Abstract
Significance: Opioid use disorder and transition to injection drug use (IDU) are an urgent, nationwide public health crisis. Wounds and skin and soft tissue infections (SSTIs) are common complications of IDU that disproportionately affect people who inject drugs (PWID) and are a major source of morbidity and mortality for this population.
Critical Issues: Injections in a nonsterile environment and reusing or sharing needles facilitates bacterial inoculation, with subsequent risk of serious complications such as sepsis, gangrene, amputation, and death. PWID are susceptible to infections with a wide spectrum of organisms beyond common culprits of SSTI, including Clostridium and Bacillus spp., as well as Candida.
Recent Advances: Syringe services programs (SSPs) are cost-effective and successful in reducing harms associated with IDU. SSPs provide new equipment to PWID and aid in discarding used equipment. SSPs aim to reduce the risks of unhygienic injecting practices, which are associated with transmission of infections and blood-borne pathogens.
Future Directions: Concurrently run SSPs and wound care clinics are uniquely positioned to facilitate care to PWID. Providing new, sterile equipment as well as early wound care intervention can reduce morbidity and mortality as well as health care expenditures by reducing the number of SSTI and injection-related wounds that require hospital admission. Establishment of wound care clinics as part of an SSP represents an untapped potential to reduce harm.
Keywords: wounds, skin and soft tissue infections, injection drug use, syringe services programs

Daniela P. Sanchez, BS
Scope and Significance
Opioid use disorder and addiction, along with transition to injection drug use (IDU) and its associated complications, have developed into an urgent, nationwide public health crisis.1 IDU and its associated morbidity and mortality are on the rise, not only in the United States, but worldwide.2 A meta-analysis study revealed that people who inject drugs (PWID) have mortality rates 13 times higher than their peers.2 The main causes of premature death among PWID are direct consequences of IDU, such as fatal drug overdose, suicide, trauma, and infection with human immunodeficiency virus (HIV), and other blood-borne viruses transmitted through shared injection equipment.2,3 Wounds and skin and soft tissue infection (SSTI) are a major source of morbidity and mortality for this population as well.4–6 Wounds and SSTIs are cited as the most common reasons PWID visit the emergency department (ED), and they are a risk factor for readmission as well as death among PWID presenting to the ED.6
Translational Relevance
PWID are susceptible to infections with a wide variety of microorganisms.7,8 Standard infectious disease workup and broad spectrum antibiotic therapy may not suffice in the treatment of wounds and infections in this population. Metagenomic next-generation sequencing (mNGS), the analysis of microbial genetic material in patient samples, has the capacity to detect all the potential pathogens (bacteria, fungi, viruses, and parasites) in a clinical sample, making it a potentially pivotal diagnostic tool in infectious disease. Utilizing mNGS for detecting a broad range of human pathogens can represent a novel point of care approach to guide diagnosis and treatment in PWID specifically, given the broad spectrum of pathogens identified in PWID wounds.9
Clinical Relevance
PWID are faced with many health risks, including increased risk of premature mortality, HIV, hepatitis C virus (HCV), hepatitis B virus (HBV), injection-related wounds,10 and SSTIs.2,11 Wounds and SSTIs are common complications of IDU and disproportionately affect PWID.4,10,12 Wounds and SSTIs in PWID may contribute further to addiction as patients seek to reduce the pain and may lead to worse outcomes (e.g., sepsis, gangrene, amputation).5,12 The synergistic provision of wound care at syringe services program (SSP) is proposed as a unique upstream access solution for this high priority population.
Overview
Injection-related wounds and SSTIs constitute not only a substantial problem for PWID but also a major financial burden to the health care system.11 We hypothesize that early detection of wounds and SSTI in this population can lead to harm reduction and improved health outcomes. In this study, we review and summarize the literature available on cutaneous wounds and SSTIs in PWID and present SSPs as a possible strategic model that can be implemented to prevent and manage consequences of IDU in PWID as part of an overarching effort to decrease morbidity, mortality, and cost associated with these preventable wounds and associated infections.
IDU: infections, wounds, and other implications
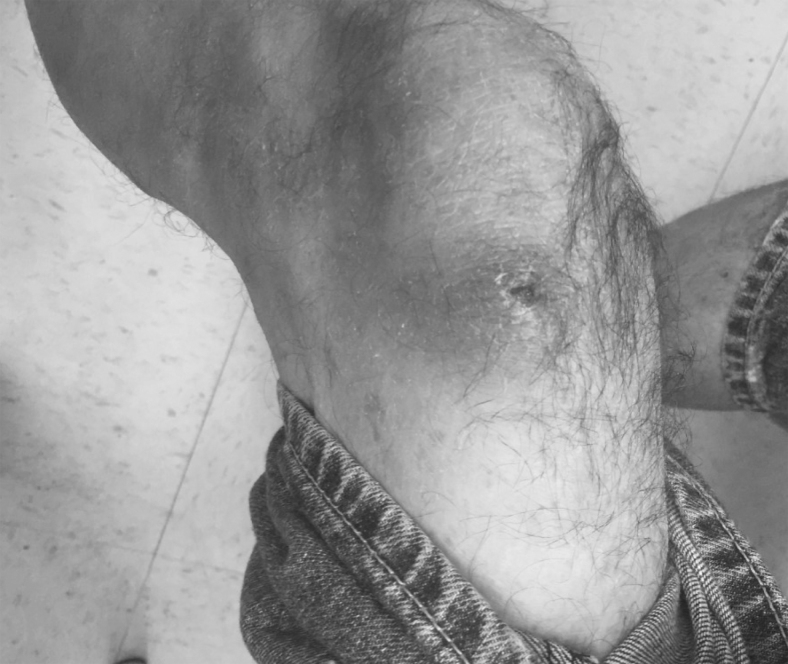
Wounds and SSTI disproportionately affect PWID and are a major source of morbidity and mortality for this population.4,12,13 Hospitalizations for IDU-related SSTI are rising in the United States and have doubled between 2000 and 2010.14 Abscesses, cellulitis, and injection-related wounds are highly prevalent among PWID (Fig. 1), likely due to repeated injection into the same anatomical site, resulting in venous and other tissue trauma.12 Injections in a nonsterile environment and practices such as reusing needles or sharing used equipment facilitate local soft tissue bacterial inoculation, with subsequent risk of serious complications such as sepsis, gangrene, amputation, and death.5 Figure 2 illustrates these and other common etiologies of chronic wounds and SSTI in PWID, further discussed below.
Figure 1.
IDU-related lower extremity wound. IDU, injection drug use.
Figure 2.
Common etiologies of wounds and SSTIs in PWID. PWID, people who inject drugs; SSTIs, skin and soft tissue infections.
Accidental subcutaneous and intramuscular injections (“missed hits”) occur with high frequency among those who aim to inject intravenously and are also associated with high rates of infection. One study found that ever reporting symptoms of an injection site infection were twice as likely among those who reported that they had had “missed hits.”15 Factors associated with “missed hits” include poor injection technique and/or hygiene as well as poor vascular access.16 “Skin popping” or intentional subcutaneous injection of drugs, often in patients with poor vascular access, has also been associated with increased risk of infection.16 Frequency of injection has a direct relationship with infection risk as well,17 with reduced injection frequency leading to reduced risk of bacterial infections.10 Type of drug injected also affects the risk of SSTI, with heroin and speedball (combined heroin and cocaine) posing a greater risk of infection and methamphetamines decreasing this risk.18 Black tar heroin has specifically been associated with higher rates of skin infections.14
Furthermore, the direct and indirect effects of IDU may result in nerve injury and subsequent neuropathy among PWID.19 The toxicity of the substances injected and the effects of the needles themselves are directly damaging to the nerves, indirectly causing local inflammation.19 A single case report depicting neuropathy after self-injection of heroin describes nerve damage, muscle fibrosis, and lymph node enlargement (changes attributed to diffuse effects of drug spread), visible on ultrasound examination.19 Neurophysiological manifestations of IDU-mediated neuropathy such as the case described above are thought to be due to extravascular heroin dissemination; however, the exact pathogenesis is not well defined.19
The pathophysiology of vascular injury following intra-arterial injection is thought to be multifactorial. Direct toxic effects from the injected drugs themselves likely produce a chemical endarteritis resulting in endothelial injury, platelet activation, and localized thrombosis.20 In addition, common heroin diluting agents, such as quinine, serve to compound injury by contributing to local vasospasm and thrombosis, thus further compromising blood flow and promoting tissue necrosis.21 Arterial vasospasm in the setting of IDU is transient and thought to be due to local release of norepinephrine as well as direct chemical effects of the injected drug on blood vessels.22 Myocyte necrosis as well as interstitial edema with arterial and capillary thrombosis may be seen histologically demonstrating the end organ damage from IDU.20
IDU also has deleterious effects on the veins of the lower extremities and damage inflicted progresses even after active IDU has ceased.23 As a result, PWID have been found to have an increased risk of chronic venous disease (CVD).24,25 CVD is associated with progressively debilitating sequelae such as lower extremity edema, varicose veins, skin changes, refractory ulcers, and chronic pain.26 IDU has been reported to augment the typical risk factors associated with CVD, such as deep venous thrombosis (DVT). Relative to the general population, PWID have an increased prevalence of DVT, which increases the risk of CVD by 25-fold.24 IDU also has implications for mobility and balance; overall physical activity among PWID is low, and in a stuporous drug state, PWID may have inactive lower extremity muscles with resultant diminished venous return.27 Injecting into the lower extremities may additionally cause nerve and muscle damage, with resultant impaired calf muscle and ankle joint function; compromised walking mobility has been found to have a direct relationship with severity of CVD in this population.25 Mobility deficits, such as difficulty walking, climbing stairs, and working, are thus a largely underrecognized consequence of IDU.26 A study among 713 participants with a history of IDU found that 25% of their population had abnormal Tinetti balance and gait scores, with 20% of the participants being at risk for falls and 7.7% at high risk for falls.25
Injection into the femoral vein (groin injecting) is another common practice, with approximately half of PWID reporting injecting into the groin within the last month.28 Groin injecting has been associated with significant risk of injury to the femoral vein and artery, as well as increased risk of blood borne and bacterial infections.29 One study of 855 patients found groin injecting to be associated with higher levels of sepsis (twice as common among those injecting into the groin), DVT (more than three times as common), and hepatitis C infection.28 PWID in the extremities are ∼35 times more likely to develop venous ulcers compared with those who never injected.30
Chronic, painful wounds, along with their accompanying malodor and stigma, can further marginalize this population and make it increasingly difficult for PWID to integrate themselves into society, obtain and maintain employment and financial security, and create a social support system.12 Numerous publications have demonstrated that patients with chronic wounds, regardless of etiology, suffer from and manifest depression and anxiety symptoms at three times the rate of the population without wounds.31 This is often attributed to wound odor, wound duration, and associated pain, immobility, and social isolation.31 Pain, depression, and social marginalization may perpetuate a cycle of continued drug use, as patients seek to ameliorate these symptoms.12 Patients using opioids to control pain may further hinder their wound healing capacity, as evidence suggests that use of systemic opioids has a negative impact on wound healing.32
PWID disproportionately suffer from protein and calorie malnutrition, further impairing their wound healing capacity.33–35 One study on 140 PWID without acute organic pathology found that 92% were underweight and 56% had experienced weight loss of greater than 5% of their body mass.34 Nutritional status was very poor; 18% were deeply malnourished by subjective nutritional assessment.34 PWID have also been found to have high rates of food insecurity (55% compared to 11.1% of all Americans), which has been associated with increased rates of behaviors that may increase the likelihood of HIV/HCV transmission, such as sharing and reutilizing injection equipment.36,37 SSPs may be an ideal venue for addressing PWID's food-related needs in addition to reducing rates of sharing and reusing injection equipment.
Homelessness has consistently been associated with IDU and is yet another complicating factor for healing wounds and SSTIs in PWID.38,39 One study of 2,396 PWID noted that 66% of participants had experienced primary homelessness at least once in their lifetime.40 Homelessness sets PWID at a disadvantage with regard to wound healing as it can limit access to clean running water and limit follow-up care options. Hygiene, including regular cleaning of wounds and SSTIs, is often compromised among those who are homeless, and patients may often find themselves reutilizing dirty dressings out of necessity.
The burden of wounds and SSTIs on health care costs is significant; PWID are disproportionately uninsured, with a study of 349 patients reporting 36% were uninsured.11 Most (56%) of PWID rely on Medicaid or Medicare.11 This estimate done at a safety net hospital in Miami determined that the single-center annual cost of treating preventable wounds and bacterial infections in this population was $11.4 million during the span of one single year.11 While the majority of the cohort had SSTIs, complicated infections such as endocarditis/bacteremia contributed to the majority of associated health care costs.11
Complexity of wound infections in PWID
Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA), is among the most prevalent bacterial pathogens found in wounds and SSTI in PWID.41 As a prominent member of the cutaneous microbiome,42,43 S. aureus has developed multiple mechanisms to overcome skin innate immunity.44–46 Most SSTI originate from a site of colonization in the skin, making S. aureus the leading culprit in most of these infections.44
The high prevalence of MRSA in PWID raises a concern of antimicrobial resistance among this population from a public health standpoint.41 Transmission of multidrug-resistant organisms among PWID is enabled by substandard sanitation and lack of access to clean water and quality antimicrobials.47 As these patients present to the ED and become hospitalized for an average of 5–7 days,48 they can introduce multidrug-resistant organisms to other vulnerable populations in the health care system, further driving antimicrobial resistance.47
Additional gram-positive pathogens, such as Streptococcus pyogenes, have been associated with altering pain and neural regulatory pathways of the immune response to promote bacterial survival in skin.49 S. pyogenes is often the culprit in SSTI and is the leading cause of necrotizing fasciitis, and secretes streptolysin S, which can directly activate nociceptive neurons to produce increased pain during infection.49 Once activated, nociceptors release calcitonin gene-related peptide into infected tissues, which inhibits neutrophilic recruitment and dampens the inflammatory response against S. pyogenes.49 Increased pain from wounds and SSTI may act as contributing factors to perpetuate the addiction cycle in PWID, as these patients may turn to illicit drugs in an effort to self-manage their pain.50
PWID are susceptible to infections with wide a spectrum of microorganisms beyond common culprits of SSTI, including spore-forming Clostridium and Bacillus spp.6 Spores are typically found in the environment (soil, dust, and water), remain viable for long periods of time, and can contaminate illicit drugs, needles, and other injection equipment. Black tar heroin, for example, when cut with organic materials (such as coffee grounds or dirt) has an increased contamination risk from Clostridium botulinum and other organisms that contribute to tissue damage and toxicity.51 Once injected, these organisms may initially cause localized infections, including necrotizing fasciitis in immunocompromised patients52; however, injected spores can germinate and release neurotoxins that may cause serious systemic illness.6 In addition, skin or muscle popping (injecting directly into skin or muscle) may lead to soft tissue damage and necrosis, resulting in a suitable environment for anaerobic bacteria such as Clostridium spp. to thrive. Infections with spore-forming bacteria among PWID are common in the United States. For example, IDU account for most cases of tetanus in New York since the 1950s.6 In the United Kingdom, ∼300 severe infections, including 52 deaths, caused by spore-forming bacteria have been reported among PWID since the year 2000.6
Invasive fungal infections have been increasing among PWID; IDU has reemerged as a risk factor for candidemia.8 A retrospective review of 198 patients in a tertiary center in Massachusetts with candidemia revealed that 24 had a history of IDU; these patients were more likely to be coinfected with HCV and have end-organ involvement, including endocarditis and osteomyelitis.8 Severe cases of diagnostically challenging chronic meningitis caused by Candida dubliniensis associated with previous drug use have been reported as well.9 Extensive infectious disease diagnostic workup (including magnetic resonance imaging, repeated cisternal cerebrospinal fluid (CSF) analyses, and lumbar meningeal biopsy) proved to be unrevealing in these cases, until CSF mNGS revealed the presence of Candida species.9 Wounds, SSTIs, and other infections associated with antimicrobial-resistant microorganisms and unpredictable bacterial and fungal contaminants in PWID underline the importance for future utilization of culture independent methods, such as mNGS,53 to guide treatment and antibiotic therapy in this at-risk underserved population.
Syringe services programs
Given the high number of those affected by the opioid crisis and the numerous health risks PWID are faced with, it is of great public health interest to find strategies for harm reduction.1 Harm reduction is an evidence-based approach to ameliorate substance use disorders, where drug consumption is not necessarily decreased, but the direct and indirect harms associated with illicit drug use are mitigated.54 Harm reduction practices are designed to meet PWID “where they are at,” and arm PWID with knowledge and practical strategies with the goal of reducing the negative consequences that often accompany drug use.55 Evidence has demonstrated that harm reduction programs (e.g., SSPs and opioid overdose education and naloxone distribution programs) are cost-effective and successful in reducing harms associated with drug use.54,56 Nonetheless, these strategies have historically been met with controversy and resistance by health care professionals, law makers, and the general public alike.54
PWID have grown to trust SSPs because of the nonjudgmental, nonabstinence-based harm reduction philosophy on which they are based and the central tenet of meeting PWID where they are.57 SSPs provide new, unused equipment to PWID, aid in discarding used and contaminated equipment, and provide a variety of other health services. These programs aim to reduce the risks of unhygienic injecting practices, such as needle sharing or reusing, which are associated with transmission of infections and blood-borne pathogens, including HIV and HCV.56 According to the CDC, there were over 44,000 acute cases of HCV in the United States in 2017, and IDU was the most common risk factor.56,58 The CDC also reports PWID accounted for 10% of all new HIV diagnoses in 2018.59
In addition to reducing transmissible infections, SSPs can serve as an early intervention wound care resource to decrease morbidity and mortality from injection-related wounds and associated infections.60 Evidence suggests the prevalence of wounds in PWID is between 55% and 68%.60 Many PWID delay seeking care for their wounds, possibly due to socioeconomic barriers such as cost, access to care, and pervasive stigma.61–63 If left untreated, these wounds can lead to serious complications such as gangrene, sepsis, endocarditis, and death.17,60 Mobile outreach community-based SSPs that serve as a setting for wound care and infection screening can help reduce health care costs and improve the health of PWID.60 These programs provide a safe space where PWID can receive wound dressing and assessments, cleaning, incision and drainage of acute abscesses, compression treatment of venous ulcers, and prescription antibiotics as deemed clinically appropriate by the supervising provider. The Baltimore Needle Exchange Program Wound Clinic found that, on average, each visit at their community-based SSP cost $146.45, which was substantially less than the cost of clinic or hospital-based treatment, especially considering that this population has disproportionately high rates of ED utilization.60 Another specialized clinic providing accessible and cost-effective medical care to patients with SSTIs, the majority (61%) of which are PWID, is the Integrated Soft Tissue Infection Services (ISIS) Clinic in San Francisco, California.64 In its first year of operation, the ISIS Clinic successfully treated SSTIs and decreased utilization of hospital resources previously allotted to treating these patients, saving ∼$8,765,200 by reducing ED visits and inpatient acute care bed days by 33%, admissions to the surgical service by almost 50%, and operating room procedures by over 70%.64
Another example of an SSP coupled with wound care services is our Infectious Disease Elimination Act (IDEA) SSP in Miami, Florida. The IDEA SSP, the only legal SSP in Florida, hosts a weekly wound care clinic that has served 222 patients between August 2017 and December 2019. The IDEA SSP provides early screening, topical antimicrobials, and other wound care interventions for PWID who otherwise may not have sought care until later in their disease course. At the IDEA SSP, the approach to wound care begins with routine screening and referral at the time of syringe exchange. Once a patient presents to this student-run wound care clinic, a team of medical students conducts a thorough history and physical examination, focusing on evaluating patients' wounds or SSTIs in addition to any other complaints they may have.65 The students present to an attending physician, who then evaluates the patient and may conduct point of care ultrasound. A management plan is formulated, and low-barrier immediate access to on-site incision and drainage, on-site antibiotics, or referral to a higher level of care (e.g., for hand and neck abscesses) occurs. Appropriate wound care supplies are dispensed and follow-up is scheduled on an ad hoc basis.66
As is illustrated by IDEA's model, SSP may also reduce harm by providing a point of access to the health care system in this vulnerable population that generally has limited engagement with conventional health care services due to frequent experiences of stigma and mistreatment in health care settings.61 PWID have described some encounters with health care personnel as stigmatizing and embarrassing, which discourages them from accessing and utilizing health services.62 Having an acute wound or SSTI may prompt PWID to utilize health care resources and SSP are optimally positioned to leverage the trust gained and connect patients to appropriate health care services.
Discussion
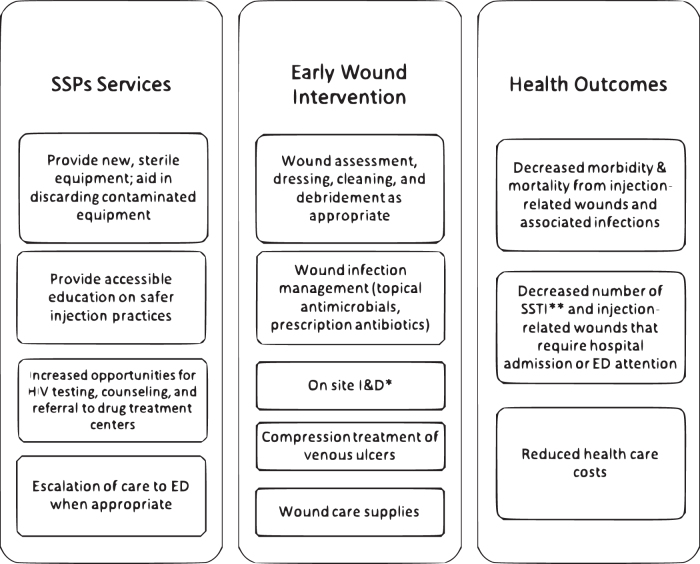
The high prevalence of wounds and SSTI in PWID coupled with disproportionately high, yet delayed, ED utilization60 suggests that there is an unmet need for early primary intervention in this population. Concurrently run SSP and wound care clinics are uniquely positioned to facilitate care to the underserved PWID population, who typically do not seek wound care in a timely manner unless it is offered as an ancillary service to a trusted SSP. Such programs have the ability to provide cost-effective treatment for injection-related wounds and SSTIs, wound care supplies, and education in safer injection practices leading to reduced risk of transmission of blood-borne pathogens, such as HIV, HCV, and HBV.57,67–70 Some high-risk injection practices that have been associated with higher rates of SSTIs are directly targeted by SSPs.18 Providing new, sterile equipment as well as early wound care intervention, including wound debridement and incision and drainage of abscesses, can reduce morbidity and mortality in PWID as well as health care expenditures by reducing the volume of SSTI and injection-related wounds that require hospital admission or ED attention (Fig. 3).60,64 Utilizing mNGS for detecting a broad range of pathogens represents a novel point of care approach to guide diagnosis and treatment in PWID, who are susceptible to infection by a wide variety of organisms. When deemed appropriate by on-site clinicians, escalation of care with warm hand offs to the ED can also be coordinated by SSPs. PWID can benefit from additional innovative services such as point of care ultrasound. Ultrasound has been demonstrated to improve the accuracy of diagnosis, and thus treatment, of cellulitis and cutaneous abscesses, which can sometimes appear similar in clinical presentation.71
Figure 3.
Relationship between combined SSPs and early wound intervention with health outcomes of PWID. SSPs, syringe services programs.
Based on the principles of harm reduction, our IDEA SSP distributes to its community materials about safer injection techniques, which are coupled with counseling on risks of different types of injection practices as well as general safety information.72 For instance, PWID are warned about the risk of blood-borne infections with intravenous injections as well as the risks of deep abscesses with intramuscular injections.72 Vein care, sterile practices, and overdose risks are also emphasized. Other harm reduction efforts include the Harm Reduction Coalition (HRC), which is a national advocacy organization that aims to promote the health of PWID.55 Services provided by HRC include educational materials, such as the Getting Off Right Safety Manual, created in collaboration by providers and PWID, which includes basic medical information regarding overdose and infection risks as well as injection techniques and other strategies to keep the community safer.
Increasing the frequency of interactions between PWID and health care providers in a comfortable, familiar, environment also allows increased opportunities for HIV testing and counseling as well as referral to drug treatment centers.57,68 A 2,000 study found that PWID utilizing an SSP were five times more likely to enter methadone treatment and 60% more likely to remain in treatment when compared to non-SSP users.73 These programs tend to be affiliated with or located near academic centers in urban settings, which also allows for further opportunities for linkage to care as well as cutting-edge research that may help to prevent wounds/SSTI in this population.74
One of the main concerns surrounding SSPs is the thought that providing free injection equipment will promote IDU; however, research data suggest that this is not the case.68 There is no evidence to support the idea that SSPs increase the duration, frequency, or initiation of illicit drug use.68 On the contrary, SSPs have been demonstrated to improve public health.57 SSPs that are coupled with wound care, early SSTI intervention, as well as access to other health care resources and social services can reduce health care costs,54,56,60 improve the quality of life of PWID, and reduce the morbidity and mortality of injection-related wounds and infections.57,60,67–70 Due to syndemic factors75 associated with barriers to care for PWID internationally, it is possible that coronavirus disease 2019 (COVID-19) may exacerbate social isolation, poverty, untreated mental health problems, and other factors that may lead to increased drug use as a coping mechanism in this population. Thus, there is a need for a swift, potent, feasible, acceptable, and sustainable (i.e., cost-effective) intervention for PWID immediately. Utilizing a harm reduction approach for PWID in a syndemic framework to enhance access to integrated care delivered through an SSP is timely and innovative and has the potential to reduce the morbidity and mortality of IDU-associated wounds.
Best practice recommendations for IDU-related wound management
Guidelines for IDU-related wound care in SSP do not exist. Herein, we summarize the best practice recommendations that have emerged from our experience. Successful treatment begins with preparation, and we recommend having, at a minimum, protective equipment for staff (gloves, face shields, etc.) and all needed clinic equipments to maintain safety (e.g., biohazard sharps disposal containers). We suggest stocking incision and drainage kits, wound care products (e.g., nonadherent gauze, tape, skin cleansing materials), and a few basic oral antibiotics (e.g., doxycycline, trimethoprim/sulfamethoxazole, cefalexin). Following the usual SSP intake, comprehensive history and physical examination is mandatory, and given the risk factors associated, we strongly encourage all patients to undergo any point of care testing available (e.g., HIV, hepatitis C). Abscesses should be drained at the level of comfort and expertise of the treating clinician. Generally, very large abscesses and ones located too close to major named arteries should be referred to the ED (e.g., neck). Portable ultrasound device is not mandatory, but can be useful to best characterize the anatomical relationship of the abscess to blood vessels. Wound care should be kept simple (e.g., clean the wound/skin daily and apply a clean dressing) and supplies provided. Since follow-up visits are unlikely, the clinician should exercise caution regarding application of compression given the high prevalence of arterial disease. Packing of superficial wounds is not needed.
All patients who appear septic (i.e., febrile, lethargic) or who present outside of clinic hours should be referred the nearest ED with the warmest handoff possible (e.g., written referral, ambulance, walking the patient over to the ED if practical, see Fig. 4). Our recommendations are limited to our experience at the IDEA SSP and our unique academic medical center environment. Our best practices are an introduction to comprehensive care delivered via an SSP, and it is expected that based on these principles, each clinic will establish specific standard operating procedures that best fit its unique scenario and leads to best outcomes.
Figure 4.
Best practice recommendations for SSP for primary and secondary prevention of wounds and SSTIs in PWID.
Summary
Establishment of wound care clinics as part of an SSP represents an untapped potential to reduce harm. Based on the findings of this review and our own clinical practice at the IDEA SSP, we have developed Fig. 4 to summarize our best practice recommendations for prevention, early diagnosis, and treatment of wounds in PWID. First, we recommend establishment of a wound care clinic associated with an SSP to offer a welcoming, low-barrier environment in a location frequented by PWID.57 Integration of medical education into the provision of services is essential to improve diagnostic skills of health care workers in training, to provide early access to marginalized populations, and combat stigma associated with PWID within our health care workforce. Both health care workers in training and PWID should be educated on safe injection practices with the provided resources.72 Point of care ultrasound can aid in SSTI diagnosis and treatment and provides another opportunity for medical education.71 On site incision, drainage, and debridement of abscesses and wounds can prevent complications from SSTIs and decrease ED utilization. Provision of onsite wound care supplies and antibiotics further decreases barriers to care among PWID.60 Currently, we recommend broad spectrum antibiotic coverage against staphylococcus and streptococcus species, but there is urgent need for future studies on wound/SSTI microbiome in this population to elucidate additional bacterial species and/or yeast responsible for infections as well as the spread of antibiotic resistance genes in PWID.
Take-Home Messages
Wounds and SSTIs disproportionately affect PWID and are a major source of morbidity and mortality for this population.
Injection-related wounds and SSTIs represent a major problem for PWID as well as a financial burden to the health care system.
SSPs aim to reduce the risks of unhygienic injecting practices, such as needle sharing or reusing, which are associated with transmission of infections and blood-borne pathogens, such as HIV, HCV, and HBV.
Providing new, sterile equipment as well as early wound care intervention in the setting of SSPs can reduce morbidity and mortality in PWID as well as health care expenditures, improve the quality of life of PWID, and reduce the morbidity and mortality of injection-related wounds and infections.
PWID are susceptible to infections with a wide spectrum of organisms.
Wounds, SSTIs, and other infections, associated with antimicrobial-resistant microorganisms and unpredictable contaminants in PWID, underline the importance for future utilization of culture-independent methods to guide treatment and antibiotic therapy in this at-risk underserved population.
Abbreviations and Acronyms
- CDC
Centers for Disease Control
- CNCP
chronic noncancer pain
- CSF
cerebrospinal fluid
- CVD
chronic venous disease
- DVT
deep venous thrombosis
- ED
emergency department
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- HRC
Harm Reduction Coalition
- I&D
incision and drainage
- IDU
injection drug use
- ISIS
Integrated Soft Tissue Infection Services
- mNGS
metagenomic next-generation sequencing
- MRSA
methicillin-resistant Staphylococcus aureus
- PWID
people who inject drugs
- SSP
syringe services program
- SSTI
skin and soft tissue infections
Authors' Contributions
H.T., I.P., and H.L.-T. conceived the idea presented. D.P.S. devised the outline and wrote the article with input from all listed authors. All authors commented on best practice recommendations and worked on editing the article.
Acknowledgments and Funding Sources
No funding was received.
Author Disclosure and Ghostwriting
All authors confirm no conflict of interest, and no ghost writers were used to write this article.
About the Authors
Daniela Sanchez, BS, is a Research Fellow at the University of Miami Dr. Phillip Frost Department of Dermatology. She is completing the final year of her medical education at Boston University School of Medicine. Ms. Sanchez graduated with a B.S. in Microbiology and Cell Science from the University of Florida. Hansel Tookes, MD, MPH, is an Assistant Professor in the Department of Medicine at the University of Miami Miller School of Medicine. He joined the faculty in the Division of Infectious Diseases after completing his residency at Jackson Memorial Hospital. Irena Pastar, PhD, is a Research Associate Professor of Dermatology at the University of Miami Miller School of Medicine. Hadar Lev-Tov, MD, is an Assistant Professor and board-certified dermatologist at the University of Miami Dr. Phillip Frost Department of Dermatology. He completed his residency in Dermatology at the Albert Einstein College of Medicine. Dr. Lev-Tov completed a clinical and translational research fellowship with focus on treatments for chronic leg ulcers at the University of California, Davis. He holds a Master's degree in clinical research from the University of California, Davis and serves as the Director of the Wound Healing Fellowship program.
References
- 1. Compton WM, Boyle M, Wargo E. Prescription opioid abuse: problems and responses. Prev Med 2015;80:5–9 [DOI] [PubMed] [Google Scholar]
- 2. Degenhardt L, Hall W, Warner-Smith M. Using cohort studies to estimate mortality among injecting drug users that is not attributable to AIDS. Sex Transm Infect 2006;82 Suppl 3(Suppl 3):iii56–iii63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: a systematic review and meta-analysis. Bull World Health Organ 2013;91:102–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention (CDC). Soft tissue infections among injection drug users—San Francisco, California, 1996–2000. MMWR Morb Mortal Wkly Rep 2001;50:381–384 [PubMed] [Google Scholar]
- 5. Annie FH, Bates MC, Uejio CK, Bhagat A, Kochar T, Embrey S. The impact of the drug epidemic on the incidence of sepsis in West Virginia. Cureus 2018;10:e3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Binswanger IA, Takahashi TA, Bradley K, Dellit TH, Benton KL, Merrill JO. Drug users seeking emergency care for soft tissue infection at high risk for subsequent hospitalization and death. J Stud Alcohol Drugs 2008;69:924–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palmateer NE, Hope VD, Roy K, et al. Infections with spore-forming bacteria in persons who inject drugs, 2000–2009. Emerg Infect Dis 2013;19:29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Poowanawittayakom N, Dutta A, Stock S, et al. Reemergence of intravenous drug use as risk factor for Candidemia, Massachusetts, USA. Emerg Infect Dis 2018;24:631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilson MR, O'Donovan BD, Gelfand JM, et al. Chronic meningitis investigated via metagenomic next-generation sequencing. JAMA Neurol 2018;75:947–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cahn BA, Bartholomew TS, Hardik PP, Pastar I, Tookes HE, Lev-Tov H. Correlates of injection-related wounds and skin infections amongst persons who inject drugs and use a syringe service programme: A single center study. Int Wound J. Epub ahead of print. DOI: 10.1111/iwj.13572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tookes H, Diaz C, Li H, Khalid R, Doblecki-Lewis S. A cost analysis of hospitalizations for infections related to injection drug use at a county safety-net hospital in Miami, Florida. PLoS One 2015;10:e0129360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith ME, Robinowitz N, Chaulk P, Johnson KE. High rates of abscesses and chronic wounds in community-recruited injection drug users and associated risk factors. J Addict Med 2015;9:87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dahlman D, Berge J, Björkman P, Nilsson AC, Håkansson A. Both localized and systemic bacterial infections are predicted by injection drug use: a prospective follow-up study in Swedish criminal justice clients. PLoS One 2018;13:e0196944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ciccarone D, Unick GJ, Cohen JK, Mars SG, Rosenblum D. Nationwide increase in hospitalizations for heroin-related soft tissue infections: associations with structural market conditions. Drug Alcohol Depend 2016;163:126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hope VD, Parry JV, Ncube F, Hickman M. Not in the vein: ‘missed hits', subcutaneous and intramuscular injections and associated harms among people who inject psychoactive drugs in Bristol, United Kingdom. Int J Drug Policy 2016;28:83–90 [DOI] [PubMed] [Google Scholar]
- 16. Brown PD, Ebright JR. Skin and soft tissue infections in injection drug users. Curr Infect Dis Rep 2002;4:415–419 [DOI] [PubMed] [Google Scholar]
- 17. Larney S, Peacock A, Mathers BM, Hickman M, Degenhardt L. A systematic review of injecting-related injury and disease among people who inject drugs. Drug Alcohol Depend 2017;171:39–49 [DOI] [PubMed] [Google Scholar]
- 18. Phillips KT, Stein MD. Risk practices associated with bacterial infections among injection drug users in Denver, Colorado. Am J Drug Alcohol Abuse 2010;36:92–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coraci D, Paolasso I, Santilli V, Padua L. Extravascular heroin injection causing neuropathy: ultrasound picture. Neurol Sci 2016;37:1887–1888 [DOI] [PubMed] [Google Scholar]
- 20. Coughlin PA, Mavor AI. Arterial consequences of recreational drug use. Eur J Vasc Endovasc Surg 2006;32:389–396 [DOI] [PubMed] [Google Scholar]
- 21. Quinine. Internationally peer reviewed chemical safety information. www.inchem.org/documents/pims/pharm/pim464.htm#SubSectionTitle:9.1.5%20%20Parenteral%20exposure (last accessed April7, 2021)
- 22. Silverman SH, Turner WW Jr. Intraarterial drug abuse: new treatment options. J Vasc Surg 1991;14:111–116 [DOI] [PubMed] [Google Scholar]
- 23. Pieper B, Kirsner RS, Templin TN, Birk TJ. Chronic venous disease and injection drug use. Arch Intern Med 2007;167:1807. [DOI] [PubMed] [Google Scholar]
- 24. Pieper B, Kirsner RS, Templin TN, Birk TJ. Injection drug use: an understudied cause of venous disease. Arch Dermatol 2007;143:1305–1309 [DOI] [PubMed] [Google Scholar]
- 25. Pieper B, Templin TN, Kirsner RS, Birk TJ. Injection-related venous disease and walking mobility. J Addict Dis 2010;29:481–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pieper B, Templin TN, Birk TJ, Kirsner RS. The standing heel-rise test: relation to chronic venous disorders and balance, gait, and walk time in injection drug users. Ostomy Wound Manage 2008;54:18–22 [PubMed] [Google Scholar]
- 27. Pieper B, Templin TN, Kirsner RS, Birk TJ. The impact of vascular leg disorders on physical activity in methadone-maintained adults. Res Nurs Health 2010;33:426–440 [DOI] [PubMed] [Google Scholar]
- 28. Hope VD, Scott J, Cullen KJ, Parry JV, Ncube F, Hickman M. Going into the groin: injection into the femoral vein among people who inject drugs in three urban areas of England. Drug Alcohol Depend 2015;152:239–245 [DOI] [PubMed] [Google Scholar]
- 29. Senbanjo R, Tipping T, Hunt N, Strang J. Injecting drug use via femoral vein puncture: preliminary findings of a point-of-care ultrasound service for opioid-dependent groin injectors in treatment. Harm Reduct J 2012;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pieper B, Templin TN, Kirsner RS, Birk TJ. Impact of injection drug use on distribution and severity of chronic venous disorders. Wound Repair Regen 2009;17:485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Renner R, Erfurt-Berge C. Depression and quality of life in patients with chronic wounds: ways to measure their influence and their effect on daily life. Chron Wound Care Manage Res 2017;4:143–151 [Google Scholar]
- 32. Herskovitz I, MacQuhae FE, Dickerson JE Jr, et al. Opioids' effect on healing of venous leg ulcers. J Invest Dermatol 2017;137:2646–2649 [DOI] [PubMed] [Google Scholar]
- 33. Stechmiller JK. Understanding the role of nutrition and wound healing. Nutr Clin Pract 2010;25:61–68 [DOI] [PubMed] [Google Scholar]
- 34. Santolaria-Fernández FJ, Gómez-Sirvent JL, González-Reimers CE, et al. Nutritional assessment of drug addicts. Drug Alcohol Depend 1995;38:11–18 [DOI] [PubMed] [Google Scholar]
- 35. Forrester JE. Nutritional alterations in drug abusers with and without HIV. Am J Infect Dis 2006;2:173–179 [PMC free article] [PubMed] [Google Scholar]
- 36. Coleman-Jensen, Alisha, Matthew P. Rabbitt, Christian A. Gregory, and Anita Singh. Household Food Security in the United States in 2018, ERR-270, U.S. Department of Agriculture, Economic Research Service, 2019 [Google Scholar]
- 37. Strike C, Rudzinski K, Patterson J, Millson M. Frequent food insecurity among injection drug users: correlates and concerns. BMC Public Health 2012;12:1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Linton SL, Celentano DD, Kirk GD, Mehta SH. The longitudinal association between homelessness, injection drug use, and injection-related risk behavior among persons with a history of injection drug use in Baltimore, MD. Drug Alcohol Depend 2013;132:457–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. National Health Care for the Homeless Council. Expanding Our Vision of the Possibilities for Preventive Care. Healing Hands, 21:3. Melissa Jean, Writer. Nashville, TN, 2017. Available at: www.nhchc.org [Google Scholar]
- 40. Topp L, Iversen J, Baldry E, Maher L; Collaboration of Australian NSPs. Housing instability among people who inject drugs: results from the Australian needle and syringe program survey. J Urban Health 2013;90:699–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bassetti S, Battegay M. Staphylococcus aureus infections in injection drug users: risk factors and prevention strategies. Infection 2004;32:163–169 [DOI] [PubMed] [Google Scholar]
- 42. Findley K, Grice EA. The skin microbiome: a focus on pathogens and their association with skin disease. PLoS Pathog 2014;10:e1004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grice EA, Kong HH, Conlan S, et al. Topographical and temporal diversity of the human skin microbiome. Science 2009;324:1190–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krishna S, Miller LS. Innate and adaptive immune responses against Staphylococcus aureus skin infections. Semin Immunopathol 2012;34:261–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Strbo N, Pastar I, Romero L, et al. Single cell analyses reveal specific distribution of anti-bacterial molecule Perforin-2 in human skin and its modulation by wounding and Staphylococcus aureus infection. Exp Dermatol 2019;28:225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Otto M. Staphylococcus colonization of the skin and antimicrobial peptides. Expert Rev Dermatol 2010;5:183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Holmes AH, Moore LS, Sundsfjord A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016;387:176–187 [DOI] [PubMed] [Google Scholar]
- 48. Palepu A, Tyndall MW, Leon H, et al. Hospital utilization and costs in a cohort of injection drug users. CMAJ 2001;165:415–420 [PMC free article] [PubMed] [Google Scholar]
- 49. Pinho-Ribeiro FA, Baddal B, Haarsma R, et al. Blocking neuronal signaling to immune cells treats streptococcal invasive infection. Cell 2018;173:1083–1097.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Voon P, Callon C, Nguyen P, et al. Self-management of pain among people who inject drugs in Vancouver. Pain Manag 2014;4:27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gonzales y Tucker RD, Frazee B. View from the front lines: an emergency medicine perspective on clostridial infections in injection drug users. Anaerobe 2014;30:108–115 [DOI] [PubMed] [Google Scholar]
- 52. Boulinguez S, Viraben R. Cutaneous Bacillus cereus infection in an immunocompetent patient. J Am Acad Dermatol 2002;47:324–325 [DOI] [PubMed] [Google Scholar]
- 53. Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet 2019;20:341–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vearrier L. The value of harm reduction for injection drug use: a clinical and public health ethics analysis. Dis Mon 2019;65:119–141 [DOI] [PubMed] [Google Scholar]
- 55. Harm Reduction Coalition: Getting Off Right Safety Manual. https://harmreduction.org/drugs-and-drug-users/drug-tools/getting-off-right (last accessed May8, 2020)
- 56. Sawangjit R, Khan TM, Chaiyakunapruk N. Effectiveness of pharmacy-based needle/syringe exchange programme for people who inject drugs: a systematic review and meta-analysis. Addiction 2017;112:236–247 [DOI] [PubMed] [Google Scholar]
- 57. Grau LE, Arevalo S, Catchpool C, Heimer R. Expanding harm reduction services through a wound and abscess clinic. Am J Public Health 2002;92:1915–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Centers for Disease Control: Surveillance for Viral Hepatitis—United States, 2017. https://www.cdc.gov/hepatitis/statistics/2017surveillance/pdfs/2017HepSurveillanceRpt.pdf (last accessed May8, 2020)
- 59. Centers for Disease HIV Surveillance Reports. https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (last accessed May8, 2020)
- 60. Robinowitz N, Smith ME, Serio-Chapman C, Chaulk P, Johnson KE. Wounds on wheels: implementing a specialized wound clinic within an established syringe exchange program in Baltimore, Maryland. Am J Public Health 2014;104:2057–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Biancarelli DL, Biello KB, Childs E, et al. Strategies used by people who inject drugs to avoid stigma in healthcare settings. Drug Alcohol Depend 2019;198:80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Paquette CE, Syvertsen JL, Pollini RA. Stigma at every turn: health services experiences among people who inject drugs. Int J Drug Policy 2018;57:104–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend 2013;131:23–35 [DOI] [PubMed] [Google Scholar]
- 64. Harris HW, Young DM. Care of injection drug users with soft tissue infections in San Francisco, California. Arch Surg 2002;137:1217–1222 [DOI] [PubMed] [Google Scholar]
- 65. Castillo M, Ginoza ME, Bartholomew TS, et al. When is an abscess more than an abscess? Syringe services programs and the harm reduction safety-net: a case report. Harm Reduct J 2020;17:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ginoza ME, Tomita-Barber J, Onugha J, et al. Student-run free clinic at a syringe Services Program, Miami, Florida, 2017–2019. AJPH 2020;110:988–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Des Jarlais DC, Marmor M, Paone D, et al. HIV incidence among injecting drug users in New York City syringe-exchange programmes. Lancet 1996;348:987–991 [DOI] [PubMed] [Google Scholar]
- 68. Wodak A, Cooney A. Do needle syringe programs reduce HIV infection among injecting drug users: a comprehensive review of the international evidence. Subst Use Misuse 2006;41:777–813 [DOI] [PubMed] [Google Scholar]
- 69. Kerr T, Small W, Buchner C, et al. Syringe sharing and HIV incidence among injection drug users and increased access to sterile syringes. Am J Public Health 2010;100:1449–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hagan H, Jarlais DC, Friedman SR, Purchase D, Alter MJ. Reduced risk of hepatitis B and hepatitis C among injection drug users in the Tacoma syringe exchange program. Am J Public Health 1995;85:1531–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Comer AB. Point-of-care ultrasound for skin and soft tissue infections. Adv Emerg Nurs J 2018;40:296–303 [DOI] [PubMed] [Google Scholar]
- 72. Merchants Quay Ireland: Safer Injecting Tips. https://www.drugsandalcohol.ie/12915/1/mqi_safer_injecting_guide.pdf (last accessed May8, 2020)
- 73. Hagan H, McGough JP, Thiede H, Hopkins S, Duchin J, Alexander ER. Reduced injection frequency and increased entry and retention in drug treatment associated with needle-exchange participation in Seattle drug injectors. J Subst Abuse Treat 2000;19:247–252 [DOI] [PubMed] [Google Scholar]
- 74. Centers for Disease Control: Syringe Service Programs for Persons Who Inject Drugs in Urban, Suburban, and Rural Areas—United States, 2013. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6448a3.htm (last accessed May8, 2020)
- 75. Singer M. John Wiley & Sons, 2009. Introduction to syndemics: a critical systems approach to public and community health. https://www.wiley.com/en-us/Introduction±to±Syndemics%3A±A±Critical±Systems±Approach±to±Public±and±Community±Health-p-9780470472033 (Iast accessed April7, 2021)