Abstract
Alcoholism and high fat diet (HFD)-induced obesity individually promote insulin resistance and glucose intolerance in clinical populations, increasing risk for metabolic diseases. HFD can also stimulate alcohol intake in short term clinical studies. Unfortunately, there is currently a disconnect between animal models and the clinical findings as animal studies typically show that HFD decreases ethanol intake while ethanol intake mitigates HFD-induced effects on insulin and glucose dysfunction. However, most previous animal studies utilized forced or continuous HFD and/or ethanol. In three experiments we sought to determine if HFD (HFD=60% calories from fat) vs control diet (Chow=16% fat) alters voluntary two-bottle choice ethanol intake in male C57Bl/6J mice given differing access schedules for 6-7 weeks and assessed resultant impact on metabolic function via insulin and glucose tolerance tests. Experiment 1: Unlimited Access Ethanol+HFD (UAE+HFD; n=15; 10% ethanol v/v, ad libitum diet and ethanol) or UAE+Chow (n=15). Experiment 2: Limited Access Ethanol+HFD (LAE+HFD; n=15; ethanol= 4 hrs/d; 3 d/wk, ad libitum diet) or LAE+Chow (n=15) with increasing ethanol concentrations (10%, 15%, 20%). Experiment 3: Intermittent HFD with limited access to ethanol (iHFD-E; HFD= single 24 hr session/week; ethanol= 4hrs/d; 4 d/wk) (n=10). UAE+HFD mice consumed significantly less ethanol and were insulin resistant and hyperglycemic compared with UAE+Chow mice. LAE+HFD mice consumed ethanol similarly to LAE+Chow mice, but exhibited hyperglycemia, insulin resistance, and glucose intolerance. iHFD-E mice displayed binge eating-like behaviors and consumed significantly more ethanol than mice given ad libitum chow or HFD. iHFD-E mice did not have significantly altered body composition, but developed insulin insensitivity and glucose intolerance. These findings suggest access schedules influence HFD effects on ethanol consumption and resultant metabolic dysfunction, ethanol intake does not improve HFD-induced metabolic dysfunction, and binge eating-like behaviors can transfer to binge drinking behaviors.
Keywords: Ethanol, High Fat Diet, Metabolic Dysfunction, Binging, Ingestive Behaviors
Introduction
Obesity and alcohol use disorder (AUD) are two of the most common chronic conditions in the United States. Clinically, diet-induced obesity, typical to western diets high in fat, is associated with insulin resistance, glucose intolerance, and increased risk for developing Type II diabetes (Haslam and James, 2005). Chronic ethanol drinking is also a risk factor for insulin dysfunction (Fan et al, 2006; Papachristou et al, 2006). Such clinical and epidemiologic findings suggest an overlap in the mechanisms by which high fat diets (HFD) and ethanol exposure modulate insulin action and glucose homeostasis and that, together, co-morbid chronic HFD and ethanol intake may increase risk for subsequent disease states such as Type II diabetes (Steiner et al, 2015). The majority of epidemiologic evidence, however, suggests that moderate ethanol intake has protective effects on insulin sensitivity (Traversy and Chaput, 2015), but this may only occur in non-obese patients (Yokoyama, 2011). Most preclinical studies also suggest that ethanol consumption mitigates HFD-induced metabolic dysfunction, which may be related to the moderate ethanol intake found in these models (Feng et al, 2012; Gelineau et al, 2017; Hong et al, 2009; Paulson et al, 2010). More recent epidemiologic studies, however, have brought these assumptions back up for debate (Griswold et al, 2018).
Numerous clinical studies show increased desire, cravings, and intake of high fat foods during and after ethanol drinking episodes (Breslow et al, 2013; Caton et al, 2004; Piazza-Gardner and Barry, 2014). Although less is known clinically about the potential for high fat foods to increase ethanol intake, it has been shown that obesity-related eating behaviors- which are associated with increased intake of high density/fat food- are associated with increased ethanol consumption in cross-sectional studies (Muñoz-Pareja et al, 2013), suggesting HFD may be a risk factor for escalation of ethanol intake. A similar positive relationship for ethanol-induced increases in HFD intake has been shown in some animal models (Barson et al, 2009). However, while the inverse relationship of acute HFD exposure stimulating ethanol intake has been suggested in some animal models (Carrillo et al, 2004), the majority of findings indicate that HFD exposure decreases ethanol consumption in rodent models (Feng et al, 2012; Gelineau et al, 2017; Sirohi et al, 2017a, 2017b). The majority of these previous studies, however, have either examined ethanol intake in the face of HFD access without specific focus on metabolic function, or examined metabolic effects of ethanol and HFD without taking intake behaviors into account. While the studies above and others (Guo et al, 2018) have greatly advanced our understanding of the impact that HFD and ethanol have on metabolic and end-organ function, these models typically do not replicate the escalation of drinking behaviors common to human AUD.
It is now well characterized that limited access scheduling increases ethanol intake in animal models in an escalating fashion akin to human AUD development (Melendez, 2011). The impact of HFD on this type of scheduled ethanol access, however, has not been examined. Intermittent HFD access has been shown to induce binge eating-like behaviors in mice (Czyzyk et al, 2010; Hardaway et al, 2016). Since acute HFD can increase ethanol intake (Carrillo et al, 2004), and vice versa (Barson et al, 2009), it stands to reason that repeated acute HFD access (i.e. intermittent HFD) might lead to increased escalation of ethanol intake under intermittent ethanol access conditions. Therefore, the overall goals of these studies were to determine: (1) if HFD alters ethanol intake in mice consuming ethanol with limited or unlimited access schedules; and (2) how such access schedules modulate the interaction of HFD and ethanol on metabolic function in male C57Bl/6J mice. Our findings suggest that continuous HFD reduces ethanol intake when ethanol is freely available, but that HFD does not alter ethanol intake when access to ethanol is limited. Furthermore, intermittent HFD significantly increases ethanol intake when ethanol is also available on an intermittent schedule. Contrary to previous work, our study suggests that moderate intake of freely-available ethanol does not mitigate the ability of HFD to promote insulin resistance. In HFD-fed mice, higher levels of ethanol intake in the limited access models failed to improve insulin sensitivity and worsened glucose tolerance, suggesting scheduled HFD and ethanol intake may interact to disrupt insulin action and glucose homeostasis.
Materials and Methods
Animals
Six-week old male C57Bl/6J mice were purchased from The Jackson Laboratory (stock # 000664; Bar Harbor, ME). Upon arrival, mice were individually housed and given standard chow diet for a four-day acclimation period. Mice were weight-matched and separated into groups as described below. All mice were kept in a temperature- and humidity-controlled room on a 12-hour light/dark cycle (lights on at 7 am, lights off at 7 pm). Limited access ethanol and all metabolic testing procedures were performed during the light cycle and were approved by the Institutional Animal Care and Use Committee at Penn State University College of Medicine (Hershey, PA).
Experiment 1: Effects of ad libitum diet on Unlimited Access to Ethanol (UAE model)
To examine effects of continuous two-bottle choice ethanol drinking in the presence of ad libitum HFD (60% calories from fat (lard), 26% calories from carbohydrates, 14% calories from protein; Bioserv F3282, Flemington, NJ) or control diet (16% calories from fat, 63% calories from carbohydrates, 21% calories from protein; Bioserv F4031, Flemington, NJ), mice were weight-matched prior to any manipulation and randomly assigned into an unlimited 24-hour access to ethanol group receiving either HFD (UAE+HFD; n=15) or control diet (UAE+Chow; n=15). Following an initial 2-week HFD exposure period to establish a consistent eating phenotype and the ethanol ramp period (see description of ethanol ramp below), UAE+HFD and UAE+Chow groups had home-cage 24-hour access to two-bottle choice of tap water and 10% ethanol (vol/vol in water) throughout the remainder of the experiment. Body mass, ethanol and water intake, and ethanol preference were assessed every 24 hours for six weeks. Summary of timeline provided in Fig 1.
Fig 1. Summary of Unlimited Access Ethanol (UAE), free-choice model timeline.
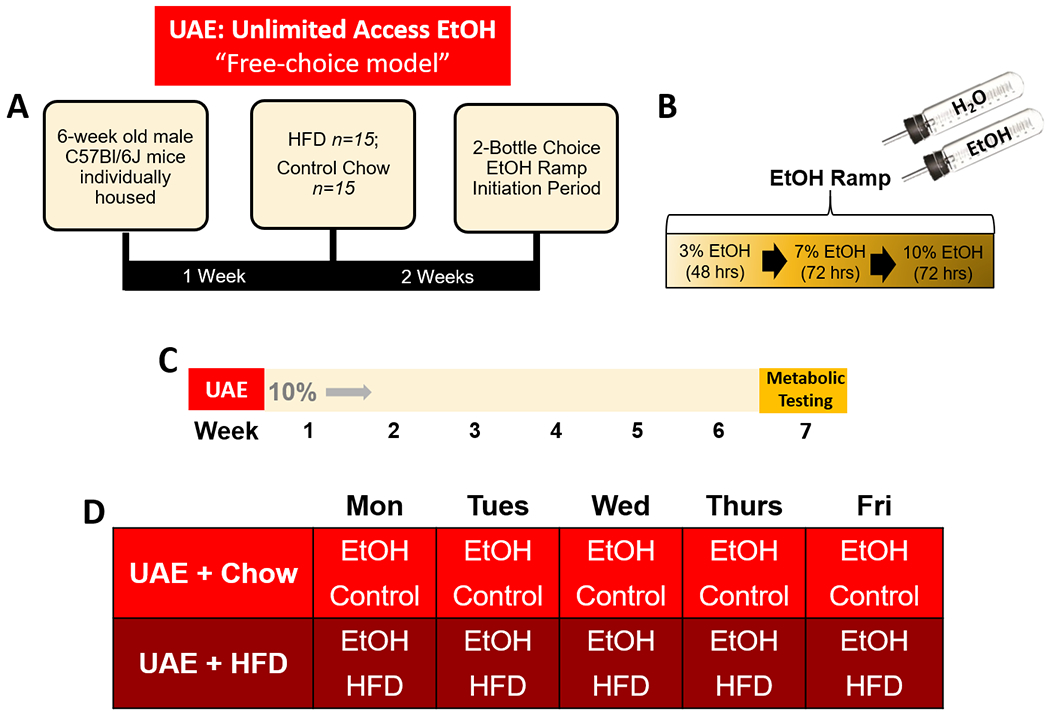
A) Description of initial HFD exposure period relative to EtOH ramp period. B) Description of two-bottle choice EtOH ramp initiation period. C) Timeline of study: following EtOH ramp period, groups had two-bottle choice access to 10% EtOH for six weeks then underwent metabolic testing. D) Weekly EtOH two-bottle choice and diet schedules for study groups.
Experiment 2: Effects of ad libitum diet on Limited Access to Ethanol (LAE model)
To examine effects of limited access two-bottle choice ethanol drinking in the presence of ad libitum HFD or control diet, mice were weight-matched and randomly assigned to groups eventually receiving either HFD (LAE+HFD; n=15) or control diet (LAE+Chow; n=15). Diets are the same as described above, except that mice had an initial 48-hour HFD access period (or control Chow) prior to the ethanol-ramp at which point they returned to control diet. Following the ethanol-ramp initiation period, appropriate diets were then provided ad libitum for the remainder of the study. LAE+HFD and LAE+Chow groups had home-cage two-bottle choice of tap water and ethanol (vol/vol in water) limited to 4-hour access periods on Monday, Wednesday, and Friday beginning at 10am and ending at 2pm. These groups had access to 10% ethanol for three weeks, followed by 15% ethanol for two weeks, and 20% ethanol for two weeks. Body mass, ethanol and water intake, and ethanol preference were assessed after each drinking session.
Experiment 3: Effects of intermittent HFD access on limited access to ethanol (iHFD-E model)
To examine effects of limited two-bottle choice ethanol drinking in the presence of intermittent HFD, mice were weight-matched and randomly assigned to groups eventually receiving either intermittent HFD (iHFD-E; n=10), ad libitum HFD (HFD-E; n=10) or ad libitum control chow diet (Chow-E; n=10). After an initial 48-hour HFD access period (or control Chow), all mice were placed on control diet and ethanol-ramp initiation period began. Following ethanol ramp, all groups began their appropriate diet regimens. iHFD-E mice had a single 24-hour HFD access period beginning at 10 am on each Monday per week (Czyzyk et al, 2010). Mice then began home-cage two-bottle choice of tap water and ethanol (vol/vol in water) limited to 4-hour access periods on Tuesday, Wednesday, Thursday, and Friday beginning at 10am and ending at 2pm. These groups had access to 10% ethanol for three weeks, followed by 15% ethanol for two weeks, and 20% ethanol for two weeks. Body mass, ethanol and water intake, and ethanol preference were assessed after each drinking session.
Metabolic Testing
Once ethanol intake studies concluded, mice underwent standardized insulin tolerance (ITT) and glucose tolerance (GTT) tests. For the ITT, mice were fasted for four hours and then injected intraperitoneally with insulin (0.75 units/kg of regular U-100 insulin in 1x PBS; Novolin, Plainsboro, NJ). A tail vein blood sample was taken at baseline (immediately prior to insulin injection) and at 15, 30, 60, 90, and 120 minutes post-insulin injection to measure blood glucose with a glucometer (Prodigy AutoCode, Charlotte, NC). For the GTT, mice were fasted overnight and then injected intraperitoneally with dextrose (2 g/kg of 50% dextrose; Hospira, Inc., Lake Forest, IL). Blood glucose was measured at baseline (immediately prior to dextrose injection) and at 15, 30, 60, 90, and 120 minutes post-dextrose injection. Body composition (fat, lean, and fluid masses) was measured in conscious mice using a quantitative nuclear magnetic analyzer (Bruker Minispec, Billerica, MA). Age- and weight-matched mice receiving HFD (n=10), control diet (n=10), or iHFD (n=10) for similar periods but without ethanol exposure were used as controls.
Ethanol Ramp
Following initial HFD exposure periods as described in the individual experiments above, all mice underwent an ethanol-ramp initiation period to promote consistent ethanol intake while avoiding potential confounds of ethanol taste aversion. The ethanol two-bottle choice ramp procedure consisted of home-cage 24-hour access to a bottle containing tap water and another bottle containing 3% ethanol for 48 hours, 7% ethanol for 72 hours, and 10% ethanol for 72 hours (all ethanol concentrations are vol/vol in tap water). Water and ethanol solutions were administered via inverted 50 mL conical tubes (Fisher Scientific, Waltham, MA) and sealed with a rubber stopper (#5.5; Fisher Scientific, Waltham, MA) containing a 2-inch stainless-steel straight sipper tube (Ancare, Bellmore, NY). Ethanol solution was made using ethyl alcohol (190 proof; PHARMCO-AAPER, Brookfield, CT) diluted in tap water.
Statistics
Statistical analyses were performed using Microsoft Excel (Microsoft Corporation, Redmond, WA) and GraphPad Prism 8 (GraphPad Software, San Diego, CA). Mixed-effects model ANOVA was used to compare the main effects of diets and number of drinking sessions on ethanol intake, and their interaction over the study time course. One-way ANOVA was used to compare end of study metabolic metrics between diet+ethanol groups versus control groups receiving identical diets but not receiving ethanol. When applicable, one-way ANOVA or unpaired t-tests were used to compare end of study ethanol consumption metrics between diet+ethanol groups. All data are represented as mean±standard error of the mean (SEM). Significance was determined at the p<0.05 level for all analyses. GraphPad Prism 8 and Microsoft PowerPoint 2011 (Microsoft Corporation, Redmond, WA) were used for figure preparation.
Results
Experiment 1:
We determined effects of ad libitum HFD or chow diet access on body mass and ethanol intake parameters in the UAE model, which had continuous free-access to 10% ethanol and water. Mixed-effects model ANOVA indicated that UAE+HFD mice had significantly higher body mass than UAE+Chow mice over the course of the study (Diet: F(1,18)=44.91, p<0.001; Ethanol exposure sessions: F(29,522)=163.1, p<0.001; Interaction: F(29,522)=76.23, p<0.001; Fig. 4A). UAE+HFD mice consumed significantly less ethanol than UAE+Chow mice (Diet: F(1,18)=22.22, p<0.001; Ethanol exposure sessions: F(24,432)=3.927, p<0.001; Interaction: F(24,432)=1.250, p=0.194; Fig. 4B). Given that the large differences in body mass could skew evaluation of g/kg measurements, we also assessed total grams of ethanol (g/ethanol) consumed per group per day. Mixed-effects model ANOVA confirmed a reduction in g/ethanol by HFD (Diet: F(1,18)=8.80, p=0.008; Ethanol exposure sessions: F(24,432)=4.01, p<0.001; Interaction: F(24,432)=0.9, p=0.597; Fig. 4C). Cumulative g/ethanol consumption across the study was higher in UAE+Chow mice compared to UAE+HFD mice (3.11±0.36 vs 1.89±0.20 g/ethanol, respectively, t=2.966, df=18, p=0.008; Fig. 4C1). UAE+HFD mice also had lower preference towards ethanol (Diet: F(1,18)=4.7, p=0.044; Ethanol exposure sessions: F(25,450)=4.872, p<0.001; Interaction: F(25,450)=0.820; p=0.717; Fig. 4D). These findings indicate that ad libitum HFD access reduces ethanol intake and preference in unlimited access “free-choice” model.
Fig 4. Ad libitum HFD access reduces ethanol intake in unlimited access “free-choice” (UAE) model.
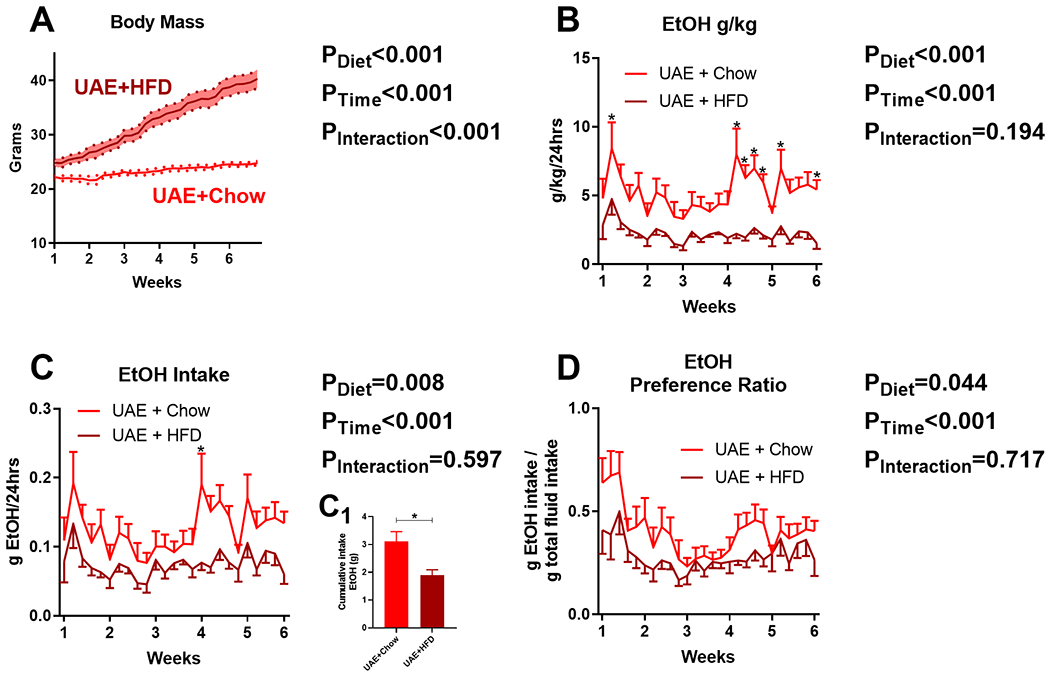
A) Time course of body mass changes by group during drinking period (n=15/group). Dark line indicates mean, shaded area with dots indicates standard error of the mean. HFD significantly reduces: B) ethanol g/kg/24hrs, C) g/ethanol consumed/24hrs, C1) cumulative ethanol intake, and D) ethanol preference. Lines indicate mean, error bars indicate standard error of the mean. * indicates significant differences between groups, as determined by mixed-effects model ANOVA or unpaired t-test (C1); p<0.05. P-values from mixed-effects ANOVA indicated to the right of each figure.
At the end of the study, mice underwent metabolic testing. Metabolic data were compared to weight- and age-matched mice given HFD or chow diet without ethanol access. One-way ANOVA (F(3,38)=26.02, p<0.001; Fig. 5A) indicated that body mass was similarly elevated in ethanol-naïve HFD (37.9±1.1 g) and UAE+HFD exposed (42.1±2.1 g) mice when compared to ethanol-naïve Chow (28.5±0.5 g) or UAE+Chow (28.4±0.3 g) mice. One-way ANOVA indicated that adiposity was significantly altered by ethanol consumption and that this was further impacted by HFD consumption (F(3,38)=27.02, p<0.001; Fig 5B). Bonferroni’s post-hoc analysis showed a significant increase in adiposity in the UAE+Chow and ethanol-naïve HFD mice compared to ethanol-naïve Chow mice and a further increase in adiposity in UAE+HFD mice compared to all other groups (Fig 5B). One-way ANOVA with Bonferroni’s post hoc analysis showed that lean mass was reduced in UAE+Chow, UAE+HFD, and ethanol-naïve HFD mice compared to ethanol-naïve Chow mice, but this reduction in lean mass was not as pronounced in UAE+Chow mice (F(3,38)=25.40, p<0.001; Fig. 5C). One-way ANOVA further showed that HFD can increase fluid mass but that this did not appear to be altered by ethanol consumption (F(3,38)=7.92, p<0.001; Fig. 5D). Overall, these data indicate that ethanol consumption may have subtle effects on HFD-induced changes in body composition in this model, with most pronounced effects on adiposity.
Fig 5. Free access ethanol consumption alters HFD-induced changes in adiposity with no change in body mass, lean mass, or fluid mass.
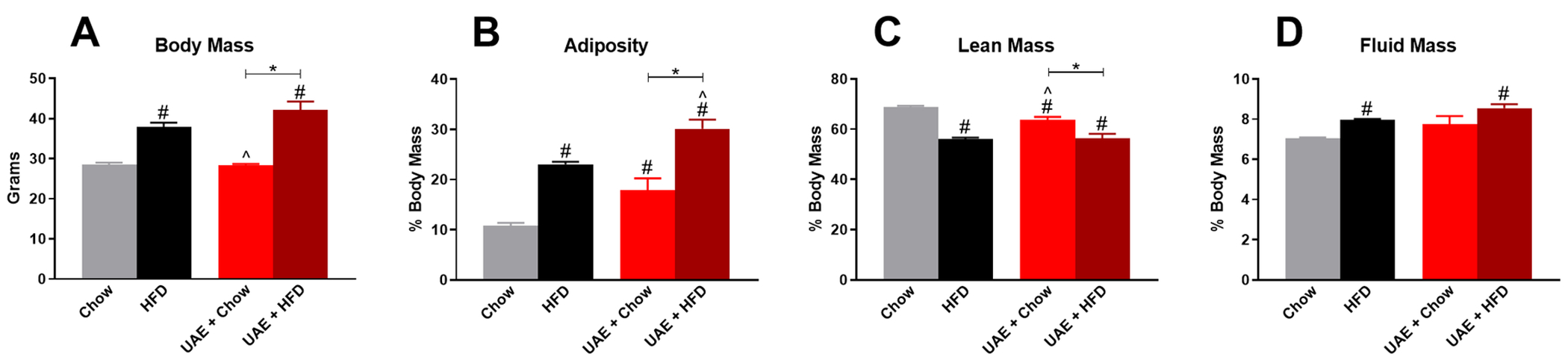
Body composition data are compared to Chow- and HFD-fed ethanol-naïve mice. A) Body mass of control mice and UAE mice prior to metabolic testing. Percent body mass of: B) adiposity, C) lean mass, and D) fluid mass. * indicates significant difference between indicated groups, # indicates significant difference from Chow, ^ indicates significant difference from HFD, as determined by one-way ANOVA with Bonferroni post-hoc analysis; p<0.05.
HFD-induced increases in body mass and adiposity are typically accompanied by insulin resistance in mouse models, and previous research indicates that ethanol consumption may mitigate these effects. We therefore performed ITT in the UAE mice and compared these results to the same age- and weight-matched controls as utilized in Fig 5. One-way ANOVA (F(3,38)=3.305, p=0.030; Fig. 6A) followed by Bonferroni’s post-hoc analysis indicated a significant increase in 4-hour fasting blood glucose levels in HFD (209±6 mg/dl) compared to Chow (166±7 mg/dl) mice, with no statistically significant differences between UAE+HFD (202±14 mg/dl) or UAE+Chow groups (182±12 mg/dl) compared to controls. Fig. 6B shows the time course of change in blood glucose levels following intraperitoneal insulin injection; data are normalized to baseline to account for differences in fasting glucose levels among groups. Fig. 6C shows the area under the curve for changes in blood glucose levels over time in response to insulin administration, with a more negative value indicating better insulin sensitivity. The ITT area under the curve indicated reduced insulin sensitivity in ethanol-naïve HFD mice (975±968 glucose mg/dl*min) compared to Chow mice (−4321±852 glucose mg/dl*min), with no effect of ethanol consumption on HFD-induced insulin resistance in UAE+HFD mice (−1282±1091 glucose mg/dl*min) (F(3,38)=4.624, p=0.008; Fig. 6C). UAE+Chow mice were not significantly different from ethanol-naïve Chow mice. Contrary to many previous findings, these results indicate that free-access ethanol consumption does not improve insulin sensitivity in HFD exposed mice.
Fig 6. Moderate ethanol consumption does not alter HFD-induced insulin resistance.
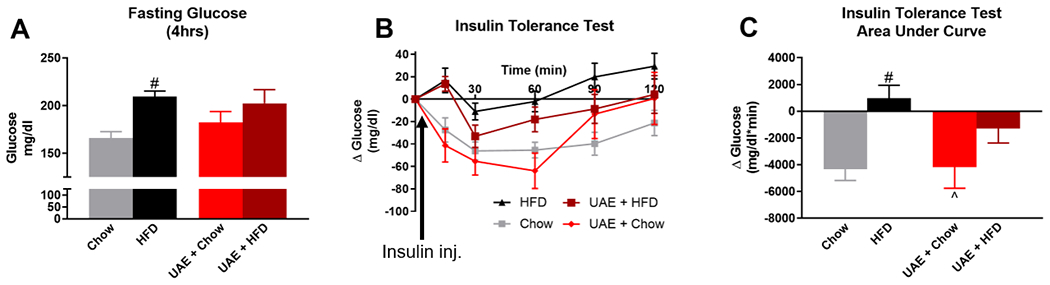
A) 4-hour fasting blood glucose levels prior to insulin tolerance test. B) Change in blood glucose levels over time following insulin injection; data are normalized to 0 at baseline. C) Area under the curve for change in blood glucose levels during insulin tolerance test. # indicates significant difference from Chow, ^ indicates significant difference from HFD, as determined by one-way ANOVA with Bonferroni post-hoc analysis; p<0.05. Arrows indicate time point at which insulin was injected following initial glucose measurements at time zero.
Experiment 2:
The above findings indicate that HFD decreases ethanol intake when provided in an unlimited “continuous-access” procedure. Previous research indicates that limiting ethanol access in an every-other-day intermittent access model increases ethanol intake in rodents compared to continuous access (Melendez, 2011). Whether HFD alters intermittent ethanol intake remains unclear. Therefore, we placed weight-matched mice in a limited access ethanol procedure (LAE; 4hr/day, Monday, Wednesday, Friday every week, see Fig. 2) and gave mice ad libitum HFD or chow. Mixed-effects model ANOVA indicated LAE+HFD mice gained significantly more body mass than LAE+Chow mice over the course of the study (Diet: F(1,28)=60.72, p<0.001; Ethanol exposure sessions: F(20,560)=522.7, p<0.001; Interaction: F(20,560)=180.8, p<0.001; Fig. 7A). LAE+HFD mice had significantly lower ethanol g/kg levels than the LAE+Chow group (Diet: F(1,28)=18.37, p<0.001; Ethanol exposure sessions: F(20,560)=52.83, p<0.001; Interaction: F(20,560)=5.734, p<0.001; Fig. 7B). Given that the large differences in body mass could skew evaluation of g/kg measurements, we also assessed total grams of ethanol (g/ethanol) consumed. Mixed-effects model ANOVA indicated a significant effect of exposure session (Ethanol exposure sessions: F(20,560)=65.20, p<0.001) with no statistically significant effect of diet on g/ethanol consumed (Diet: F(1,28)=3.902, p=0.058) but a significant interaction between the two variables (Interaction: F(20,560)=1.645, p=0.039; Fig. 7C). However, there was no significant effect of diet on cumulative g/ethanol consumed over the course of the study between LAE+Chow vs LAE+HFD mice (1.91±0.13 vs 1.53±0.15 g/ethanol, respectively, t=1.975, df=28, p=0.058; Fig. 7C1). For ethanol preference, mixed-effects model ANOVA indicated significant main effects of diet (F(1,28)=18.85, p<0.001) and ethanol exposure sessions (F(20,560)=5.967, p<0.001), with no significant interaction (F(20,560)=1.376, p=0.127; Fig. 7D).
Fig 2. Summary of Limited Access Ethanol (LAE), binge EtOH model timeline.
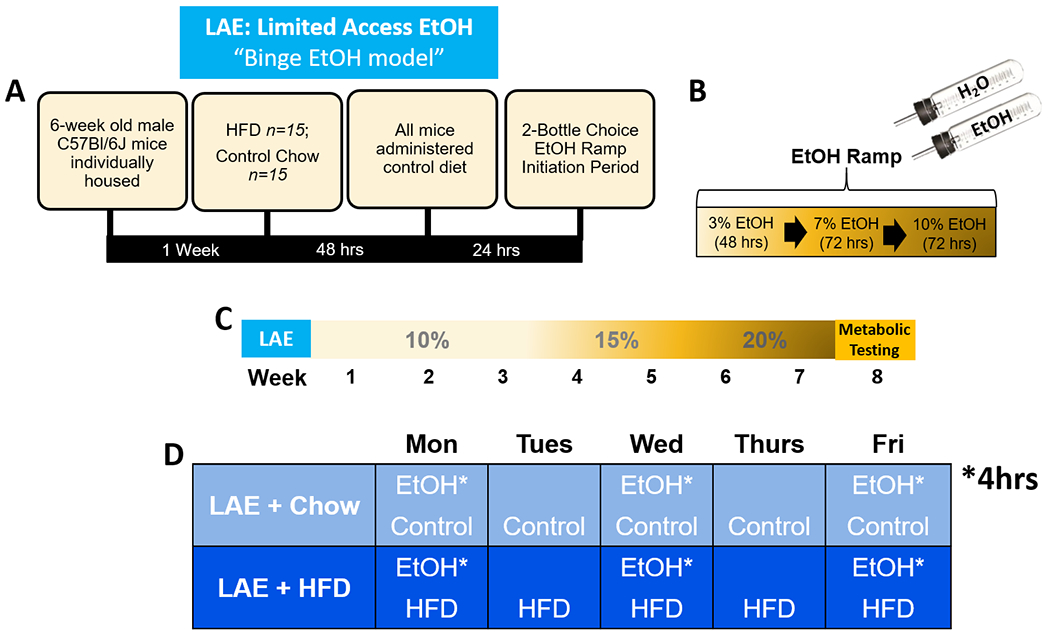
A) Description of initial HFD exposure period relative to return to control diet and initiation of the EtOH ramp. B) Description of two-bottle choice EtOH ramp initiation period. C) Timeline of study: following EtOH ramp period, groups had two-bottle choice access to 10% EtOH for three weeks, 15% EtOH for two weeks, 20% EtOH for two weeks, then underwent metabolic testing. D) Weekly EtOH two-bottle choice and diet schedules for study groups. EtOH is limited to a 4hr session on access days.
Fig 7. HFD effects on ethanol intake in the limited access “binge EtOH” (LAE) model.
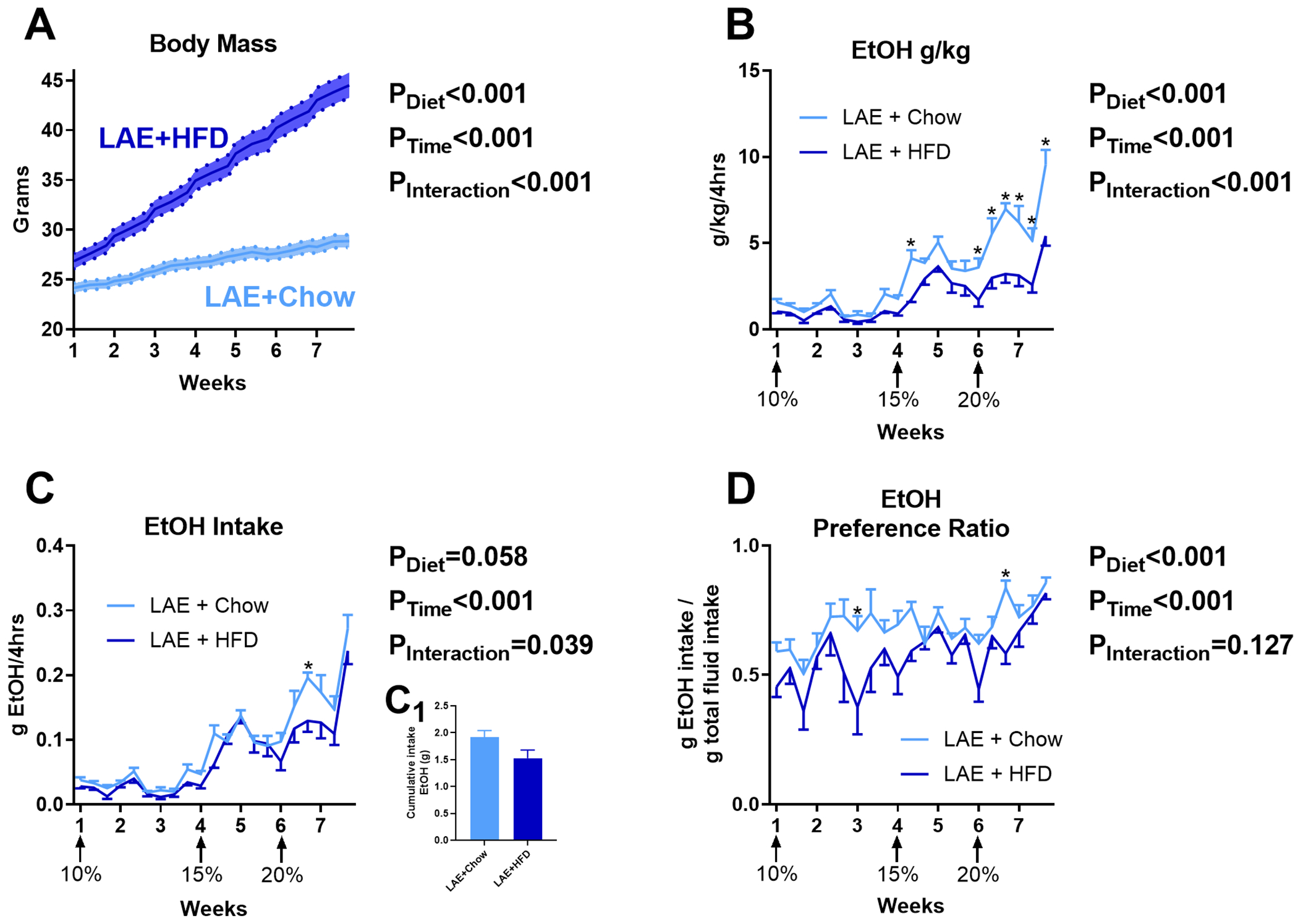
A) Time course of body mass changes by group during drinking period (n=15/group). HFD decreases EtOH g/kg (B) but does not significantly alter total g/ethanol consumed over the course of the study (C, C1) and alters ethanol preference (D). * indicates significant differences between groups on individual ethanol access sessions, as determined by mixed-effects model ANOVA with Bonferroni post-hoc analysis; p<0.05. P-values from mixed-effects model ANOVA indicated to the right of each figure.
For metabolic testing, results from LAE mice were compared to the same ethanol-naïve HFD and Chow control mice utilized in the previous experiment. One-way ANOVA showed body mass was elevated in both ethanol-naïve HFD (37.9±1.1 g) and LAE+HFD (43.8±1.1 g) mice when compared to LAE+Chow (28.1±0.6 g) or chow-fed ethanol-naïve mice (28.5±0.5 g) (F(3,46)=77.62, p<0.001; Fig. 8A). In contrast to the UAE model, limited ethanol access in the LAE+HFD mice led to a significantly increased body mass compared to ethanol-naïve HFD mice. Since ethanol intake did not alter body mass in LAE+Chow mice compared to ethanol-naïve Chow mice, these findings suggest a potential synergistic interaction between HFD and limited-access ethanol intake on body mass. One-way ANOVA indicated that HFD and ethanol consumption significantly altered adiposity (F(3,46)=288.9, p<0.001; Fig. 8B), lean mass (F(3,46)=280.6, p<0.001; Fig. 8C), and fluid mass (F(3,46)=87.68, p<0.001; Fig. 8D). For each measure tested, potential synergistic interactions between HFD and ethanol on body mass were further reflected by significantly increased adiposity and fluid mass in LAE+HFD vs ethanol-naïve HFD mice and decreased lean mass between these two groups. LAE+Chow mice were not significantly different from ethanol-naïve Chow mice on any of these measures.
Fig 8. Limited Access Ethanol (LAE) consumption worsens HFD-induced changes to body mass, adiposity, lean mass, and fluid mass.
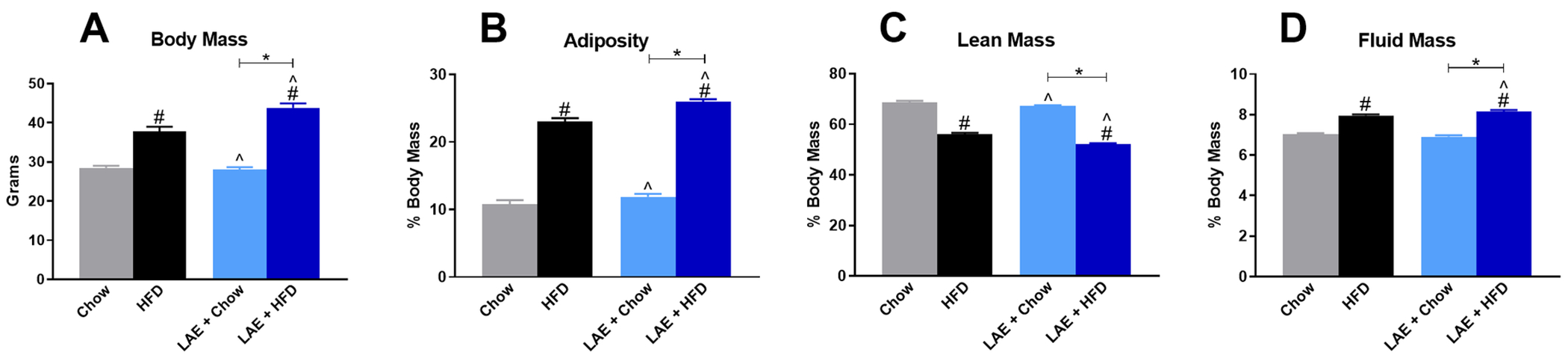
Body composition data of LAE mice compared to Chow- and HFD-fed ethanol-naïve mice. A) Body mass of control mice and LAE mice prior to metabolic testing. Percent body mass of: B) adiposity, C) lean mass, and D) fluid mass. * indicates significant difference between indicated groups, # indicates significant difference from Chow, ^ indicates significant difference from HFD, as determined by one-way ANOVA with Bonferroni post-hoc analysis; p<0.05.
For metabolic testing, one-way ANOVA (F(3,46)=31.70, p<0.001; Fig. 9A) followed by Bonferroni’s post-hoc test indicated 4-hour fasting blood glucose was elevated in the ethanol-naïve HFD (209±6 mg/dl) and LAE+HFD (250±8 mg/dl) mice compared to ethanol-naïve Chow (166±7 mg/dl) and LAE+Chow (173±7 mg/dl) mice. Similar to body composition data, fasting blood glucose was significantly higher in the LAE+HFD mice compared to ethanol-naïve HFD mice although LAE+Chow mice were not significantly different from ethanol-naïve Chow mice. In the ITT, One-way ANOVA (F(3,44)=5.229, p=0.004; Fig. 9B,C) indicated that insulin sensitivity was reduced in the ethanol-naïve HFD group (975±968 glucose mg/dl*min) compared to ethanol-naïve Chow mice (−4321±852 glucose mg/dl*min). There was no significant difference in insulin sensitivity between LAE+Chow (−3583±460 glucose mg/dl*min) and ethanol-naïve Chow mice. Surprisingly, although there was a significant increase in fasting glucose in LAE+HFD mice, there was no significant difference in insulin sensitivity in these mice (−1885±1281 glucose mg/dl*min) compared to any other group.
Fig 9. Limited Access Ethanol (LAE) does not improve insulin sensitivity or glucose tolerance in HFD-fed mice.
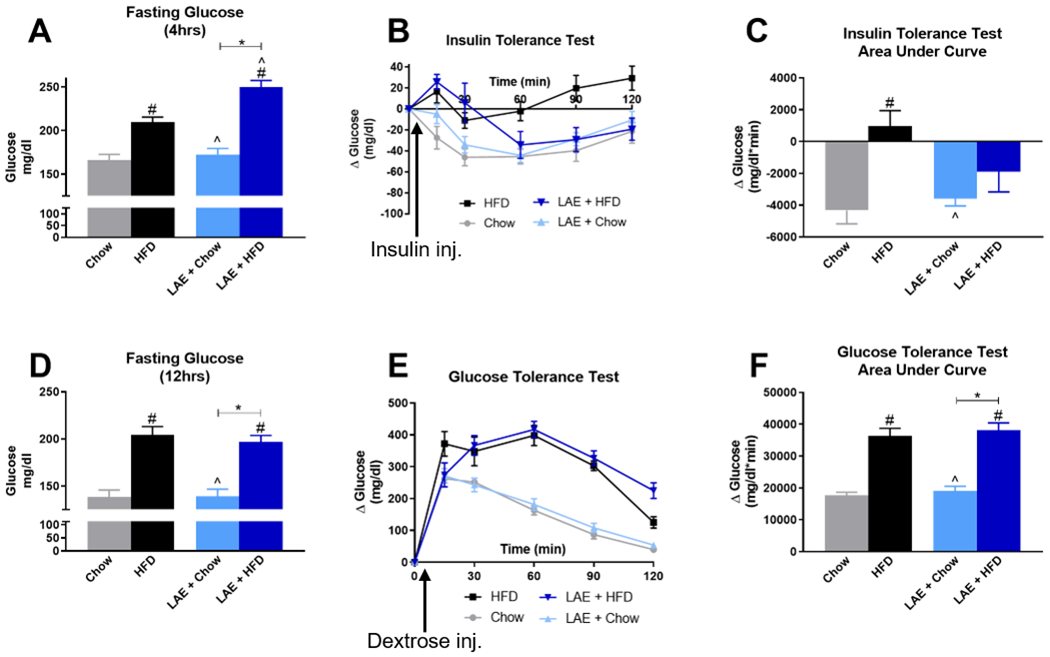
A) 4-hour fasting blood glucose levels prior to insulin tolerance test. B) Change in blood glucose levels over time following insulin injection; data are normalized to 0 at baseline. C) Area under the curve for change in blood glucose levels during insulin tolerance test. D) 12-hour fasting blood glucose levels prior to glucose tolerance test. E) Change in blood glucose levels over time following dextrose injection; data are normalized to 0 at baseline. F) Area under the curve for change in blood glucose levels during glucose tolerance test. * indicates significant difference between indicated groups, # indicates significant difference from Chow, ^ indicates significant difference from HFD, as determined by one-way ANOVA with Bonferroni post-hoc analysis; p<0.05. Arrows indicate time point of insulin or dextrose injection following initial glucose measurements at time zero.
Given differences in fasting blood glucose levels between groups, we next performed GTT to determine the impact of HFD and ethanol on the ability to dissipate changes in blood glucose in response to a glucose load. One-way ANOVA indicated that 12-hour fasting blood glucose levels were elevated in HFD compared to chow-fed mice (F(3,46)=22.36, p<0.001; Fig. 9D), but ethanol intake did not alter HFD-induced changes in blood glucose as LAE+HFD (197±7 mg/dl) was not statistically different from ethanol-naïve HFD mice (205±9 mg/dl). The increase in blood glucose levels in response to exogenous dextrose administration over the 120-minute study period is shown in Fig. 9E. These data were summarized as an area under the curve in Fig. 9F, with a more positive value indicating glucose intolerance. One-way ANOVA (F(3,46)=30.09, p<0.001; Fig. 9E,F) indicated a significant increase in the GTT area under the curve in LAE+HFD mice (38047±2397 glucose mg/dl*min) and ethanol-naïve HFD mice (36322±2421 glucose mg/dl*min) compared to LAE+Chow (19028±1493 glucose mg/dl*min) and ethanol-naïve Chow (17699±938 glucose mg/dl*min) mice.
Experiment 3:
Since limiting access to HFD can induce binge eating-like behaviors (Czyzyk et al, 2010; Hardaway et al, 2016), we hypothesized that such binge-like intake behaviors toward food would transfer to ethanol directed behaviors to increase ethanol intake. We therefore sought to examine the effects of intermittent HFD access on limited access two-bottle choice ethanol drinking. We placed weight-matched mice in a limited access ethanol procedure (4hr/day, Tuesday, Wednesday, Thursday, Friday every week, see Fig 3) and gave mice intermittent access to HFD (a single 24hr period/week; iHFD-E), ad libitum chow (Chow-E), or ad libitum HFD (HFD-E). Mixed-effects model ANOVA indicated HFD-E mice gained significantly more body mass than Chow-E or iHFD-E mice over the course of the study (Diet: F(2,27)=81.04, p<0.001; Ethanol exposure sessions: F(34,918)=363.4, p<0.001; Interaction: F(68,918)=89.98, p<0.001; Fig. 10A). Interestingly, although weight gain was predominantly linear in HFD-E and Chow-E mice, iHFD-E mice showed weight cycling, indicating a binge-like eating pattern where most of their food intake each week occurred on the HFD exposure day (Fig. 10B) with reduced food intake the rest of the week (Fig. 10C). Over the course of the study, iHFD-E mice gained significantly more body mass following a single 24hr HFD access period compared to the ad libitum fed Chow-E and HFD-E mice (One-way ANOVA, F(2,18)=32.14, p<0.001; Fig. 10B), suggesting binge-like behavior toward HFD. When HFD was not present, body mass significantly decreased in iHFD mice compared to the other groups (Fig. 10C). Mixed-effects model ANOVA indicated that iHFD-E mice had significantly higher ethanol g/kg levels (Diet: F(2,27)=33.08, p<0.001; Ethanol exposure sessions: F(27,729)=25.02, p<0.001, Interaction: F(54,729)=12.5, p<0.001) and higher g/ethanol consumed (Diet: F(2,27)=26.55, p<0.001; Ethanol exposure sessions: F(27,729)=25.12, p<0.001; Interaction: F(54,729)=11.17, p<0.001) than Chow-E or HFD-E (Fig. 10D,E). Total ethanol consumption over the course of the study was also significantly higher in iHFD-E mice than Chow-E or HFD-E mice (One-way ANOVA, F(2,27)=26.55, p<0.001; Fig. 10E1) while post-hoc analysis showed no significant difference in total ethanol consumption between Chow-E and HFD-E mice. Mixed-effects model ANOVA also indicated that although there was no significant effect of diet on ethanol preference (F(2,27)=2.663, p=0.088), there was a significant effect of ethanol exposure sessions (F(27,729)=2.938, p<0.001) and diet by ethanol interaction (F(54,729)=3.295, p<0.001; Fig. 10F).
Fig 3. Summary of intermittent access to HFD with limited access to Ethanol (iHFD-E), binge-like HFD model timeline.
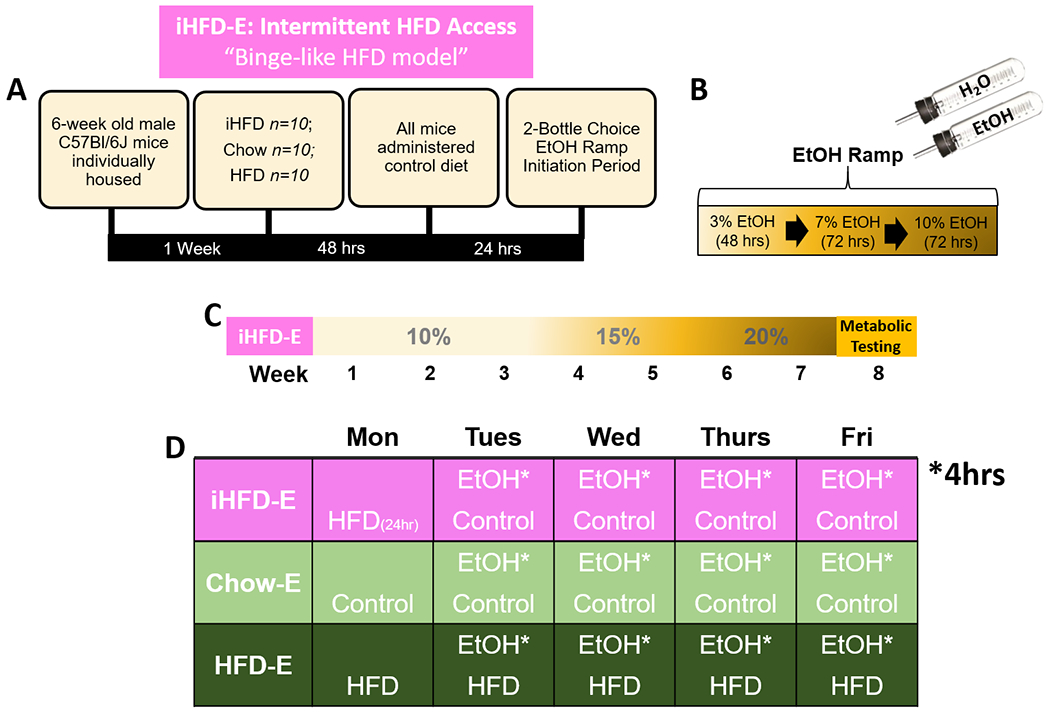
A) Description of initial HFD exposure period relative to return to control diet and initiation of ethanol ramp. B) Description of two-bottle choice EtOH ramp initiation period. C) Timeline of study: following EtOH ramp period, groups had two-bottle choice access to 10% EtOH for three weeks, 15% EtOH for two weeks, 20% EtOH for two weeks, then underwent metabolic testing. D) Weekly EtOH two-bottle choice and diet schedules for study groups. EtOH is limited to a 4hr session on access days.
Fig 10. Intermittent HFD effects on limited access to ethanol “binge-like HFD” (iHFD-E) model.
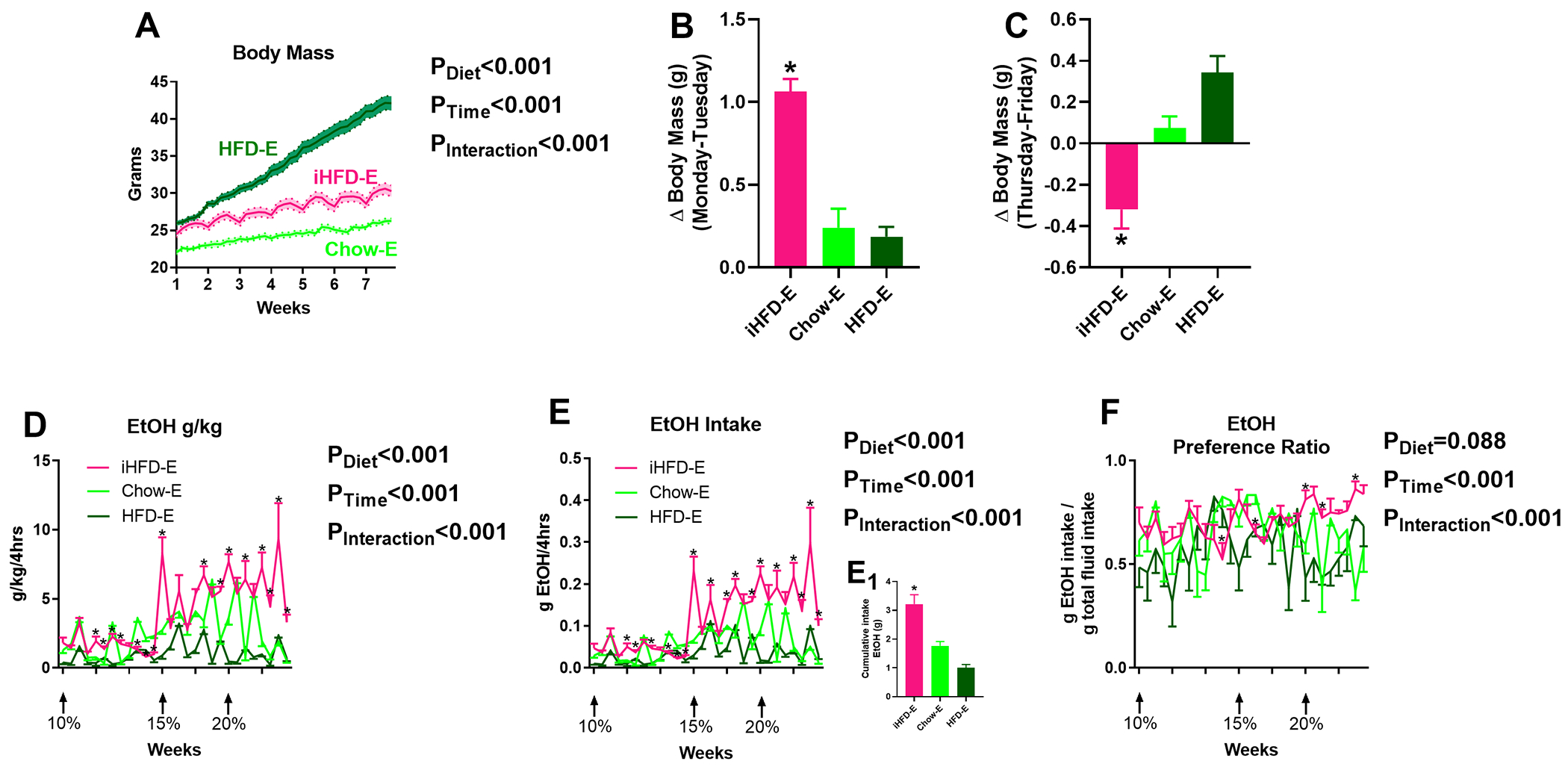
A) Time course of body mass changes by group during drinking period (n=10/group). B) Average change in body mass following weekly 24-hour HFD exposure in iHFD-E group compared to change in body mass over the same time point in the Chow-E and HFD-E groups. C) Average change in body mass during the last day of the week for each group (i.e. after iHFD-E mice had solely chow access for four days). D-F) iHFD-E increases: D) ethanol g/kg, E) g/ethanol consumed over the course of the study, E1) cumulative ethanol intake, and F) ethanol preference. * indicates significant differences between groups on individual ethanol access sessions, as determined by mixed-effects model ANOVA with Bonferroni post-hoc analysis; p<0.05. P-values from mixed-effects ANOVA indicated to the right of each figure.
For metabolic testing, results from iHFD-E, Chow-E, and HFD-E mice were compared to the same ethanol-naïve HFD and Chow control mice utilized in the previous experiments in addition to an iHFD ethanol-naïve control group. One-way ANOVA showed body mass was elevated in both ethanol-naïve HFD (37.9±1.1 g) and HFD-E (42.7±1.0 g) mice when compared to Chow-E (27.0±0.4 g), iHFD-E (29.4±0.8 g), ethanol-naïve Chow mice (28.5±0.5 g), or ethanol-naïve iHFD mice (31.2±0.8 g) (F(5,53)=58.74, p<0.001; Fig. 11A). Body mass did not differ between Chow-E, iHFD-E, or ethanol-naïve Chow mice, but similar to findings in Experiment 2, HFD-E mice gained significantly more body mass than ethanol-naïve HFD mice. Overall, one-way ANOVA indicated a significant change of adiposity (F(5,53)=62.80, p<0.001; Fig. 11B), lean mass (F(5,53)=67.61, p<0.001; Fig. 11C), and fluid mass (F(5,53)=87.86, p<0.001; Fig. 11D). For individual groups, there appeared to be no significant effect of ethanol consumption in the Chow-E group on any body composition measurement compared to ethanol-naïve Chow mice. Ethanol-naïve iHFD were not significantly difference from ethanol-naïve Chow mice on any measure except for increased fluid mass. Ethanol-naïve HFD mice had increased body mass, adiposity, and fluid mass, and reduced lean mass (compared to Chow mice), effects that were exacerbated by ethanol consumption in the HFD-E mice. iHFD-E mice had a reduction in fluid mass compared to ethanol-naïve iHFD mice, but otherwise had no significant changes to body mass, adiposity, or fluid mass and no significant differences on any measure compared to ethanol-naïve Chow mice.
Fig 11. Intermittent HFD does not alter body mass, adiposity, lean mass, or fluid mass.
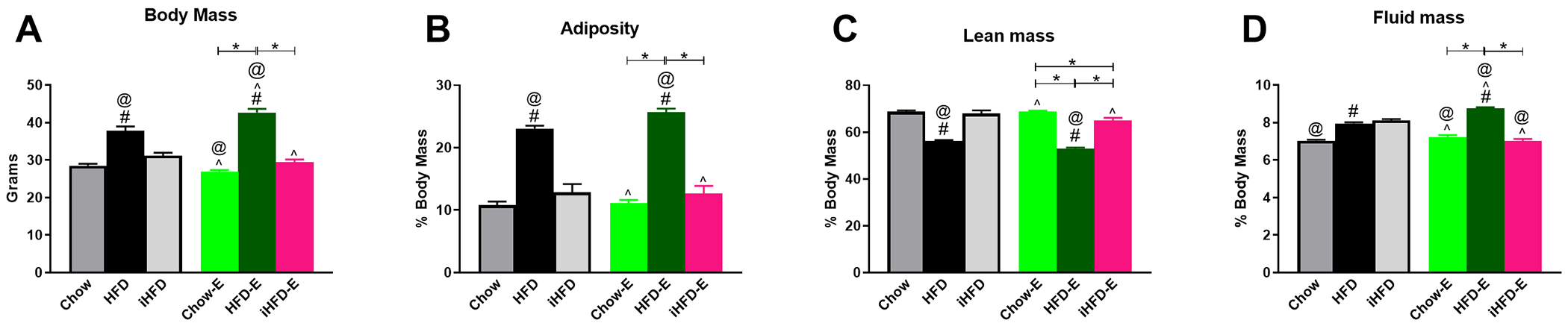
Body composition data of ethanol exposed mice compared to iHFD-, Chow-, and HFD-fed ethanol-naïve mice. A) Body mass of control mice and experimental mice prior to metabolic testing. Percent body mass of: B) adiposity, C) lean mass, and D) fluid mass. * indicates significant difference between indicated groups, # indicates significant difference from Chow, ^ indicates significant difference from HFD, @ indicates significant difference from iHFD, as determined by one-way ANOVA with Bonferroni post-hoc analysis; p<0.05.
For the ITT, one-way ANOVA (F(5,53)=28.59, p<0.001; Fig. 12A) followed by Bonferroni’s post-hoc test indicated that 4-hour fasting glucose was elevated in the ethanol-naïve HFD (209±6 mg/dl) mice compared to Chow-E (162±5 mg/dl), iHFD-E (157±10 mg/dl), and ethanol-naïve Chow mice (166±7 mg/dl), with an even greater increase in HFD-E mice (271±10 mg/dl). One-way ANOVA (F(5,53)=8.147, p<0.001; Fig. 12B,C,D) indicated that insulin sensitivity was reduced in the ethanol-naïve HFD group (975±968 glucose mg/dl*min) compared to ethanol-naïve Chow mice (−4321±852 glucose mg/dl*min), ethanol-naïve iHFD mice (−5968±906 glucose mg/dl*min), Chow-E mice (−3823±809 glucose mg/dl*min), and HFD-E mice (−3513±882 glucose mg/dl*min). Interestingly, ethanol-naïve iHFD mice (−5968±906 glucose mg/dl*min) had significantly greater insulin sensitivity compared to ethanol-naïve HFD mice (975±968 glucose mg/dl*min) and iHFD-E mice (−1699±559 glucose mg/dl*min).
Fig 12. Intermittent HFD promotes insulin resistance and glucose intolerance.
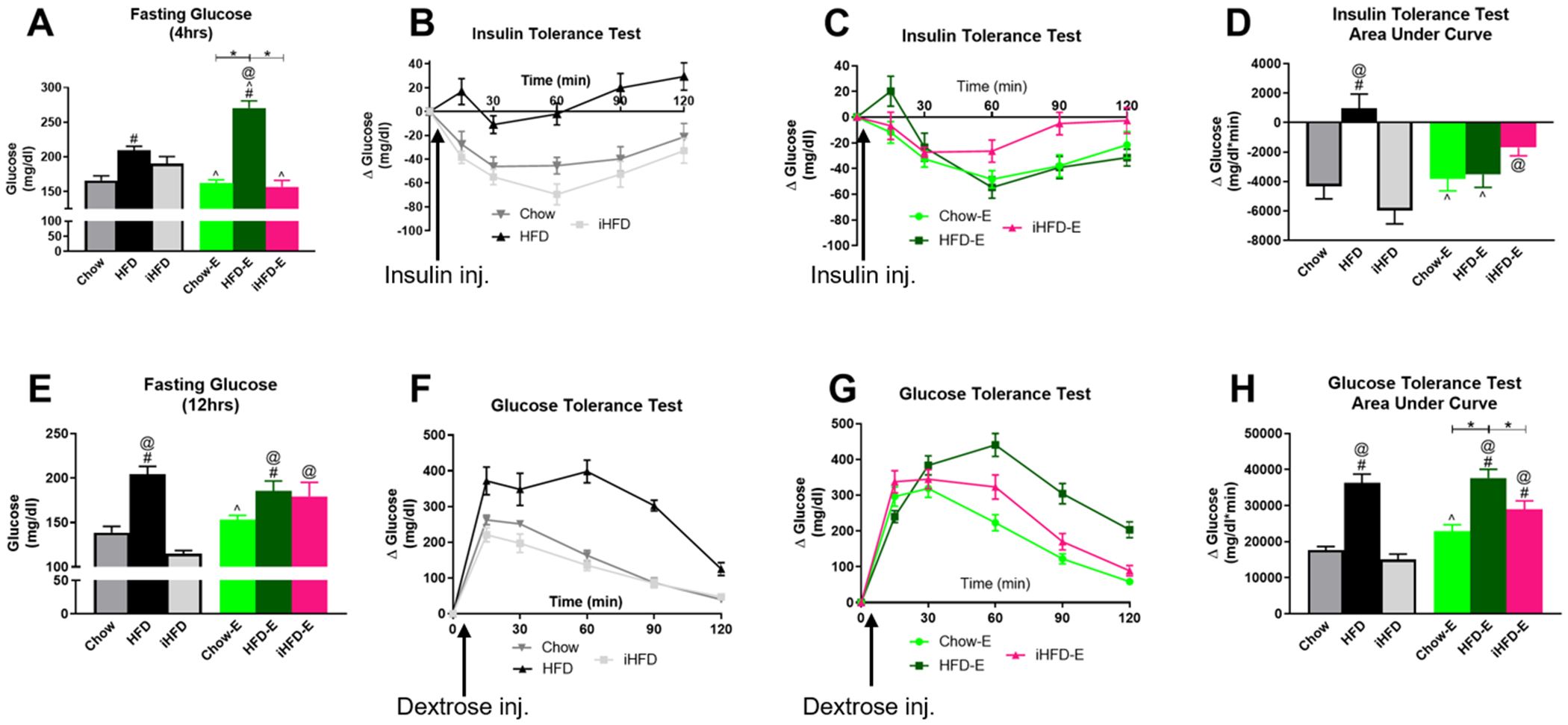
A) 4-hour fasting blood glucose levels prior to insulin tolerance test. Change in blood glucose levels over time following insulin injection in control groups (B) and experimental groups (C); data are normalized to 0 at baseline. D) Area under the curve for change in blood glucose levels during insulin tolerance test. E) 12-hour fasting blood glucose levels prior to glucose tolerance test. Change in blood glucose levels over time following dextrose injection in control groups (F) and experimental groups (G); data are normalized to 0 at baseline. H) Area under the curve for change in blood glucose levels during glucose tolerance test. * indicates significant difference between indicated groups, # indicates significant difference from Chow, ^ indicates significant difference from HFD, @ indicates significant difference from iHFD, as determined by one-way ANOVA with Bonferroni post-hoc analysis; p<0.05. Arrows indicate time point of insulin or dextrose injection following initial glucose measurements at time zero.
For the GTT, one-way ANOVA (F(5,53)=11.65, p<0.001; Fig. 12E) indicated that 12-hour fasting glucose levels were reduced in ethanol-naïve iHFD mice (115±3 mg/dl) compared to ethanol-naïve HFD mice (205±9 mg/dl), HFD-E mice (186±11 mg/dl), and iHFD-E mice (179±16 mg/dl). One-way ANOVA (F(5,53)=22.02, p<0.001; Fig. 12F,G,H) indicated a significant increase in the GTT area under the curve in HFD-E (37634±2428 glucose mg/dl*min) compared to Chow-E mice (22871±1827 glucose mg/dl*min), iHFD-E mice (28952±2342 glucose mg/dl*min), ethanol-naïve Chow mice (17699±938 glucose mg/dl*min), and ethanol-naïve iHFD mice (15086±1488 glucose mg/dl*min). There was also a significant increase in GTT AUC in iHFD-E mice (28952±2342 glucose mg/dl*min) compared to ethanol-naïve Chow mice (17699±938 glucose mg/dl*min) and ethanol-naïve iHFD mice (15086±1488 glucose mg/dl*min).
Discussion
The current study aimed to examine the impact of HFD on ethanol intake in distinct scheduled access periods. Overall, the findings indicate that ad libitum HFD access only altered ethanol intake when ethanol intake was ad libitum (UAE+HFD mice, Experiment 1). Ad libitum HFD however, did not significantly alter ethanol intake when ethanol access was limited (LAE+HFD, Experiment 2; HFD-E, Experiment 3). Intermittent HFD scheduling (iHFD-E, Experiment 3) induced binge eating-like behaviors that resulted in escalated ethanol intake on days in which HFD was not available. These findings suggest that scheduling access is an important factor in determining the role of HFD on ethanol intake. Furthermore, these findings suggest that HFD intake binge-like behaviors in a limited access model can transfer towards binge ethanol intake behaviors.
Following HFD and ethanol intake periods, body composition and glucose and insulin function was then assessed in these mice. Although UAE+HFD mice in Experiment 1 had lower ethanol intake than controls, this moderate level of ethanol intake did not alter HFD-induced changes in body mass, adiposity, lean mass, fluid mass, or insulin sensitivity compared to mice given HFD for the same amount of time without ethanol. LAE+HFD mice in Experiment 2 had similar levels of ethanol intake compared to LAE+Chow mice, and this level of ethanol intake appears to synergize with HFD consumption to promote increased body mass, adiposity, lean mass, or fluid mass, with similar levels of insulin resistance and glucose intolerance compared to HFD control mice. Similar results were found in the HFD-E mice in Experiment 3, where ethanol consumption further enhanced HFD-induced changes in body composition and fasting glucose levels. Interestingly, while iHFD-E mice in Experiment 3 remained lean and had body composition similar to Chow control mice, iHFD-E mice had insulin and glucose sensitivity similar to HFD mice. This suggests even moderate amounts of HFD consumption in the face of enhanced ethanol intake may synergize to produce metabolic deficits even with a lack of body composition changes. Overall, the total of the findings here indicate that ethanol access schedules are critical in mediating the impact of HFD on ethanol intake, while patterned ethanol intake in the face of HFD may synergize to produce more profound metabolic disturbances than HFD alone.
Our finding that ad libitum HFD access decreases ad libitum ethanol intake (UAE+HFD group) is a common finding in the literature in both male and female rodents (Feng et al, 2012; Gelineau et al, 2017). This may be due to a number of factors. One possibility is that rodents find HFD a rewarding food choice and prefer this to the potential rewarding effects of ethanol. Although not directly assessed here, animals exposed to continuous HFD typically undergo an initial hyperphagic response (Hariri and Thibault, 2010), suggesting this diet has some rewarding value leading to escalation of intake at least short-term. Further research supports this hypothesis by showing that HFD exposure can alter dopamine activity in the nucleus accumbens (Fordahl et al, 2016; Rada et al, 2012), ventral tegmental area (Valdivia et al, 2015), and alter neuronal signaling in other key brain regions mediating reward processing (Barson et al, 2012; Sharma et al, 2013; Valdivia et al, 2014). The current finding that UAE+HFD mice have a lower ethanol preference than UAE+Chow mice further supports this hypothesis. We only examined 10% ethanol intake in the UAE cohort, however, and it will be important to examine the preference and intake of ethanol at higher concentrations such as was performed in the LAE cohort. Another possibility is that ethanol metabolism and/or clearance may have been altered in UAE+HFD mice due to changes in body composition (increased adiposity and decreased lean mass) when compared with UAE+Chow mice (Feldstein, 1978; Reed and Kalant, 1977). C57Bl/6J mice typically consume enough ethanol to reach pharmacologically relevant BEC levels in relatively short access periods (~ two hours) (Becker and Lopez, 2004). C57Bl/6J mice have also been shown to have numerous drinking bouts when ethanol is provided over extended time periods (Risinger et al, 1998). Therefore, although not directly examined here, it is plausible that the UAE+Chow mice may have had numerous bouts of drinking over the 24-hour access period. If UAE+HFD mice had similar initial bouts but prolonged ethanol clearance/metabolism due to changes in adiposity or lean mass, then they may not seek as much ethanol in subsequent bouts to maintain pharmacologically relevant BECs, thus lowering their total ethanol consumption over the 24-hour access period. This possibility will be fully addressed in future studies.
Extending the hypothesis that HFD has a higher relative reward value than ethanol, LAE+HFD mice had significantly lower ethanol preference compared to controls. This effect appeared to be more pronounced at 10% ethanol, with less differences in ethanol preference at 15% and 20% ethanol. This finding may suggest that the rewarding value of 10% ethanol was lower in HFD exposed mice across the two models, but that this may be overcome at higher ethanol doses. Overall though, 10% ethanol consumption was low in the LAE model but increased significantly at higher concentrations, suggesting that the overall reward value of 10% ethanol may have generally been low when given in a 4-hour access period. Therefore, caution must be used when directly comparing the UAE and LAE models given the differences in ethanol access schedules (24 vs 4 hrs, continuously vs intermittently). Importantly, findings from the LAE model suggest that HFD does not greatly alter ethanol intake when ethanol access is limited. This is an important consideration given the previous preclinical literature indicating a reduction in ethanol intake by HFD, but numerous clinical findings indicate that ethanol stimulates HFD intake and vice versa (Breslow et al, 2013; Caton et al, 2004; Feng et al, 2012; Gelineau et al, 2017; Piazza-Gardner and Barry, 2014).
Intriguingly, mice that had HFD on a limited, intermittent schedule (iHFD-E mice) developed binge eating-like patterns that appeared to trigger increased ethanol consumption in a limited access schedule. This model may better recapitulate the clinical findings above that HFD and ethanol may stimulate over-consumption of both reward modalities. Given the shared neurocircuitry involved in reward value of palatable diets and ethanol (Kenny, 2011), it is possible that binge-like HFD consumption may have sensitized this shared neurocircuitry to produce higher ethanol intake levels. Previous research, however, shows intermittent high fat diet exposure decreases ethanol intake in rats (Sirohi et al, 2017b, 2017a; Villavasso et al, 2019), suggesting the iHFD model described here may not translate across species. It is important to note, though, that these previous studies in rats used multiple HFD access periods per week and included multiple weeks of intermittent HFD access prior to the initiation of ethanol intake studies, as opposed to our model which utilized a 48 hr initial HFD period prior to ethanol initiation and then a single 24hr HFD access period per week interspersed with ethanol access. Together, these findings suggest the number and length of HFD binge-like episodes in relation to ethanol exposures may be a critical factor in cross-sensitization of binge behaviors across reward modalities. Examination of these hypotheses will be of great interest in future studies.
Extensive previous research has shown moderate ethanol consumption improves insulin sensitivity in both clinical (Traversy and Chaput, 2015) and preclinical settings (Hong et al, 2009). Therefore, it was surprising that UAE+HFD mice, which consumed a moderate amount of ethanol on a per day basis for this mouse strain, had insulin resistance of a similar magnitude to HFD mice without ethanol intake history. The level of HFD-induced insulin resistance in control HFD mice here is similar to our previous research (Loloi et al, 2018; Williams et al, 2016). Consistent with the finding for insulin resistance, 4-hour fasting glucose in UAE+HFD mice was elevated to a similar degree as observed in the HFD mice. The reason for the lack of replication between our current study and previous research indicating ethanol consumption improves insulin sensitivity in HFD exposed animals is unclear, but previous research does indicate that twice daily intra-gastric ethanol exposure was more beneficial to improve insulin sensitivity in HFD-fed rats than continuous free access intake, even when total daily ethanol dosage (5g/kg) was accounted (Feng et al, 2012). This was due to differences in peak plasma ethanol concentrations of the different modes of exposure. Since daily ethanol intake in the UAE+HFD mice was generally between 2-3g/kg per day, this level of ethanol intake may not have been high enough to produce beneficial effects on insulin sensitivity.
In contrast to this hypothesis, LAE+HFD mice, which had higher ethanol intake levels than UAE+HFD mice (~5g/kg per day over the last three weeks of the study) did not have improved insulin sensitivity compared to HFD controls. Body composition and glucose tolerance was also drastically disturbed in the LAE+HFD. Specifically, LAE+HFD mice had increased body mass, adiposity, and fluid mass, with decreased lean mass compared to ethanol-naïve HFD mice. In addition, these mice had elevated 4-hour fasting glucose levels and were glucose intolerant. Such changes in body composition and insulin and glucose function were not seen in LAE+Chow mice, suggesting that the enhanced metabolic disturbances in LAE+HFD were not just additive effects of ethanol on top of those seen in HFD mice, but potentially a synergistic effect. HFD-E mice in the Experiment 3 also had similar, and potentially synergistic, effects of ethanol and HFD consumption on body composition and insulin and glucose function. iHFD-E mice, which drank significantly more ethanol than HFD-E mice but did not gain as much body mass, also had trends toward insulin insensitivity and had significant glucose intolerance. It is possible that such disturbances would have continued to worsen over longer exposure periods in this model, further suggesting synergistic HFD and ethanol interactions. Although the current data cannot speak to the precise mechanism of this potential synergistic action, previous findings indicate that ethanol can greatly impact glucose metabolism by a variety of mechanisms including impairments in intestinal glucose absorption, endogenous pancreatic insulin secretion, glucose effectiveness, and counter-regulatory responses (Steiner et al, 2015). Future studies using sophisticated hyperinsulinemic-euglycemic clamp with isotropic tracer methods will be needed to identify tissue-specific disturbances in insulin and glucose action as seen in the LAE+HFD and iHFD-E models.
Overall, the models of combined ethanol and HFD consumption described here point to little benefit of ethanol in the face of metabolic dysfunction, at least in male mice. The concept that moderate ethanol drinking has beneficial health effects has come under increased scrutiny in the past few years (Griswold et al, 2018) and brings back into debate the potential interactive role of ethanol and HFD in the development of metabolic diseases, such as Type II diabetes. Indeed, given the well-established roles of ethanol and HFD as individual risk factors for the development of metabolic disturbances, and the increasing understanding that chronic ethanol and HFD have similar effects on peripheral and central signaling mechanisms, it is surprising that clinical evidence points to moderate ethanol consumption as a mitigating factor in HFD-induced metabolic disturbances. The findings here suggest that there are many factors that may influence how ethanol and HFD interact to promote or mitigate metabolic disturbances, such as frequency and duration of ethanol access. However, this was only tested in male mice and needs to be extended to female mice and other species. It should be noted that several studies report a U- or J-shaped relationship between ethanol and insulin function (Kiechl et al, 1996; Lazarus et al, 1997; Villegas et al, 2004), or that beneficial metabolic effects of ethanol may only be seen in those individuals without obesity or insulin resistance (Yokoyama, 2011). Such findings further suggest the need to better examine the interactions of ethanol and HFD on insulin action and glucose tolerance both clinically and pre-clinically while controlling for time course, duration, and frequency of both ethanol and HFD exposures, as well as sex as a biological variable.
Acknowledgement
Funding: This work was supported by the National Institutes of Health [grant numbers R01 AA026865 and F31 AA027697].
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
References
- Barson JR, Karatayev O, Chang GQ, Johnson DF, Bocarsly ME, Hoebel BG, et al. (2009). Positive relationship between dietary fat, ethanol intake, triglycerides, and hypothalamic peptides: counteraction by lipid-lowering drugs. Alcohol 43: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Morganstern I, Leibowitz SF (2012). Neurobiology of consummatory behavior: mechanisms underlying overeating and drug use. ILAR J 53: 35–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF (2004). Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res 28: 1829–38. [DOI] [PubMed] [Google Scholar]
- Breslow RA, Chen CM, Graubard BI, Jacobovits T, Kant AK (2013). Diets of drinkers on drinking and nondrinking days: NHANES 2003-20081-3. Am J Clin Nutr 97: 1068–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo CA, Leibowitz SF, Karatayev O, Hoebel BG (2004). A high-fat meal or injection of lipids stimulates ethanol intake. Alcohol 34: 197–202. [DOI] [PubMed] [Google Scholar]
- Caton SJ, Ball M, Ahern A, Hetherington MM (2004). Dose-dependent effects of alcohol on appetite and food intake. Physiol Behav 81: 51–58. [DOI] [PubMed] [Google Scholar]
- Czyzyk TA, Sahr AE, Statnick MA (2010). A Model of Binge-Like Eating Behavior in Mice That Does Not Require Food Deprivation or Stress. Obesity 18: 1710–1717. [DOI] [PubMed] [Google Scholar]
- Fan AZ, Russell M, Dorn J, Freudenheim JL, Nochajski T, Hovey K, et al. (2006). Lifetime alcohol drinking pattern is related to the prevalence of metabolic syndrome. The Western New York Health Study (WNYHS). Eur J Epidemiol 21: 129–138. [DOI] [PubMed] [Google Scholar]
- Feldstein A (1978). The Metabolism of Alcohol: On the Validity of the Widmark Equations, in Obesity, and in Racial and Ethnic Groups. J o[ Stud Alcohol 39: . [DOI] [PubMed] [Google Scholar]
- Feng L, Han B, Wang R, Li Q, Bian D, Ma C, et al. (2012). The frequency of daily ethanol consumption influences the effect of ethanol on insulin sensitivity in rats fed a high-fat diet. Br J Nutr 107: 850–7. [DOI] [PubMed] [Google Scholar]
- Fordahl SC, Locke JL, Jones SR (2016). High fat diet augments amphetamine sensitization in mice: Role of feeding pattern, obesity, and dopamine terminal changes. Neuropharmacology 109: 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelineau RR, Arruda NL, Hicks JA, Monteiro De Pina I, Hatzidis A, Seggio JA (2017). The behavioral and physiological effects of high-fat diet and alcohol consumption: Sex differences in C57BL6/J mice. Brain Behav 7: e00708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SRM, Tymeson HD, et al. (2018). Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 392: 1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Zheng K, Benedé-Ubieto R, Cubero FJ, Nevzorova YA (2018). The Lieber-DeCarli Diet-A Flagship Model for Experimental Alcoholic Liver Disease. Alcohol Clin Exp Res 42: 1828–1840. [DOI] [PubMed] [Google Scholar]
- Hardaway JA, Jensen J, Kim M, Mazzone CM, Sugam JA, Diberto JF, et al. (2016). Nociceptin receptor antagonist SB 612111 decreases high fat diet binge eating. Behav Brain Res 307: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri N, Thibault L (2010). High-fat diet-induced obesity in animal models. Nutr Res Rev 23: 270–299. [DOI] [PubMed] [Google Scholar]
- Haslam DW, James WPT (2005). Obesity. Lancet 366: 1197–1209. [DOI] [PubMed] [Google Scholar]
- Hong J, Smith RR, Harvey a E, Núñez NP (2009). Alcohol consumption promotes insulin sensitivity without affecting body fat levels. Int J Obes (Lond) 33: 197–203. [DOI] [PubMed] [Google Scholar]
- Kenny PJ (2011). Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci 12: 638–651. [DOI] [PubMed] [Google Scholar]
- Kiechl S, Willeit J, Poewe W, Egger G, Oberhollenzer F, Muggeo M, et al. (1996). Insulin sensitivity and regular alcohol consumption: large, prospective, cross sectional population study (Bruneck study). BMJ 313: 1040–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus R, Sparrow D, Weiss ST (1997). Alcohol Intake and Insulin Levels: The Normative Aging Study. Am J Epidemiol 145: 909–916. [DOI] [PubMed] [Google Scholar]
- Loloi J, Miller AJ, Bingaman SS, Silberman Y, Arnold AC (2018). Angiotensin-(1-7) Contributes to Insulin-Sensitizing Effects of Angiotensin Converting Enzyme Inhibition in Obese Mice. Am J Physiol Metab ajpendo.00281.2018doi: 10.1152/ajpendo.00281.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI (2011). Intermittent (Every-Other-Day) Drinking Induces Rapid Escalation of Ethanol Intake and Preference in Adolescent and Adult C57BL/6J Mice. Alcohol Clin Exp Res 35: 652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Pareja M, Guallar-Castillón P, Mesas AE, López-García E, Rodríguez-Artalejo F (2013). Obesity-Related Eating Behaviors Are Associated with Higher Food Energy Density and Higher Consumption of Sugary and Alcoholic Beverages: A Cross-Sectional Study. PLoS One 8: e77137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papachristou GI, Papachristou DJ, Morinville VD, Slivka A, Whitcomb DC (2006). Chronic alcohol consumption is a major risk factor for pancreatic necrosis in acute pancreatitis. Am J Gastroenterol 101: 2605–10. [DOI] [PubMed] [Google Scholar]
- Paulson QX, Hong J, Holcomb VB, Nunez N p (2010). Effects of body weight and alcohol consumption on insulin sensitvity. Nutr J 14: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza-Gardner AK, Barry AE (2014). A Qualitative Investigation of the Relationship Between Consumption, Physical Activity, Eating Disorders, and Weight Consciousness. Am J Heal Educ 45: 174–182. [Google Scholar]
- Rada P, Avena NM, Barson JR, Hoebel BG, Leibowitz SF (2012). A High-Fat Meal, or Intraperitoneal Administration of a Fat Emulsion, Increases Extracellular Dopamine in the Nucleus Accumbens. Brain Sci 2: 242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed TE, Kalant H (1977). Bias in calculated rate of alcohol metabolism due to variation in relative amounts of adipose tissue. J Stud Alcohol 38: 1773–1776. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Brown MM, Doan AM, Oakes RA (1998). Mouse strain differences in oral operant ethanol reinforcement under continuous access conditions. Alcohol Clin Exp Res 22: 677–84. [DOI] [PubMed] [Google Scholar]
- Sharma S, Fernandes MF, Fulton S (2013). Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. Int J Obes 37: 1183–1191. [DOI] [PubMed] [Google Scholar]
- Sirohi S, Cleef A Van, Davis JF (2017a). Binge-like intake of HFD attenuates alcohol intake in rats. Physiol Behav 178: 187–195. [DOI] [PubMed] [Google Scholar]
- Sirohi S, Cleef A Van, Davis JF (2017b). Intermittent access to a nutritionally complete high-fat diet attenuates alcohol drinking in rats. Pharmacol Biochem Behav 153: 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JL, Crowell KT, Lang CH (2015). Impact of alcohol on glycemic control and insulin action. Biomolecules 5: 2223–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversy G, Chaput JP (2015). Alcohol Consumption and Obesity: An Update. Curr Obes Rep 4: 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia S, Cornejo MP, Reynaldo M, Francesco PN De, Perello M (2015). Escalation in high fat intake in a binge eating model differentially engages dopamine neurons of the ventral tegmental area and requires ghrelin signaling. Psychoneuroendocrinology 60: 206–216. [DOI] [PubMed] [Google Scholar]
- Valdivia S, Patrone A, Reynaldo M, Perello M (2014). Acute High Fat Diet Consumption Activates the Mesolimbic Circuit and Requires Orexin Signaling in a Mouse Model. PLoS One 9: e87478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villavasso S, Shaw C, Skripnikova E, Shah K, Davis JF, Sirohi S (2019). Nutritional contingency reduces alcohol drinking by altering central neurotransmitter receptor gene expression in rats. Nutrients 11: 2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas R, Salim A, O’Halloran D, Perry IJ (2004). Alcohol intake and insulin resistance. A cross-sectional study. Nutr Metab Cardiovasc Dis 14: 233–40. [DOI] [PubMed] [Google Scholar]
- Williams IM, Otero YF, Bracy DP, Wasserman DH, Biaggioni I, Arnold AC (2016). Chronic Angiotensin-(1–7) Improves Insulin Sensitivity in High-Fat Fed Mice Independent of Blood PressureNovelty and Significance. Hypertension 67: 983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama H (2011). Beneficial effects of ethanol consumption on insulin resistance are only applicable to subjects without obesity or insulin resistance; drinking is not necessarily a remedy for metabolic syndrome. Int J Environ Res Public Health 8: 3019–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


