleft status (affected vs. unaffected with cleft) and sex had an impact on the DFT and dft mean scores; therefore, these factors were included as covariates in the genetic analyses.
Genetic results
Manhattan plots illustrating the GWAS results for the primary dft and permanent DFT are shown in Fig. 1a, c. The genomic inflation factor (λ) for the primary dentition analysis was 0.98, and for the permanent dentition analysis was 0.99, indicating no inflation of p values (Fig. 1b, d). Note that associations identified under the GWAS approach do not specify which SNP at that locus is the causal SNP (cause the association) or which gene is affected by the causal SNP. Here we report any known biological functions of genes near our significant and suggestive results that have plausible roles in dental caries or have some biological relevance to tooth development and/or oral health.
Fig. 1.
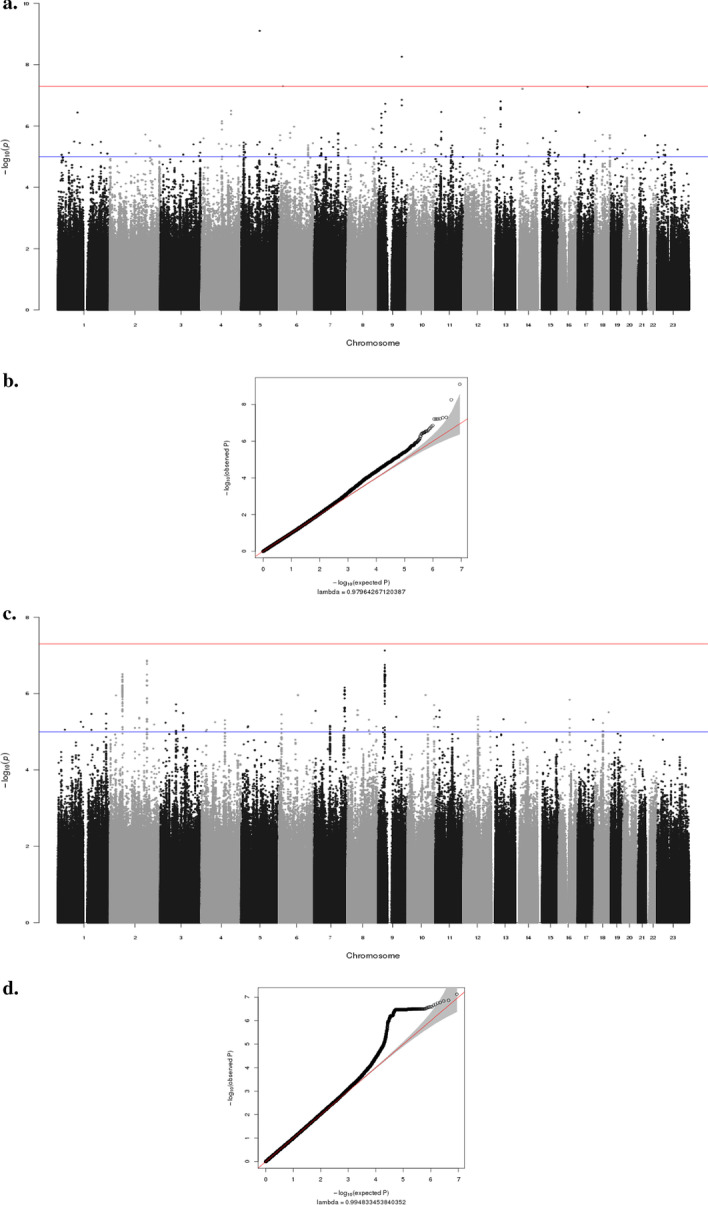
Manhattan plots and (Q-Q) plots showing GWAS results for the analyses. Red lines represent thresholds for genome-wide significance (p value < 5 × 10−8). Blue lines represent thresholds for suggestive significance (p value < 5 × 10−6). a Manhattan plots for primary dft show negative log10-transformed p values (y-axis) across the whole genome (x-axis). Genotyped and imputed SNPs are plotted together. b The quantile–quantile plot (Q-Q) for GWAS of Primary dft. The genomic inflation factor (λ) is 0.98. c Manhattan plots for permanent DFT show negative log10-transformed p values (y-axis) across the whole genome (x-axis). Genotyped and imputed SNPs are plotted together. d The quantile–quantile plot (Q–Q) for GWAS of permanent DFT. The genomic inflation factor (λ) is 0.99
Primary dentition
For the primary dft, SNPs at multiple loci exceeded the threshold for genome-wide significance (P < 5 × 10−8) and others exhibited suggestive significance (5 × 10−8 < P < 1 × 10−6). The biological roles of potential candidate genes at these loci are described in Table 5. Genes at two of the genome-wide significant loci have no known role in cariogenesis and warrant further investigation. One of the lead SNPs (rs113021760, P = 3.36 × 10−8), is located in the intron of the SUSD1 gene (Sushi Domain Containing 1), so it might play a regulatory role. In addition, this SNP shows enhancer chromatin marks in different tissues, including osteoblasts, which are found in the periodontal ligament and alveolar bone in the oral cavity.
Table 5.
Significant and suggestive SNPs across the analyses
| Lead SNP | Chr band | BP | Beta | P | Nearby Gene(s) | dbSNP Annotation | Biological Role* |
|---|---|---|---|---|---|---|---|
| Primary analysis | |||||||
| rs80177293 | 5q14.3 | 88,865,345 | − 2.91 | 8.23e−10 | MIR3660 | Non-coding | Essential for post- transcriptional regulation of gene expression in different organism [Hansen, et al. 2010] |
| rs113021760 | 9p23 | 114,805,385 | − 2.67 | 3.36e−09 |
SUSD1 UGCG |
Intronic |
A protein coding gene that has been associated with Venous thromboembolism (VTE), a cardiovascular disease [Tang et al. 2013] Involved in the biosynthesis of glycosphingolipid (Ichikawa, et al. 1996) |
| rs75833698 | 6p22.3 | 19,866,520 | − 2.17 | 4.93e−08 | ID4 | Non-coding | Inhibitor of DNA binding 4 has been, regulate different cellular processes [Benezra, et al. 2001] |
| rs16948495 | 17q21.33 | 48,005,748 | − 2.15 | 5.16e−08 |
DLX3 DLX4 |
Non-coding |
Mutations in this gene have been associated with tricho-dento-osseous syndrome (TDO), which cause enamel hypoplasia [Y.li et al. 2015] Play a role in forebrain and craniofacial development. Mutations cause non-syndromic orofacial cleft [Wu et al. 2015] |
| rs75459295 | 14q13.1 | 34,767,100 | − 2.26 | 6.08e−08 | SPTSSA | Non-coding | Involved in sphingolipid biosynthesis (Han, et al. 2009) |
| chr9:35,753,170 | 9p13.3 | 35,753,170 | − 2.27 | 1.89e−07 |
CA9 TLN1 |
Non-coding |
Participates in a variety of biological processes, including regulation of pH, and can be found in gastrointestinal mucosa [Yang et al. 2017] Plays a role migration of various cell types, including fibroblasts and osteoclasts [Monkley et al. 2001, Zou et al. 2013] |
| Permanent analysis | |||||||
| rs17226825 | 9p21.1 | 33,010,337 | − 1.42 | 7.47e−08 |
APTAX NFX1 |
Non-coding |
Repair of DNA damage in cells [Sano et al. 2004] Regulating the duration of an inflammatory response [Yamashita et al. 2016] |
| rs6708025 | 2p24.2 | 178,577,642 | − 3.00 | 1.36e−07 | PDE11A | Intronic | Messenger in different signal transduction pathways [Fawcett et al. 2000] |
| rs11686767 | 2p16.1 | 60,990,128 | − 0.63 | 3.09e−07 |
PAPOLG REL |
Intronic |
Catalyzing template-independent extension of a DNA/RNA strand [Topalian et al. 2001] Regulate inflammation, immune response & apoptosis [Shono et al. 2014] |
| rs288547958 | 7q35 | 145,203,344 | − 176 | 6.93e−07 | CNTNAP2 | Non-coding | Role in neuronal migration, dendritic arborisation and synaptic transmission [Anderson et al. 2012] |
| rs73753796 | 6q15 | 92,190,076 | − 2.91 | 1.079e−06 | MIR4643 | Non-coding | Involved in post-transcriptional regulation of gene expression [Ghanbari et al. 2015] |
| rs111979811 | 7q34 | 141,658,200 | − 2.07 | 1.11e−06 |
TAS2R38, TAS2R3, TAS2R4, TASR25 OR9A4 |
Non-coding |
Linked to loci influence bitter perception. Significant association for caries risk [Wendell et al. 2010] Activate the neural response that initiate the perception of smell [Niimura, 2012] |
*Although no known role of some of the presented genes in cariogenesis and/or odontogenesis, further investigations is needed to discover if some of these genes could have possible role in dental caries and/or odontogenesis
One of the strongest suggestive association signals for the primary dft was at 17q21.33 (rs16948495, P = 5.16 × 10−8) which is less than 100 kb downstream from DLX3 and upstream from DLX4 (see Fig. 2a). These genes belong to the Distal-less homeobox family, known to be expressed in cranial neural crest cells and in the craniofacial mesenchyme; and important in tooth development [18–20]. DLX3, which plays an essential role in skeletal formation and development, is also required for tooth morphogenesis [21, 22]. Furthermore, a recent study also showed that DLX3 plays an important role in dentinogenesis. Mutations in this gene cause tricho-dento-osseous (TDO) syndrome, a rare syndrome that affects the teeth and bones, including enamel hypoplasia and dentin hypoplasia [23]. These severe outcomes of DLX3 mutations emphasize the important role DLX3 plays in the development of bones and teeth.
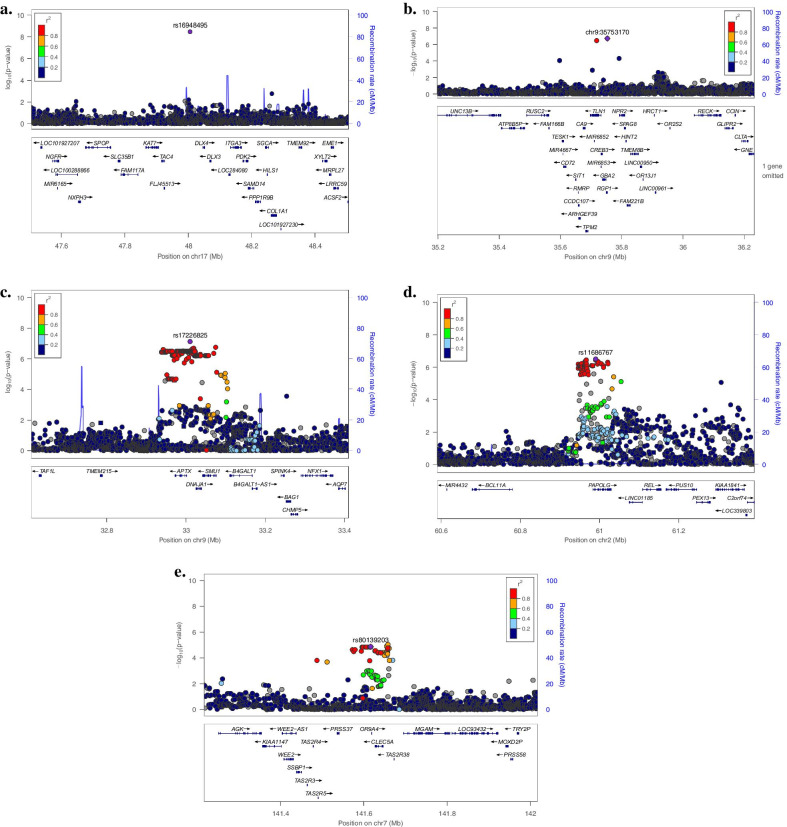
Fig. 2.
LocusZoom of regions of Interest. Panels a, b and c are association results from the primary dentition GWAS, panels d and e are from the permanent dentition GWAS. a suggestive locus near DLX3 and DLX4 genes on chromosome 17. b suggestive locus near TLN1 and CA9 on chromosome 9. c suggestive locus near NFX1 gene on chromosome 9. d suggestive locus near REL gene on chromosome 2. e suggestive locus near Taste receptor genes (TAS2R38, TAS2R3, TAS2R4, TASR25) on chromosome 7. The genome build used for the recombination rate was based on 1000 Genomes November 2014 EUR data. All of the gene positions and directions of transcription are annotated on the plots
DLX4 is highly expressed in human dental pulp cells (DPCs) and is associated with abnormal human tooth formation [24]. DLX4 also plays an important role in forebrain and craniofacial and development. A mutation in DLX4 was reported to cause a non-syndromic form of cleft lip and palate [25]. Rs16948495 shows enhancer chromatin marks in osteoblasts. These lines of evidence suggest that both DLX3 and DLX4 may play important roles in the dental caries process by the regulation tooth development [21–23, 25].
There was also a strong suggestive association signal for primary dft and a SNP at 9p13.3 (chr9:35,753,170, P = 1.89 × 10−7, Fig. 2b). The gene CA9 (CA IX) is located less than 200 kb upstream from the lead SNP at this locus: CA9 encodes a family of enzymes with at least 14 different isoforms that participate in several biological processes including the secretion of saliva and pH homeostasis [26]. In candidate gene studies, a different member of the CA family (CA VI) was investigated due to its role in saliva secretion and regulating salivary pH and was found to be significantly associated with dental caries in children [27–29]. When we looked-up CA6 gene in our association results, we did find a SNP (rs58579969), 300 kb downstream from it. However, this SNP only showed nominal evidence of an association with primary dft (P = 1.2 × 10−3).
Another gene at the 9p13.3 locus is TLN1; a cytoskeletal protein that has been implicated in several biological functions, including migration of fibroblasts and osteoclasts [30, 31]. The expression of tln1 in zebrafish was observed in the craniofacial cartilage structures, including the palate. Tln1 mutant zebrafish had malformation in their palate and craniofacial muscles, which indicate the possible role of tln1 in craniofacial morphogenesis [32]. Neither TLN1 nor CA9 genes have been implicated in dental caries before, however, their plausible roles in craniofacial morphogenesis and pH regulation could suggest that they may influence cariogenesis.
Permanent dentition
In the permanent dentition analysis, there were no SNPs meeting the genome-wide significance level, but there were results with multiple loci that were within the range for the genome-wide suggestive level (5 × 10−8 < P < 1 × 10−6). Summaries of the lead SNPs and nearby genes from the permanent analysis are presented in Table 5.
One of the strongest suggestive association signals for the permanent DFT was at the 9p21.1 locus (rs17226825, P = 7.47 × 10−8) which is located within APTAX (Fig. 2c), and the encoded protein of this gene is involved in the repair of DNA damage in cells [33]; however, no role is currently known for this gene in odontogenesis or dental caries. The lead SNP is a strong eQTL (expression quantitative trait locus) for APTAX (per an (eQTL) database [34]. It is also a strong eQTL for other genes (B4GALT1, SMU1, RP11-54K16.2) [16]. The lead SNP is also approximately 300 kb upstream from the NFX1 gene, which plays an important role in regulating the duration of inflammatory responses [35].
Another suggestive association signal at 2p16.1 (rs11686767, P = 3.11 × 10−8), is intronic to PAPOLG (Fig. 2d) which plays a role in catalyzing template-independent extension of a DNA/RNA strand [36] The SNP is a strong eQTL for PAPOLG [16], however, no known role yet of this gene in odontogenesis or dental caries. Finally, rs11686767 was also 100 kb upstream to REL gene. The protein encoded by this gene, proto-oncogene c-Rel, is involved in many important cellular processes such as apoptosis, inflammation, and the immune response [37]. Those genes; NFX1 and REL could contribute to dental caries by influencing host susceptibility to oral microorganism.
An interesting suggestive association for the permanent dentition DFT was at 7q34 (lead SNP rs11197981; P = 1.11 × 10−6), approximately 200 kb downstream of taste receptor genes (TAS2R38, TAS2R3, TAS2R4, TASR25) (Fig. 2e), which have plausible roles in dental caries. Specifically, TAS2R38 as has been associated with dental caries in previous studies [38–41]. Further, taste receptor genes, specifically TAS2R38, influence bitter perception and dietary habits that are hypothesized to lead to consuming more sweets in the diet and thus leading to dental caries. The lead SNP at this locus is also within the OR9A4 gene, which is responsible for activating the neural response that initiates the perception of smell [42].
To further investigate these significant or suggestive SNPs, we used the FUMA platform (Functional Mapping and Annotation of Genome-Wide Association Studies) [43] to prioritize, annotate, and interpret the genomic variants and genes from the GWAS results of both primary and permanent analyses. Gene-based testing using GWAS results was computed by MAGMA (Multi-marker Analysis of GenoMic Annotation) using the default settings implemented in FUMA. After mapping the top SNPs from the GWAS analyses to 19,182 protein coding genes, the genome-wide significance level was calculated based on the number of tested genes and it was set at 0.05/19182 = 2.61 × 10−6. However, none of the genes in both analyses reached the genome wide significance level. FUMA results of the risk genomic loci for both analyses are presented in the Additional file 1: Tables S1 and S2.
Discussion
Dental caries is one of the most common chronic diseases that affects both children and adults, and is influenced by a complex interplay of diet, bacteria, salivary flow, genetic factors and other environmental factors. Untreated dental caries could lead to tooth loss which can negatively impact the oral health quality of life (OHRQoL) because it affects function, aesthetics and satisfaction. OHRQoL is a multidimensional concept that includes the impact of dental health on the individual’s functional well-being, emotional health, satisfaction, and self-esteem. OHRQoL is very important because of its usefulness in addressing the impact of oral health disparities and access to care on overall health and quality of life to communicate it with policymakers to improve oral health by increasing access to care [44, 45].
There are now a number of effective preventive methods for dental caries (good oral hygiene, balanced diet, regular dental care) [46, 47]. Most notably, stannous fluoride, such as in toothpaste and water, has been an important public health advance by reducing the prevalence of dental caries [48]. However, dental caries is still wide spread worldwide [3], requiring additional approaches to understanding risk factors for dental caries.
The current study took a genome-wide approach to investigate genetic factors contributing to dental caries in the primary and permanent dentitions. Several previous genetic studies have investigated and identified the role of different genes in cariogenesis. Most of these past studies involved samples that were ethnically homogeneous, mainly of European descent [49–53], unlike the current multiethnic study (see also Table 1).
Notably, results in the primary dentition nominated a few SNPs that showed association at genome-wide significance, and multiple loci that showed suggestive evidence for association. In the permanent dentition, no variants reached genome-wide significance, but several reached suggestive significances. Importantly, none of the top associated SNPs in either of the analyses (dft and DFT) were replicated in the other one, full results are presented in the Additional file 1: Tables S3 and S4. This confirms that genetic loci that influence the susceptibility to dental caries differ between primary and permanent dentitions [54]. Although none of the genome-wide significant association signals were near genes with known roles in dental caries or tooth development, some of the suggestive signals were near genes with potential roles in dental caries.
Some of the new associations identified in this study were in or near genes that have important roles in the inflammatory process, such as NFX1 and REL genes (permanent dentition). Those genes could contribute to dental caries by influencing host susceptibility to oral microorganism. This study also identified association signals near genes that have a confirmed role in tooth and craniofacial development, such as TLN1 and DLX genes. Heterozygous variants in DLX4 have been implicated before in Orofacial clefting [25]. This could indicate that both dental caries and OFC share common genetic risk loci. This is plausible because some of the genes that had been previously implicated in dental caries and OFCs are craniofacial development genes. PKD2 gene is one of the genes that has been associated with dental caries and had an implication in craniofacial development [8] and mutation in PKD2 has been linked to craniofacial anomalies and tooth loose in mice [55]. Thus, our future investigations will focus on exploring the relationship between dental caries and OFCs on both phenotypic and genetic levels.
This study also supported the role of taste receptor genes in dental caries (TAS2R38, TAS2R3, TAS2R4, TASR25, see Table 5), which have been associated with dental caries in previous studies [38–41]. On the other hand, several loci/genes associated with dental caries from previous large meta-GWAS studies of dental caries [50, 53] were reviewed in our results (the lead SNPs ± 500 kb, a total of 5935 SNPs), but none of these variants reached statistical significance (see Additional file 1: Tables S5, S6 for details).
In the permanent dentition subset of the current study population, females had a significantly higher rate of dental caries than males (P = 0.000234) consistent with earlier studies [56, 57]. However, there was no gender difference in the primary dentition. Possible explanations for the increased rates of dental caries among adult females include (i) the fact that teeth tend to erupt earlier in females, which allows for a longer exposure to a cariogenic oral environment, and (ii) hormonal changes during pregnancy affect saliva pH leading to a decrease in the concentration of calcium, phosphorus, magnesium, and chloride, which, in turn might lead to increased caries. (iii) other behavioral/environmental factors during pregnancy such as consuming a diet high in sucrose and poor oral hygiene could also increase the risk of developing more dental caries.
Conclusion
In summary, this study found multiple associations near genes that may play important roles in dental caries, such as TAS2R38, TAS2R3, TAS2R4, TASR25, DLX3 and DLX4, several of which have plausible biological functions relevant to tooth development and cariogenesis. These findings contribute to our understanding of the genetic mechanisms of cariogenesis, providing targets for follow-up translational studies that will eventually improve prevention and treatment of this highly prevalent chronic disease worldwide.
Supplementary Information
Additional file 1. Supplementary material.
Acknowledgements
Many thanks to the participating families world-wide who made this project possible. The tireless efforts by the dedicated field staff and collaborators was similarly essential to the success of this study. Special thanks to Margaret Cooper, Judith Resick, Dr. Eduardo Castilla and Dr. Andrew Czeizel.
Abbreviations
- dft
Number of decayed or filled primary tooth
- DFT
Number of decayed or filled permanent teeth
- GWAS
Genome-wide association study
- MAF
Minor allele frequency
- SNP
Single nucleotide polymorphism
Authors' contributions
RNA generated the data set for the analyses. RNA, JMC analyzed data. RNA, MLM, JMC, JRS, SMW helped define outcomes to be studied. RNA wrote the first draft of the manuscript. MLM, JRS, SMW, BJH, LMU, NM, and KN critically reviewed the manuscript. RNA & MLM designed the study. RNA interpreted data. MLM, CS, FWBD, KN, CP, FAP, IMO, CJB, JTH, GLW, SMW, ARV, LMU, REL collected and interpreted data. RNA & MLM generated the final draft of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Institutes of Health (NIH) including: R01-DE016148 [MLM, SMW], X01-HG007485 [MLM, EF], U01-DE024425 [MLM], R37-DE008559 [JCM, MLM], R21-DE016930 [MLM], R01-DE012472 [MLM], R01-DE011931 [JTH], U01-DD000295 [GLW], R00-DE024571 [CJB], S21-MD001830 [CJB], U54-MD007587 [CJB], R56-DE027055 [JRS]. Genotyping and data cleaning were provided via an NIH/NIDCR contract to the Johns Hopkins Center for Inherited Disease Research: HHSN268201200008I. Additional support provided by an intramural grant from the Research Institute of the Children's Hospital of Colorado [FWD]; additional operating costs support in the Philippines was provided by the Institute of Human Genetics, National Institutes of Health, University of the Philippines, Manila [CP].
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the dbGaP repository, https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000774.v2.p1
Declarations
Ethics approval and consent to participate
Study approval was granted by the University of Pittsburgh Institutional Review Board (coordinating center approval # CR19030367-003, Pittsburgh site approval # CR19080127-00). All participant provided written informed consent for themselves and for children younger than 18 years, informed consent was obtained from their parents or their legally authorized representative. Local ethical approval was obtained at each site and all methods in this study were performed in accordance with the Institutional Review Board policies and guidelines of the University of Pittsburgh and all of the other sites.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Leal SC, Bronkhorst EM, Fan M, Frencken JE. Untreated cavitated dentine lesions: impact on children's quality of life. Caries Res. 2012;46(2):102–106. doi: 10.1159/000336387. [DOI] [PubMed] [Google Scholar]
- 2.Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcenes W, Kassebaum NJ, Bernabe E, Flaxman A, Naghavi M, Lopez A, Murray CJ. Global burden of oral conditions in 1990–2010: a systematic analysis. J Dent Res. 2013;92(7):592–597. doi: 10.1177/0022034513490168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahluwalia M, Brailsford SR, Tarelli E, Gilbert SC, Clark DT, Barnard K, Beighton D. Dental caries, oral hygiene, and oral clearance in children with craniofacial disorders. J Dent Res. 2004;83:175–179. doi: 10.1177/154405910408300218. [DOI] [PubMed] [Google Scholar]
- 5.Shaffer JR, Wang X, DeSensi RS, Wendell S, Weyant RJ, Cuenco KT, Crout R, McNeil DW, Marazita ML. Genetic susceptibility to dental caries on pit and fissure and smooth surfaces. Caries Res. 2012;46:38–46. doi: 10.1159/000335099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin B, Wilkerson AH, Patterson G, Nahar VK, Sharma M. Need for addressing oral health disparities in rural Appalachia. Health Promot Perspect. 2017;7(4):178–180. doi: 10.15171/hpp.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa SM, Martins CC, Bonfim Mde L, Zina LG, Paiva SM, Pordeus IA, Abreu MH. A systematic review of socioeconomic indicators and dental caries in adults. Int J Environ Res Public Health. 2012;9(10):3540–3574. doi: 10.3390/ijerph9103540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaffer JR, Feingold E, Wang X, Lee M, Tcuenco K, Weeks DE, Weyant RJ, Crout R, McNeil DW, Marazita ML. GWAS of dental caries patterns in the permanent dentition. J Dent Res. 2013;92(1):38–44. doi: 10.1177/0022034512463579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaffer JR, Polk DE, Feingold E, Wang X, Cuenco KT, Weeks DE, DeSensi RS, Weyant RJ, Crout R, McNeil DW, et al. Demographic, socioeconomic, and behavioral factors affecting patterns of tooth decay in the permanent dentition: principal components and factor analyses. Community Dent Oral Epidemiol. 2013;41(4):364–373. doi: 10.1111/cdoe.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaffer JR, Wang X, Feingold E, Lee M, Begum F, Weeks DE, Cuenco KT, Barmada MM, Wendell SK, Crosslin DR, et al. Genome-wide association scan for childhood caries implicates novel genes. J Dent Res. 2011;90:1457–1462. doi: 10.1177/0022034511422910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howe BJ, Cooper ME, Wehby GL, Resick JM, Nidey NL, Valencia-Ramirez LC, Lopez-Palacio AM, Rivera D, Vieira AR, Weinberg SM, et al. Dental decay phenotype in nonsyndromic orofacial clefting. J Dent Res. 2017;96:1106–1114. doi: 10.1177/0022034517709961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leslie EJ, Carlson JC, Shaffer JR, Feingold E, Wehby G, Laurie CA, Jain D, Laurie CC, Doheny KF, McHenry T, et al. A multi-ethnic genome-wide association study identifies novel loci for non-syndromic cleft lip with or without cleft palate on 2p24.2, 17q23 and 19q13. Hum Mol Genet. 2016;25(13):2862–72. [DOI] [PMC free article] [PubMed]
- 13.Zhou X, Stephens M. Efficient multivariate linear mixed model algorithms for genome-wide association studies. Nat Methods. 2014;11:407–409. doi: 10.1038/nmeth.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, Boehnke M, Abecasis GR, Willer CJ. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26(18):2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40(Database issue):D930–934. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Consortium GT Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348(6235):648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Consortium EP An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127(8):1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- 19.Duverger O, Zah A, Isaac J, Sun HW, Bartels AK, Lian JB, Berdal A, Hwang J, Morasso MI. Neural crest deletion of Dlx3 leads to major dentin defects through down-regulation of Dspp. J Biol Chem. 2012;287(15):12230–12240. doi: 10.1074/jbc.M111.326900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas BL, Tucker AS, Qui M, Ferguson CA, Hardcastle Z, Rubenstein JL, Sharpe PT. Role of Dlx-1 and Dlx-2 genes in patterning of the murine dentition. Development. 1997;124(23):4811–4818. doi: 10.1242/dev.124.23.4811. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Z, Stock D, Buchanan A, Weiss K. Expression of Dlx genes during the development of the murine dentition. Dev Genes Evol. 2000;210(5):270–275. doi: 10.1007/s004270050314. [DOI] [PubMed] [Google Scholar]
- 22.Isaac J, Erthal J, Gordon J, Duverger O, Sun HW, Lichtler AC, Stein GS, Lian JB, Morasso MI. DLX3 regulates bone mass by targeting genes supporting osteoblast differentiation and mineral homeostasis in vivo. Cell Death Differ. 2014;21(9):1365–1376. doi: 10.1038/cdd.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Han D, Zhang H, Liu H, Wong S, Zhao N, Qiu L, Feng H. Morphological analyses and a novel de novo DLX3 mutation associated with tricho-dento-osseous syndrome in a Chinese family. Eur J Oral Sci. 2015;123(4):228–234. doi: 10.1111/eos.12197. [DOI] [PubMed] [Google Scholar]
- 24.Tamaoki N, Takahashi K, Aoki H, Iida K, Kawaguchi T, Hatakeyama D, Inden M, Chosa N, Ishisaki A, Kunisada T, et al. The homeobox gene DLX4 promotes generation of human induced pluripotent stem cells. Sci Rep. 2014;4:7283–7283. doi: 10.1038/srep07283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu D, Mandal S, Choi A, Anderson A, Prochazkova M, Perry H, Gil-Da-Silva-Lopes VL, Lao R, Wan E, Tang PL, et al. DLX4 is associated with orofacial clefting and abnormal jaw development. Hum Mol Genet. 2015;24(15):4340–4352. doi: 10.1093/hmg/ddv167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Review article. J Enzyme Inhibit Med Chem. 2004;19(3):199–229. doi: 10.1080/14756360410001689540. [DOI] [PubMed] [Google Scholar]
- 27.Frasseto F, Parisotto TM, Peres RC, Marques MR, Line SR. Nobre Dos Santos M: Relationship among salivary carbonic anhydrase VI activity and flow rate, biofilm pH and caries in primary dentition. Caries Res. 2012;46(3):194–200. doi: 10.1159/000337275. [DOI] [PubMed] [Google Scholar]
- 28.Peres RC, Camargo G, Mofatto LS, Cortellazzi KL, Santos MC, Nobre-dos-Santos M, Bergamaschi CC, Line SR. Association of polymorphisms in the carbonic anhydrase 6 gene with salivary buffer capacity, dental plaque pH, and caries index in children aged 7–9 years. Pharmacogenomics J. 2010;10(2):114–119. doi: 10.1038/tpj.2009.37. [DOI] [PubMed] [Google Scholar]
- 29.Picco DCR, Lopes LM, Rocha Marques M, Line SRP, Parisotto TM. Nobre dos Santos M: Children with a higher activity of carbonic anhydrase VI in saliva are more likely to develop dental caries. Caries Res. 2017;51(4):394–401. doi: 10.1159/000470849. [DOI] [PubMed] [Google Scholar]
- 30.Monkley SJ, Pritchard CA, Critchley DR. Analysis of the mammalian talin2 gene TLN2. Biochem Biophys Res Commun. 2001;286(5):880–885. doi: 10.1006/bbrc.2001.5497. [DOI] [PubMed] [Google Scholar]
- 31.Zou W, Izawa T, Zhu T, Chappel J, Otero K, Monkley SJ, Critchley DR, Petrich BG, Morozov A, Ginsberg MH, et al. Talin1 and Rap1 are critical for osteoclast function. Mol Cellul Biol. 2013;33(4):830–844. doi: 10.1128/MCB.00790-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishii K, Mukherjee K, Okada T, Liao EC. Genetic requirement of talin1 for proliferation of cranial neural crest cells during palate development. Plast Reconstr Surg Glob Open. 2018;6(3):e1633. doi: 10.1097/GOX.0000000000001633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sano Y, Date H, Igarashi S, Onodera O, Oyake M, Takahashi T, Hayashi S, Morimatsu M, Takahashi H, Makifuchi T, et al. Aprataxin, the causative protein for EAOH is a nuclear protein with a potential role as a DNA repair protein. Ann Neurol. 2004;55(2):241–249. doi: 10.1002/ana.10808. [DOI] [PubMed] [Google Scholar]
- 34.Westra HJ, Peters MJ, Esko T, Yaghootkar H, Schurmann C, Kettunen J, Christiansen MW, Fairfax BP, Schramm K, Powell JE, et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet. 2013;45(10):1238–1243. doi: 10.1038/ng.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamashita S, Fujii K, Zhao C, Takagi H, Katakura Y. Involvement of the NFX1-repressor complex in PKC-δ-induced repression of hTERT transcription. The J Biochem. 2016;160(5):309–313. doi: 10.1093/jb/mvw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Topalian SL, Kaneko S, Gonzales MI, Bond GL, Ward Y, Manley JL. Identification and functional characterization of neo-poly(A) polymerase, an RNA processing enzyme overexpressed in human tumors. Mol Cell Biol. 2001;21(16):5614–5623. doi: 10.1128/MCB.21.16.5614-5623.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shono Y, Tuckett AZ, Ouk S, Liou H-C, Altan-Bonnet G, Tsai JJ, Oyler JE, Smith OM, West ML, Singer NV, et al. A small-molecule c-Rel inhibitor reduces alloactivation of t cells without compromising antitumor activity. Cancer Discov. 2014;4(5):578–591. doi: 10.1158/2159-8290.CD-13-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keller KL, Tepper BJ. Inherited taste sensitivity to 6-n-propylthiouracil in diet and body weight in children. Obes Res. 2004;12(6):904–912. doi: 10.1038/oby.2004.110. [DOI] [PubMed] [Google Scholar]
- 39.Mennella JA, Pepino MY, Reed DR. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics. 2005;115(2):e216–e222. doi: 10.1542/peds.2004-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wendell S, Wang X, Brown M, Cooper ME, DeSensi RS, Weyant RJ, Crout R, McNeil DW, Marazita ML. Taste genes associated with dental caries. J Dent Res. 2010;89(11):1198–1202. doi: 10.1177/0022034510381502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yildiz G, Ermis RB, Calapoglu NS, Celik EU, Türel GY. Gene-environment interactions in the etiology of dental caries. J Dent Res. 2016;95(1):74–79. doi: 10.1177/0022034515605281. [DOI] [PubMed] [Google Scholar]
- 42.Niimura Y. Olfactory receptor multigene family in vertebrates: from the viewpoint of evolutionary genomics. Curr Genomics. 2012;13(2):103–114. doi: 10.2174/138920212799860706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017;8(1):1826. doi: 10.1038/s41467-017-01261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fiorillo L. Oral health: the first step to well-being. Medicina (Kaunas) 2019;55(10):676. doi: 10.3390/medicina55100676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sischo L, Broder HL. Oral health-related quality of life: what, why, how, and future implications. J Dent Res. 2011;90(11):1264–1270. doi: 10.1177/0022034511399918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grigalauskiene R, Slabsinskiene E, Vasiliauskiene I. Biological approach of dental caries management. Stomatologija. 2015;17(4):107–112. [PubMed] [Google Scholar]
- 47.Twetman S. Prevention of dental caries as a non-communicable disease. Eur J Oral Sci. 2018;126(Suppl 1):19–25. doi: 10.1111/eos.12528. [DOI] [PubMed] [Google Scholar]
- 48.Fiorillo L, Cervino G, Herford AS, Laino L, Cicciu M. Stannous fluoride effects on enamel: a systematic review. Biomimetics (Basel) 2020;5(3):41. doi: 10.3390/biomimetics5030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gasse B, Grabar S, Lafont AG, Quinquis L, Opsahl Vital S, Davit-Béal T, Moulis E, Chabadel O, Hennequin M, Courson F, et al. Common SNPs of AmelogeninX (AMELX) and dental caries susceptibility. J Dent Res. 2013;92(5):418–424. doi: 10.1177/0022034513482941. [DOI] [PubMed] [Google Scholar]
- 50.Haworth S, Shungin D, van der Tas JT, Vucic S, Medina-Gomez C, Yakimov V, Feenstra B, Shaffer JR, Lee MK, Standl M, et al. Consortium-based genome-wide meta-analysis for childhood dental caries traits. Human Mol Genet. 2018;27(17):3113–3127. doi: 10.1093/hmg/ddy237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Izakovicova Holla L, Borilova Linhartova P, Lucanova S, Kastovsky J, Musilova K, Bartosova M, Kukletova M, Kukla L, Dusek L. GLUT2 and TAS1R2 polymorphisms and susceptibility to dental caries. Caries Res. 2015;49(4):417–424. doi: 10.1159/000430958. [DOI] [PubMed] [Google Scholar]
- 52.Kastovsky J, Borilova Linhartova P, Musilova K, Zackova L, Kukletova M, Kukla L, Izakovicova Holla L. Lack of association between BMP2/DLX3 gene polymorphisms and dental caries in primary and permanent dentitions. Caries Res. 2017;51(6):590–595. doi: 10.1159/000479828. [DOI] [PubMed] [Google Scholar]
- 53.Shungin D, Haworth S, Divaris K, Agler CS, Kamatani Y, Keun Lee M, Grinde K, Hindy G, Alaraudanjoki V, Pesonen P, et al. Genome-wide analysis of dental caries and periodontitis combining clinical and self-reported data. Nat Commun. 2019;10(1):2773–2773. doi: 10.1038/s41467-019-10630-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Shaffer JR, Zeng Z, Begum F, Vieira AR, Noel J, Anjomshoaa I, Cuenco KT, Lee MK, Beck J, et al. Genome-wide association Scan of dental caries in the permanent dentition. BMC Oral Health. 2012 doi: 10.1186/1472-6831-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khonsari RH, Ohazama A, Raouf R, Kawasaki M, Kawasaki K, Porntaveetus T, Ghafoor S, Hammond P, Suttie M, Odri GA, et al. Multiple postnatal craniofacial anomalies are characterized by conditional loss of polycystic kidney disease 2 (Pkd2) Hum Mol Genet. 2013;22(9):1873–1885. doi: 10.1093/hmg/ddt041. [DOI] [PubMed] [Google Scholar]
- 56.Ferraro M, Vieira AR. Explaining gender differences in caries: a multifactorial approach to a multifactorial disease. Int J Dent. 2010 doi: 10.1155/2010/649643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salvolini E, Di Giorgio R, Curatola A, Mazzanti L, Fratto G. Biochemical modifications of human whole saliva induced by pregnancy. Br J Obstet Gynaecol. 1998;105(6):656–660. doi: 10.1111/j.1471-0528.1998.tb10181.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary material.
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the dbGaP repository, https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000774.v2.p1