Abstract
Chimeric antigen receptors (CARs) are artificial fusion proteins that incorporate antigen-recognition domains and T cell signaling domains. CD30 is a cell surface protein expressed on Hodgkin's lymphoma, some T cell lymphomas, and some B cell lymphomas. CD30 has a restricted expression pattern in normal cells, so CD30 has good potential as a clinical target for CAR T cells. We compared three different anti-CD30 CAR designs incorporating a single-chain variable fragment derived from the 5F11 fully human monoclonal antibody. 5F11-28Z has hinge, transmembrane, and costimulatory domains from CD28 and a CD3ζ T cell activation domain. 5F11-CD828Z has hinge and transmembrane domains from CD8α, a CD28 costimulatory domain, and a CD3ζ T cell activation domain. 5F11-CD8BBZ is identical to 5F11-CD828Z, except for the replacement of the CD28 moiety with a 4-1BB moiety. We found that T cells expressing 5F11-CD8BBZ had lower levels of CD30-specific degranulation and cytokine release compared with CD28-containing CARs. When compared to the CD28-containing CARs, T cells expressing 5F11-CD8BBZ had higher levels of nonspecific functional activity, including degranulation, cytokine release, and proliferation, when stimulated with CD30-negative target cells. We established tumors in nod-scid common gamma-chain deficient (NSG) mice and treated the tumors with T cells expressing different CARs. T cells expressing 5F11-28Z were most effective at eradicating tumors. T cells expressing 5F11-CD828Z had intermediate effectiveness, and T cells expressing 5F11-CD8BBZ were least effective. CD30+ T cells are lost from cultures of T cells containing 5F11-28Z-expressing T cells. This indicated the killing of CD30+ T cells by the 5F11-28Z-expressing T cells. Despite this, the number of T cells in the cultures consistently accumulated to numbers needed for use in a clinical trial. Based on all in vitro and murine experiments comparing the different CARs, we selected 5F11-28Z for further development, and we have initiated a clinical trial testing 5F11-28Z T cells.
Keywords: CAR, CD30, chimeric antigen receptor, 4-1BB, CD28
Introduction
Chimeric antigen receptors (CARs) are fusion proteins that include an antigen-recognition domain, costimulatory domains such as CD28 and 4-1BB, and a T cell activation domain such as CD3ζ.1–7 T cells expressing anti-CD19 CARs have proven clinical efficacy in treating B cell lymphomas, which provides a rationale for the development of anti-CD30 CARs for treating CD30+ lymphomas.8–14 CD30 is also known as Ki-1 or tumor necrosis family receptor superfamily member 8 (TNFRSF8). CD30 is a type I transmembrane receptor.15,16 The function of CD30 remains unclear. Studies have suggested costimulatory functions regulating T cell responses among other context-dependent effects.17–23
CD30 is strongly expressed by the malignant Reed-Sternberg cells of Hodgkin's lymphoma (HL); CD30 is also expressed by anaplastic large cell lymphoma (ALCL), some other T cell lymphomas, and some B cell lymphomas.24–27 CD30 expression has been reported to be largely absent from non-lymphoid tissues.28 CD30 expression on B cells and T cells increases when these cells are activated.16,29 Because of the high expression of CD30 on some lymphomas and its restricted expression on non-hematopoietic tissues, CD30 is a promising target for CAR T cells.
CD30 is a target antigen that might allow CAR T cells to be utilized against a broader range of malignancies, including HL and T cell lymphomas. Brentuximab vedotin is an antibody-drug conjugate consisting of an anti-CD30 antibody conjugated to a microtubule-disrupting drug; brentuximab vedotin has clinical activity against HL and ALCL.30–33 Savoldo et al. reported preclinical results of a gamma-retroviral-encoded anti-CD30 CAR with a single-chain variable fragment (scFv) antigen-recognition domain derived from a murine antibody.34–36 In a clinical trial using the same CAR, a 39% overall response rate (ORR) was initially reported.37 A more recent update confirmed that anti-CD30 CAR T cells could induce complete remissions (CRs) of HL.38 Results from a different clinical trial of T cells expressing a lentiviral-encoded anti-CD30 CAR containing a murine scFv showed an ORR of 33% among 18 total patients, 17 with HL and 1 with ALCL.39 While these earlier clinical trials have yielded important findings, we reasoned that anti-CD30 CAR T cell therapies could be improved to increase the rate and durability of remissions. Moreover, CARs used in these prior anti-CD30 CAR clinical trials had binding domains derived from murine antibodies, which could lead to recipient-versus-CAR immune responses.40,41
Our goal was to design new anti-CD30 CARs with a binding domain derived from a fully human antibody and to select an anti-CD30 CAR design for use in a clinical trial. Because CAR design can affect CAR T cell function in mice and humans,7,8,42 we compared anti-CD30 CARs with different hinge and transmembrane domains, and we compared CARs with either CD28 or 4-1BB costimulatory domains. We designed an scFv binding domain derived from a fully human anti-CD30 antibody, and we selected an anti-CD30 CAR with favorable characteristics for clinical use.
Methods
Use of human cells and use of mice in experiments
Peripheral blood mononuclear cells (PBMCs) were from patients enrolled on National Cancer Institute (NCI) clinical trials. The use of patient samples for research was approved by the NCI Institutional Review Board. Informed consent was obtained from all patients. All animal studies were carried out on protocols approved by the NCI Animal Care and Use Committee.
PCR to quantify CD30 expression
CD30 cDNA copies were quantitated in samples of cDNA from human tissues in the Human Major Tissue quantitative polymerase chain reaction (qPCR) Panel II (Origene) by performing qPCR with a CD30-specific primer and probe set (Applied Biosystems, Foster City, CA). CD30+ HH cells served as a positive control for CD30 expression. A standard curve for the CD30 qPCR was created by amplifying dilutions of a plasmid that encoded the full-length cDNA of CD30 (Origene). β-actin cDNA copy numbers were quantified in the same tissues with a TaqMan β-actin primer and probe kit (Applied Biosystems) and used to normalize CD30 copy numbers. qPCR was conducted on a Roche LightCycler480.
We performed qPCR to assess differences in CD30 expression in LSIN-5F11-28Z-transduced T cells versus untransduced T cells from the same human donors. For these experiments, T cells were cultured and transduced as described below under “T cell cultures” and “Transductions.” On day 7 of culture, RNA was extracted from T cells and used to synthesize cDNA. The qPCR was performed on cDNA with a Roche LightCycler 96 real-time PCR system. CD30 qPCR was performed with a CD30-specific primer and probe set (Applied Biosystems). A standard curve for the qPCR was created by amplifying dilutions of a plasmid that encoded the full-length cDNA of CD30 (Origene). CD30 qPCR results were normalized to T cell β-actin and have been reported as CD30 transcript copies/100,000 actin transcript copies. For the actin qPCR, an actin plasmid (Origene plasmid ACTB NM_001101) was amplified in duplicate with a primer and probe set (Bio-rad ACTB qHsaCEP0036280) to generate a standard curve.
Design and construction of plasmids encoding anti-CD30 CARs
The sequence of the fully human anti-CD30 antibody 5F11 was obtained from a patent.43 The variable regions of 5F11 were used to design an scFv with the following pattern from N-terminus to C-terminus: light chain variable region, linker, and heavy chain variable region. The linker had the following amino acid sequence: GSTSGSGKPGSGEGSTKG.44
The first anti-CD30 CAR that we designed was designated 5F11-28Z. The 5F11-28Z sequence followed this pattern from the N-terminus to the C-terminus: CD8α signal sequence, 5F11 scFv, hinge and transmembrane domains of the CD28 molecule, the cytoplasmic region of CD28, and the cytoplasmic portion of CD3ζ. In the 5F11-CD828Z CAR, the CD28 hinge and transmembrane domains of 5F11-28Z were replaced with CD8α hinge and transmembrane domains. The 5F11-CD8BBZ CAR was identical to 5F11-CD828Z, except the cytoplasmic region of CD28 in 5F11-CD828Z was replaced by the cytoplasmic region of 4-1BB in 5F11-CD8BBZ. A negative-control CAR, SP6-CD828Z, has been previously reported.45 All of the above CARs were encoded by a self-inactivating lentivirus vector designated pRRLSIN.cPPT.MSCV.coDMF5.oPRE (LSIN).46
We constructed four CARs encoded by a gamma-retroviral vector called mouse stem cell virus-based splice-gag vector (MSGV1).47 The MSGV1-5F11-28Z plasmid encoded the same CAR as the lentiviral version of 5F11-28Z described above. We designed a CAR with an scFv derived from the murine anti-CD30 antibody HRS3.48 This CAR was designated HRS3-28Z. HRS3-28Z had the same sequence as 5F11-28Z, except for the different scFvs. We previously constructed a negative-control CAR MSGV1-SP6-CD828Z and the anti-CD19 CAR MSGV1-Hu19-CD828Z.7,41
DNA encoding all CAR sequences was codon optimized and synthesized by Invitrogen (GeneArt) with appropriate restriction sites and ligated into the appropriate vector plasmids by using standard methods.
T cell culture
T cells were cultured as described previously.44 In brief, PBMCs were thawed and washed in AIM V complete medium that contained AIM V medium (Thermo), 5% human AB serum (Valley Biomedical), 100 U/mL penicillin, and 100 μg/mL streptomycin. Before transduction, PBMCs were suspended at a concentration of 1 × 106 cells/mL in AIM V complete medium and stimulated with 50 ng/mL of the anti-CD3 monoclonal antibody OKT3 (Ortho). The medium contained 300 international units (IU)/mL of interleukin-2 (IL-2, Teceleukin; Roche). After transductions, T cell cultures were maintained in AIM V complete media with 300 IU/mL IL-2.
Transductions
To produce lentivirus-containing supernatant, 293T-17 cells (ATCC) were transfected with the following plasmids as detailed previously46: pMDG (encoding the vesicular stomatitis virus envelope), pMDLg/pRRE (encoding gag and pol), pRSV-Rev (encoding Rev), and the appropriate CAR-encoding plasmid.46
Twenty-four hours after the T cell culture initiation, transductions were conducted by combining AIM V complete medium, IL-2, lentivirus vector, and protamine sulfate with the stimulated T cells. The cells were cultured with the lentivirus vector for 48 h, and then they were washed and returned to culture. Gamma-retroviral transductions were performed as previously described 2 days after initiation of T cell cultures.41
Cell lines
The following CD30+ cell lines were used: HH (T cell lymphoma; ATCC), SUDHL-1 (lymphoma; ATCC), and L428 (HL, DSMZ). We transduced BV173 cells (leukemia, a kind gift of Dr. A. Wiestner, National Heart Lung and Blood Institute) to express full-length human CD30 (CD30-BV173). We also transduced BV-173 cells with low-affinity nerve growth factor receptor (NGFR-BV173) to serve as a CD30-negative control. The following CD30-negative cell lines were used: CCRF-CEM (T cell leukemia; ATCC), A549 (lung carcinoma; ATCC), TC71 (Ewing sarcoma, a kind gift of Dr. M. Tsokos, NCI), Sol8 (myoblast; ATCC), Panc10.05 (pancreatic carcinoma; ATCC), and MDA-MB-231 (breast carcinoma; ATCC).
CAR detection on transduced T cells and flow cytometry
T cells were washed and stained with either biotinylated protein L (GenScript) or a CD30 fragment-crystallizable (Fc) protein labeled with phycoerythrin (CD30-Fc-PE, Creative Biomart) to detect cell surface CAR molecules. Flow cytometry was performed by standard methods.44
The following antibodies were used: CD3 APC-Cy7 (Clone UCHT1; BD Biosciences), CD4 FITC/BV510 (Clone RPA-T4; BD Biosciences or Biolegend), CD8 PE-Cy7/eFluor450 (Clone RPA-T8; BD Biosciences or Thermo Scientific), and CD30 APC (Clone BerH8; BD Biosciences). Dead cells were excluded by 7-amino-actinomycin D (7-AAD; BD Biosciences). Analysis for all experiments was performed with FlowJo version 10 (Tree Star, Inc.).
CD107a degranulation assay
For each T cell culture tested, two tubes were prepared. One tube contained CD30-BV173 cells, and the other tube contained NGFR-BV173 cells. Both tubes contained CAR-transduced T cells, 1 mL of AIM-V complete medium, a titrated concentration of an anti-CD107a antibody (Clone H4A3; Thermo Scientific), and 1 μL of Golgi Stop (monensin, BD). All tubes were incubated at 37°C for 4 h and then stained for CD3, CD4, and CD8. Samples were analyzed by flow cytometry. Normalization was carried out by dividing the percentage of CD4+ or CD8+ T cells that were CD107a+ by the percentage of CD4+ or CD8+ T cells that were CAR+ by protein L staining.
Cytotoxicity assay
Cytotoxicity assays were conducted with a slightly modified version of a previously described assay.49 Cytotoxicity was measured by comparing the survival of HH CD30+ target cells relative to the survival of negative-control CCRF-CEM cells. In some experiments, CAR T cell cultures were depleted of natural killer (NK) cells by negative selection with anti-CD56 microbeads (Miltenyi Biotec).
Enzyme-linked immunosorbent assay
CD30-positive or CD30-negative target cells were combined with CAR-transduced T cells in duplicate wells of a 96-well round bottom plate in AIM-V complete medium at a 1:1 effector:target ratio. Plates were incubated at 37°C for 18–20 h. Following incubation, enzyme-linked immunosorbent assays (ELISAs) for interferon gamma (IFNγ) were performed by using standard methods as previously described.7 IL-2 and tumor necrosis factor alpha (TNFα) ELISAs (R&D Systems) were performed as recommended by the manufacturer. When two or more CARs were compared, cytokine release was normalized for CAR expression by dividing the cytokine levels by the fraction of T cells expressing a given CAR.
Proliferation assays
Carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled CAR T cells were cultured with CD30-BV173 cells or NGFR-BV173 target cells for 4 days followed by flow cytometry to assess proliferation. These assays were conducted as previously described, except for the different target cells.41
Mouse tumor experiments
All mouse experiments used immunocompromised NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (nod-scid common gamma-chain deficient [NSG]) mice at 6–8 weeks of age from NCI-Frederick or Jackson Laboratories. For experiments using CD30+ HH cells, mice were injected with 4 × 106 HH cells in a 1:1 solution of Matrigel (Corning) plus phosphate-buffered saline (PBS, Corning) intradermally. Eight days later, when measurable tumors were present, CAR T cells were injected intravenously. For the experiment with the CD30+ L428 lymphoma cell line, mice were injected with 8 × 106 L428 cells in 1:1 solution of Matrigel plus PBS intradermally. Nine days after L428 injection, mice were intravenously injected with CAR T cells. Tumors were measured using a caliper every 3 days. Tumor volume was calculated as (length × width × height)/2.
Statistics
All statistics are described in figure legends and were performed with GraphPad Prism version 10.
Results/Discussion
CD30 has a favorable expression pattern for CAR targeting
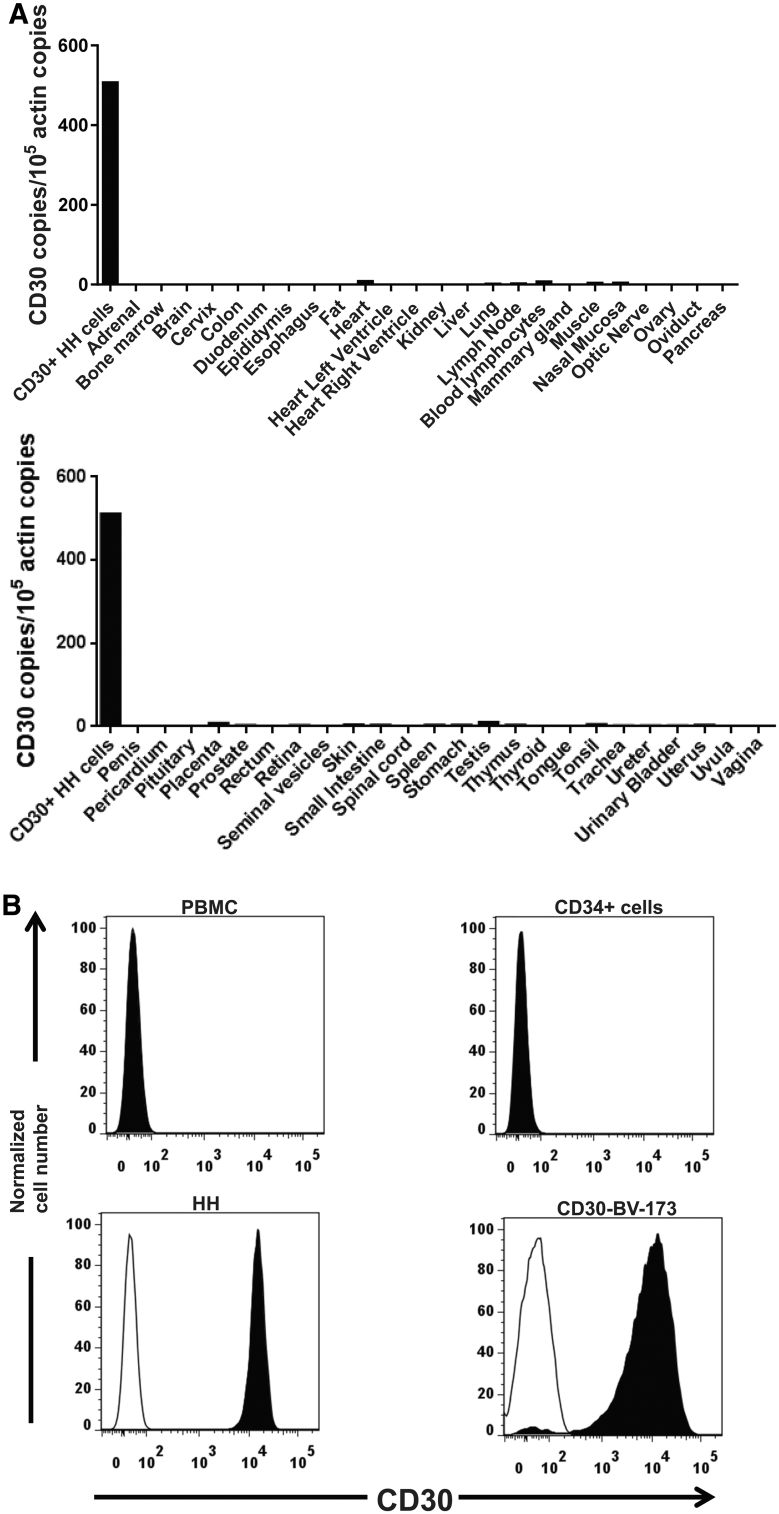
To avoid unacceptable toxicities in patients, antigens targeted by CARs should not be expressed on normal essential human cells. We assessed CD30 expression in normal tissues. CD30 RNA expression was assessed by conducting qPCR on a panel of cDNA samples. CD30 was found to be absent or expressed at very low levels in all major non-hematopoietic tissues. There was a low level of CD30 RNA expression in some lymphoid tissues, including lymph node and blood lymphocytes (Fig. 1A). Prior immunohistochemistry work conducted by other investigators showed that CD30 was not expressed on the non-hematopoietic cells of major human organs, except for decidual cells of the pregnant uterus; uterine decidual cells are present only in the uterus and only during pregnancy and the later half of the menstrual cycle.15,28,50 Low levels of CD30 RNA in some organs are likely explained by the presence of CD30+ leukocytes such as activated B cells and T cells.26 We evaluated CD30 protein expression by flow cytometry. CD30 was not detected on PBMC or CD34+ hematopoietic stem cells. In contrast, CD30 was detected on the HH and CD30-BV173 cell lines (Fig. 1B).
Figure 1.
Expression of CD30 in human tissues and cell lines. (A) CD30 RNA expression was assessed in all major normal tissues and the CD30+ lymphoma cell line HH. Quantitative PCR was performed on cDNA from each of the tissues. CD30 cDNA copies were normalized to actin cDNA copies from each tissue. (B) The indicated cell types were stained with an anti-CD30 antibody and an isotype control antibody. The plots show overlapped histograms for CD30 (solid black histograms) and isotype control antibody (open histograms). The PBMC histogram shows all PBMCs. The CD34+ plot is gated on flow-sorted CD34+ cells from peripheral blood of a patient treated with granulocyte colony-stimulating factor. Because of complete overlap between the anti-CD30 histogram and isotype histogram, the open isotype histograms are not visible for PBMCs and CD34+ cells. The HH and CD30-BV173 histograms show staining of all cells of the HH and CD30-BV173 cell lines. PBMC, peripheral blood mononuclear cell.
In a previous report by Hombach et al., CD30 was found to be expressed on ∼20% of human CD34+ cord blood cells before any ex vivo culture; CD30 expression increased on these cells after cytokine activation.51 The same study provided evidence that CD30+, CD34+ cord blood cells were resistant to destruction by CD30-directed CAR T cells.51 We did not find CD30 expression on peripheral blood CD34+ cells from an adult patient who had received granulocyte colony-stimulating factor to increase blood CD34+ cell numbers.
Anti-CD30 CARs
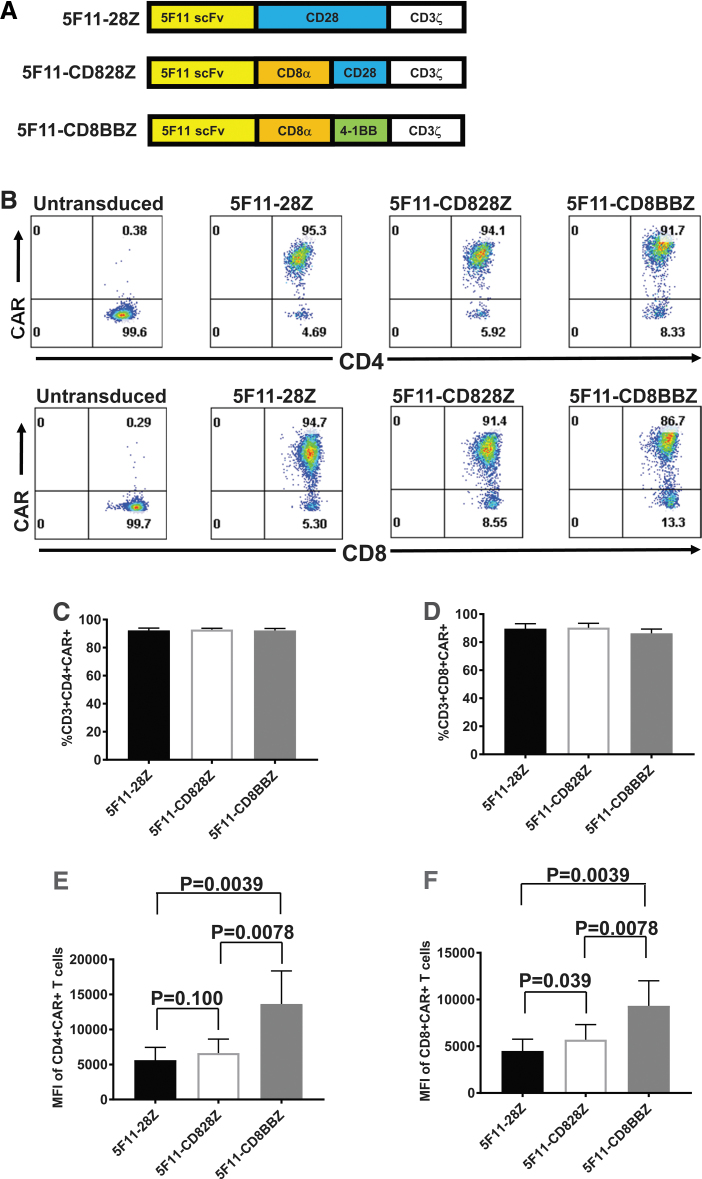
We constructed three anti-CD30 CARs incorporating scFvs derived from the fully human antibody 5F11 (Fig. 2A). All three of these CARs had CD3ζ T cell activation domains. The 5F11-28Z CAR contained hinge, transmembrane, and costimulatory domains from CD28. The 5F11-CD828Z and 5F11-CD8BBZ CARs had hinge and transmembrane domains from the CD8α molecule. The rationale for testing different hinge and transmembrane domains is that these domains can strongly affect CAR T cell function.7,8,42 5F11-CD828Z had a CD28 costimulatory domain, while 5F11-CD8BBZ had a 4-1BB costimulatory domain. The rationale for testing different costimulatory moieties is that CAR T cell function can be different based on the costimulatory molecule included in CARs, and when CD28 and 4-1BB costimulatory domains have been compared, the optimal costimulatory domain for tumor elimination has varied with different CARs and tumor models.41,52–54 The anti-CD30 CARs in Fig. 2A were encoded by a self-inactivating lentiviral vector (LSIN).46
Figure 2.
Expression of anti-CD30 CARs. (A) Three CARs were compared. All three CARs had the 5F11 scFv. 5F11-28Z had hinge, transmembrane, and costimulatory domains from CD28 and a CD3ζ T cell activation domain. 5F11-CD828Z had hinge and transmembrane domains from the CD8α molecule, a CD28 costimulatory domain, and a CD3ζ T cell activation domain. 5F11-CD8BBZ was identical to 5F11-CD828Z, except a 4-1BB domain was included instead of a CD28 domain. (B) Human PBMCs were stimulated to proliferate with anti-CD3 in IL-2-containing medium. One day after culture initiation, the cells were transduced with vectors encoding the indicated CARs, or the cells were left untransduced. Six days after transduction, the cells were stained with antibodies against CD3, CD4, and CD8. Cells were also stained with Protein L to detect CARs. Plots are gated on live CD3+ lymphocytes and then on either CD4+ or CD8+ cells. The mean + SEM percentages of (C) CD4+ T cells and (D) CD8+ T cells that expressed the indicated CARs are shown. There was no statistically significant difference between any pair of CARs for either CD4+ or CD8+ T cells. The mean + SEM MFI of CAR expression is shown for (E) CD4+CAR+ T cells and (F) CD8+CAR+ T cells. p-values are shown on the graphs with brackets connecting the groups being compared by each p-value. For (C–F), statistical analysis was by Wilcoxon matched-pairs signed rank test; n = 9. For (C–F), correction for multiple comparison was performed by the Bonferroni method, so p < 0.017 was considered statistically significant. CAR, chimeric antigen receptor; MFI, median fluorescence intensity; scFv, single-chain variable fragment; SEM, standard error of the mean.
Expression of anti-CD30 CARs
We assessed CAR expression on the surface of transduced T cells. All three 5F11-containing CARs were highly expressed on the surface of CD4+ and CD8+ T cells (Fig. 2B). There was no statistically significant difference in the percentage of T cells that were CAR+ when T cells transduced with each of the three 5F11-containing CARs were compared (Fig. 2C, D). Interestingly, T cells expressing 5F11-CD8BBZ had a significantly higher median fluorescence intensity (MFI) of CAR expression when compared with T cells expressing 5F11-28Z or 5F11-CD828Z (Fig. 2E, F).
Degranulation and cytokine release by anti-CD30 CAR T cells
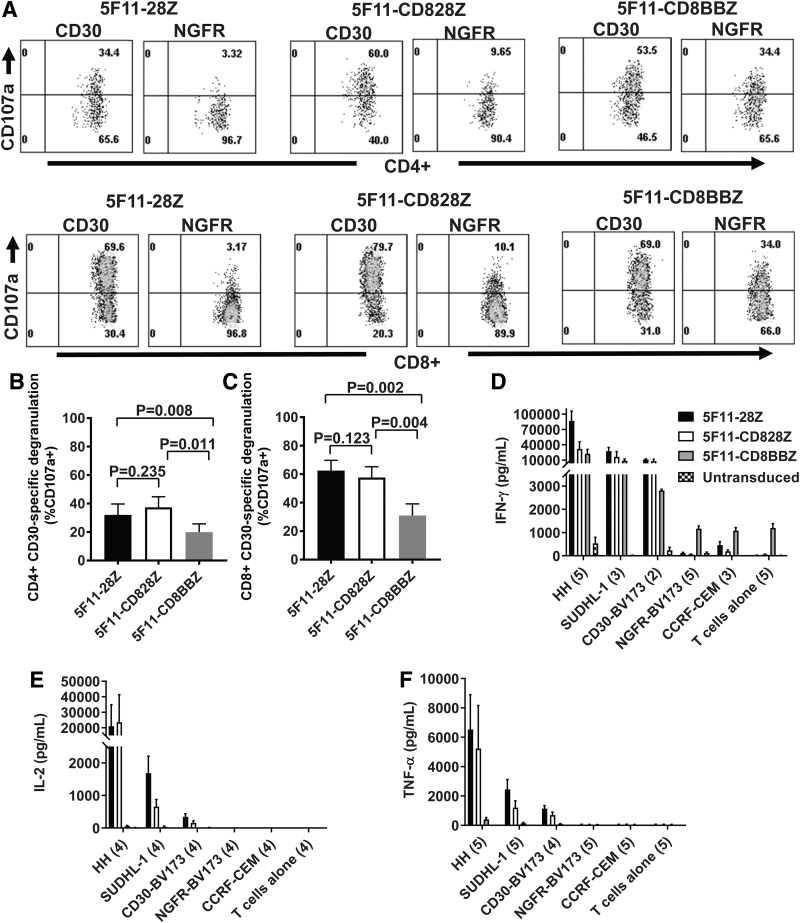
To compare degranulation of T cells expressing either 5F11-28Z, or 5F11-CD828Z, or 5F11-CD8BBZ, we stained for CD107a surface expression on CAR T cells during incubation with either CD30-BV173 as a positive target or NGFR-BV173 as a negative target. CD107a expression is upregulated with T cell degranulation. T cells expressing each of the three CARs degranulated in response to CD30-BV173 as indicated by the increased CD107a staining in both CD4+ and CD8+ T cells when the T cells were stimulated with CD30-BV173 versus NGFR-BV173 (Fig. 3A). As expected, low levels of degranulation occurred when 5F11-28Z CAR T cells or 5F11-CD828Z CAR T cells were incubated with CD30-negative NGFR-BV173 cells. When compared to T cells expressing either 5F11-28Z or 5F11-CD828Z, T cells expressing 5F11-CD8BBZ had higher levels of degranulation in response to NGFR-BV173. As summarized in Fig. 3B, C, T cells expressing 5F11-28Z or 5F11-CD828Z exhibited a similar degree of CD30-specific degranulation. For both CD4+ and CD8+ T cells, T cells expressing 5F11-CD8BBZ had significantly lower levels of CD30-specific degranulation when compared with either 5F11-28Z T cells or 5F11-CD828Z T cells. CD30-specific degranulation was calculated as degranulation with CD30-BV173 stimulation minus degranulation with NGFR-BV173 stimulation. Part of the reason for the lower level of CD30-specific degranulation by 5F11-CD8BBZ T cells is the high level of nonspecific degranulation associated with 5F11-CD8BBZ as shown in A.
Figure 3.
Degranulation and cytokine release by anti-CD30 CAR T cells. (A) To perform degranulation assays, CAR-transduced T cells were incubated with either CD30-BV173 (CD30) target cells or NGFR-BV173 (NGFR) target cells for 4 h in the presence of an antibody against CD107a. After 4 h, cells were stained for CD3, CD4, and CD8 and analyzed by flow cytometry. Plots are gated on live CD3+ lymphocytes and then on either CD4+ or CD8+ cells. CD107a degranulation assays were performed as described in A for (B) CD4+ T cells and (C) CD8+ T cells. The mean + SEM of CD30-specific degranulation is shown for the indicated CARs. CD30-specific degranulation was calculated by subtracting the %CD107a+ events with NGFR-BV173 stimulation from the %CD107a+ events with CD30-BV173 stimulation. Statistical comparison was by paired two-tailed t-test; n = 5. Correction for multiple comparison was done by the Bonferroni method; p-values <0.017 were considered statistically significant. (D–F) ELISAs were performed on the culture supernatants after overnight culture of CAR T cells with target cells. (D) IFNγ ELISA: numbers of replicate experiments with T cells from different donors are given in parentheses on the x-axis with the exceptions that n = 4 for untransduced T cells+NGFR-BV173 and untransduced T cells alone. For the ELISA results in (D–F), bars represent mean + SEM. The legend with D indicates the T cell populations analyzed, and the legend applies to the ELISAs in (D–F). Statistical comparisons were made for (D–F) with the ratio two-tailed paired t-test with correction for multiple comparisons by the Bonferroni method; p < 0.0170 was considered statistically different. In (D–F), all statistically different comparisons of cytokine release by CAR T cells are listed below. Differences that were not statistically different are not listed. IFNγ release was statistically higher for 5F11-28Z versus 5F11-CD8BBZ for HH targets (p = 0.0008). Nonspecific IFNγ release against NGFR-BV173 targets was statistically lower for 5F11-28Z (p = 0.0005) and 5F11-CD828Z (p < 0.0001) versus 5F11-CD8BBZ. Nonspecific IFNγ release against NGFR-BV173 was also statistically lower for 5F11-828Z versus 5F11-28Z (p = 0.0152). (E) IL-2 ELISA: numbers of replicate experiments with T cells from different donors is given in parentheses on the x-axis, except n = 3 for the CD30-BV173 plus 5F11-CD8BBZ combination. IL-2 release was statistically higher for 5F11-28Z versus 5F11-CD8BBZ for HH (p = 0.0107) and CD30-BV173 (p = 0.0014). IL-2 release was statistically higher for 5F11-CD828Z versus 5F11-CD8BBZ for CD30-BV173 (p = 0.0003). IL-2 release by 5F11-CD8BBZ T cells was often too low to be easily seen on the graph. (F) TNFα ELISAs were performed on culture supernatants. The number of replicate experiments with T cells from different donors is given on the x-axis. TNFα release was statistically higher for 5F11-28Z versus 5F11-CD8BBZ for HH (p = 0.0008), SUDHL-1 (p = 0.0012), and CD30-BV173 (p = 0.0055). TNFα release was statistically higher for 5F11-CD828Z versus 5F11-CD8BBZ for HH (p = 0.0041) and CD30-BV173 (p = 0.0129). ELISA, enzyme-linked immunosorbent assay; IFNγ, interferon gamma; TNFα, tumor necrosis factor alpha.
We performed ELISAs to assess cytokine release by anti-CD30 CAR T cells. Compared with 5F11-CD8BBZ CAR T cells, 5F11-28Z CAR T cells produced statistically higher levels of IFNγ when HH target cells were used (Fig. 3D). Compared with 5F11-CD8BBZ T cells, 5F11-28Z and 5F11-CD828Z CAR T cells exhibited statistically lower levels of nonspecific IFNγ release when NGFR-BV173 target cells were used. IL-2 release was statistically higher for 5F11-28Z versus 5F11-CD8BBZ when HH or CD30-BV173 target cells were used, and IL-2 release was statistically higher for 5F11-CD828Z versus 5F11-CD8BBZ when CD30-BV173 target cells were used (Fig. 3E). TNFα release was statistically higher for 5F11-28Z versus 5F11-CD8BBZ for all CD30+ target cells assessed. TNFα release was statistically higher for 5F11-CD828Z versus 5F11-CD8BBZ when HH or CD30-BV173 target cells were used (Fig. 3F). All in all, T cells expressing the 4-1BB-containing CAR released lower levels of cytokines in response to CD30+ target cells when compared to T cells expressing the CD28-containing CARs.
Anti-CD30 CAR T cells with CD28 co-stimulatory domains proliferated in an antigen-specific manner against CD30+ target cells
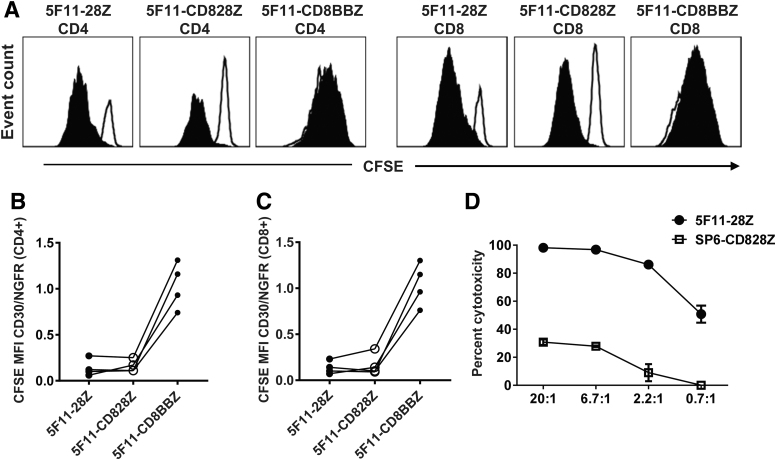
We performed in vitro proliferation assays to evaluate CD30-specific proliferation of CAR T cells based on CFSE dilution. CD30-specific proliferation was present when greater dilution of CFSE occurred with CD30-BV173 stimulation versus NGFR-BV173 stimulation. T cells expressing 5F11-28Z or 5F11-CD828Z exhibited proliferation that was more specific for CD30-BV173 when compared with T cells expressing 5F11-CD8BBZ (Fig. 4A). We calculated the ratio of CFSE MFI in CAR T cells stimulated with CD30-BV173 to CFSE MFI in CAR T cells stimulated with NGFR-BV173 (CFSE MFI CD30/NGFR). CD30-specific proliferation was greater for T cells expressing either 5F11-28Z or 5F11-CD828Z versus 5F11-CD8BBZ (Fig. 4B, C). This was due, in large part, to the nonspecific proliferation of 5F11-CD8BBZ T cells when cultured with CD30-negative NGFR-BV173 cells. This nonspecific proliferation was similar to the increased levels of non-antigen-specific functional activity with 5F11-CD8BBZ in other assays, including degranulation (Fig. 3A) and IFNγ release (Fig. 3D). This nonspecific activity of 5F11-CD8BBZ T cells is attributable to the 4-1BB domain since the rest of the sequence of 5F11-CD8BBZ is identical to the sequence of 5F11-CD828Z, which does not exhibit substantial nonspecific activity. Because of the loss of CD30 expression in anti-CD30 CAR-transduced T cells, it is difficult to compare CD30 expression in CD28 versus 4-1BB CARs. The non-antigen-specific activity of 4-1BB-containing CARs has been observed previously with CARs targeting signaling lymphocytic activation molecule F7 (SLAMF7) and CARs targeting CD19.55
Figure 4.
Proliferation and cytotoxicity of anti-CD30 CAR T cells. (A) T cells expressing 5F11-28Z, 5F11-CD828Z, or 5F11-CD8BBZ were labeled with CFSE and cultured for 4 h with CD30-BV173 (solid black histograms) or NGFR-BV173 (open histograms) for 4 days. The indicated plots were gated on either CD4+ or CD8+ live CAR+ T cells. CFSE dilution indicates proliferation. The ratio of CFSE MFI with CD30-BV173 stimulation to CFSE MFI with NGFR-BV173 stimulation (CFSE MFI CD30/NGFR) is used as a measure of CD30-specific proliferation with a lower value indicating more CD30-specific proliferation. CFSE MFI CD30/NGFR values are shown for (B) CD4+ or (C) CD8+ T cells expressing the indicated CARs. Lines connect paired results with cells from the same donor. There was a statistically greater degree of CD30-specific proliferation (lower CFSE MFI CD30/NGFR) for 5F11-28Z versus 5F11-CD8BBZ (p = 0.0031) and 5F11-CD828Z versus 5F11-CD8BBZ (p = 0.0039) for CD4+ T cells. There was a statistically greater degree of CD30-specific proliferation (lower CFSE MFI CD30/NGFR) of 5F11-28Z versus 5F11-CD8BBZ (p = 0.0032) and 5F11-CD828Z versus 5F11-CD8BBZ (p = 0.0021) for CD8+ T cells. Statistics were by two-tailed paired t-tests; n = 4. With correction for multiple comparison by the Bonferroni method, p < 0.017 is statistically significant. (D) T cells expressing 5F11-28Z exhibited cytotoxicity against the HH cell line in a 4-h assay. Graphs show mean ± SEM of duplicate wells. One of two representative experiments with different donor cells and similar results is shown. CFSE, carboxyfluorescein diacetate succinimidyl ester.
We assessed the cytotoxicity of 5F11-28Z CAR T cells in 4-h cytotoxicity assays. T cells transduced with the SP6-828Z CAR served as a negative control. 5F11-28Z CAR T cells specifically recognized and eliminated HH cells in an effector-to-target ratio-dependent manner (Fig. 4D).
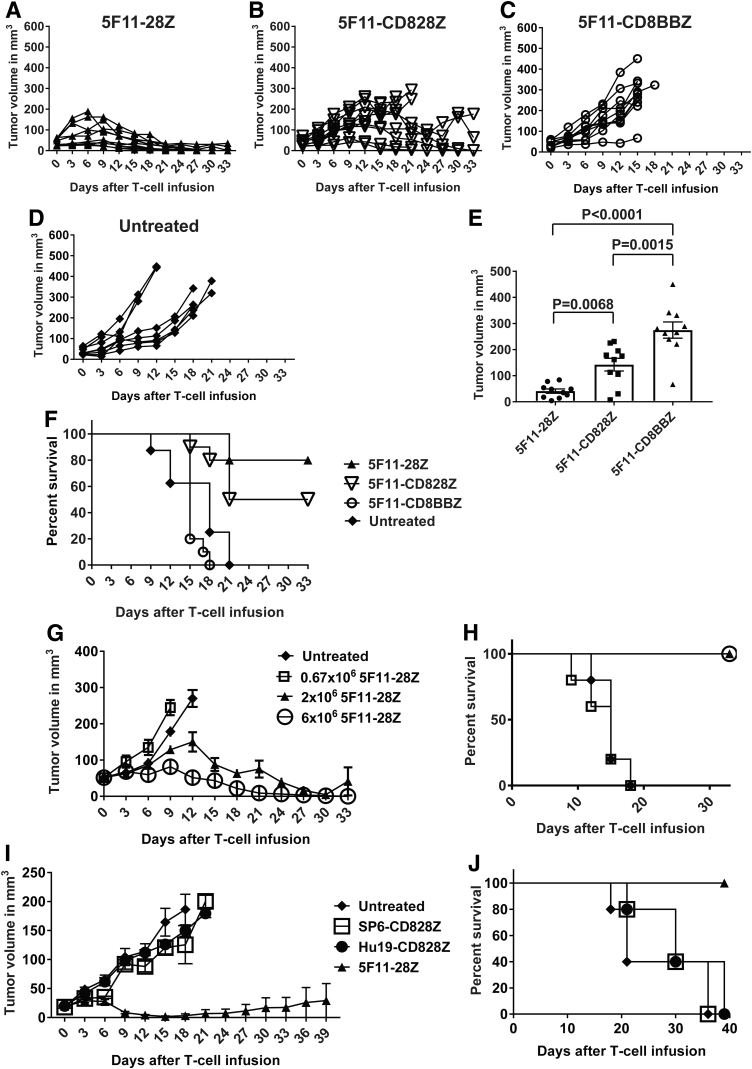
5F11-28Z CAR T cells eradicated CD30+ tumors from mice
To evaluate antitumor activity of anti-CD30 CAR T cells in murine models, we established solid tumors of CD30+ HH cells in immunocompromised NSG mice. A single intravenous infusion 2 × 106 CAR+ T cells/mouse was able to suppress HH tumor growth in a hierarchical manner, with 5F11-28Z CAR T cells being the most effective (Fig. 5A), followed by 5F11-CD828Z (Fig. 5B). In contrast, 5F11-CD8BBZ CAR T cells (Fig. 5C) and untreated mice (Fig. 5D) had progressive tumor growth. The hierarchical antitumor activity of the anti-CD30 CAR T cells was further demonstrated when comparing the tumor volume of treated mice 15 days after CAR T cell infusion (Fig. 5E). When tumor sizes were assessed 15 days after CAR T cell infusion, we found that T cells expressing the 5F11-28Z CAR-containing CD28 hinge and transmembrane domains were more effective at reducing tumor sizes when compared to T cells expressing the 5F11-CD828Z CAR-containing CD8α hinge and transmembrane domains (Fig. 5E); however, survival was not statistically different when we compared treatment with 5F11-28Z T cells versus 5F11-CD828Z T cells (Fig. 5F). Antitumor activity was consistently inferior for the 4-1BB-containing CAR 5F11-CD8BBZ when compared to T cells expressing either of the CD28-containing CARs 5F11-28Z or 5F11-CD828Z (Fig. 5E, F). Because 5F11-CD828Z and 5F11-CD8BBZ differed only in their costimulatory domains, comparison of these two CARs allowed a stringent assessment of the impact of the choice of costimulatory domain on antitumor activity in mice. Because of the superior antitumor effect of 5F11-28Z CAR T cells compared with T cells expressing the other two CAR constructs and the results of in vitro experiments, 5F11-28Z was chosen for further evaluation.
Figure 5.
Assessment of antitumor activity in mice. (A–D) Solid tumors were established on the abdomens of NSG mice by intradermal injection of 4 × 106 HH cells. After palpable tumors were established, mice received intravenous injections of 2 × 106 T cells transduced with vectors encoding 5F11-28Z, 5F11-CD828Z, or 5F11-CD8BBZ on day 0. One group of mice was left untreated. For A-D, each line represents an individual mouse, and n = 10 for all groups, except the untreated group, which had n = 8. (E) The graph shows the mean ± SEM of tumor volume on day 15 after injection of T cells expressing the indicated CARs. These results are from the same mice as in (A–C). Day 15 was chosen for this comparison because this was the last day when all mice that received CAR T cells were still alive. Statistical analysis was by Mann–Whitney test. With correction for multiple comparisons by the Bonferroni method, p < 0.0170 should be considered statistically significant. (F) Survival of the same mice seen in (A–D) is presented. Survival was not statistically different for untreated mice versus 5F11-CD8BBZ T cells; p = 0.1627. Survival was statistically decreased for 5F11-CD8BBZ when compared with 5F11-28Z T cells (p < 0.0001) and 5F11-CD828Z T cells (p < 0.0001). p < 0.0001 for the comparison of 5F11-28Z versus untreated mice. p = 0.0058 for the comparison of 5F11-CD828Z versus untreated mice. There was no statistically significant difference in survival between mice that received 5F11-28Z versus 5F11-CD828Z T cells. (G) HH cell tumors were established in NSG mice, and when tumors were established, mice received intravenous injections of the indicated doses of 5F11-28Z T cells. One group of mice was left untreated. Tumor volumes are shown as the mean of groups of five mice for each group. (H) Survival of the same mice as in G is shown. (I) NSG mice were injected intradermally with 8 × 106 CD30+ L428 cells to establish tumors. On day 0, indicated on the x-axis, 6 × 106 CAR T cells/mouse were infused intravenously. T cell infusions were 9 days after tumor cell injection. Unlike other mouse experiments, all CAR constructs in I were encoded by the MSGV1 gamma-retroviral vector. The graph shows the mean + SEM tumor size; n = 5 for all groups. (J) Kaplan-Meier plots show survival of the same mice as in I. Survival was superior for the 5F11-28Z group compared with the Hu19-CD828Z group (p = 0.0026) and the SP6-CD828Z group (p = 0.0026) and the untreated group (p = 0.0026). All p-values were calculated by log-rank tests. NSG, nod-scid common gamma-chain deficient.
In a dose-titration experiment, a single intravenous injection of 6 × 106 5F11-28Z CAR T cells/mouse completely eradicated HH solid tumors in NSG mice (Fig. 5G). HH tumor growth was suppressed by a single intravenous infusion of 2 × 106 CAR+ T cells/mouse. No antitumor activity was evident at a dose of 0.67 × 106 CAR+ T cells/mouse. Survival of the same mice followed a similar pattern (Fig. 5H). We also evaluated the antitumor activity of 5F11-28Z CAR T cells against CD30+ L428 cell solid tumors in NSG mice. In all experiments mentioned so far, the 5F11-28Z CAR was encoded by a lentiviral vector, but for the L428 tumor experiments, the 5F11-28Z CAR was encoded by the MSGV1 gamma-retroviral vector. A single intravenous injection of 6 × 106 5F11-28Z CAR T cells/mouse was able to suppress L428 tumor growth; there was a 100% survival rate of mice treated with 5F11-28Z T cells when the experiment ended 40 days after CAR T cell infusion (Fig. 5I, J).
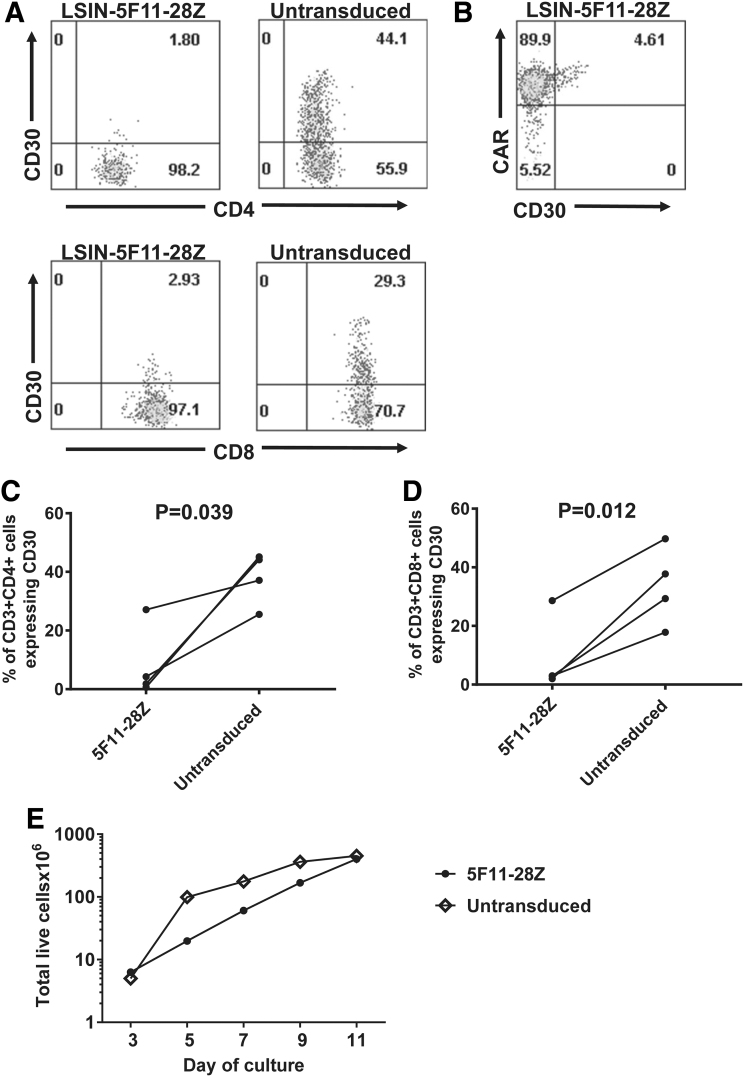
Loss of CD30+ T cells from cultures of 5F11-28Z-transduced T cells
Since activated T cells upregulate CD30 expression,56 we compared the level of CD30 expression on cultured untransduced T cells to T cells from cultures that were transduced with LSIN-5F11-28Z. Untransduced T cells expressed CD30, but both CD4+ and CD8+ subsets of LSIN-5F11-28Z CAR-transduced T cells showed only low levels of CD30 expression (Fig. 6A). In the LSIN-5F11-28Z-transduced cultures, the residual CD30+ T cells were found in the CAR+ cells, but not the CAR-negative cells (Fig. 6B). The reduced percentage of CD30+ cells among LSIN-5F11-28Z-transduced T cells versus untransduced T cells was a statistically significant finding for both CD4+ (Fig. 6C) and CD8+ (Fig. 6D) T cells. These results suggest that CD30+ T cells are eliminated by 5F11-28Z CAR T cells. Residual CD30+ cells were found among the CAR+ subset of T cells, but not the CAR-negative subset in the LSIN-5F11-28Z-transduced T-cell cultures. This demonstrates that in LSIN-5F11-28Z-transduced T-cell cultures, CD30 is not absent only from the CAR+ cells. We also performed qPCR in paired sets of cultures of LSIN-5F11-28Z-transduced T cells and untransduced T cells from three donors. We found decreased CD30 RNA expression in the LSIN-5F11-28Z-transduced T cell cultures compared with untransduced T cell cultures with all 3 donors (p = 0.029, Supplementary Fig. S1). The qPCR results agree with the flow cytometry finding of loss of CD30+ T cells in LSIN-5F11-28Z-transduced cultures. Despite the probable elimination of CD30+ T cells by 5F11-28Z CAR T cells in culture, 5F11-28Z CAR T cells accumulated in culture similar to untransduced T cells (Fig. 6E).
Figure 6.
CD30+ T cells were lost from cultures of 5F11-28Z T cells. (A) Plots show T cells 6 days after transduction with LSIN-5F11-28Z or untransduced T cells from the same donor; both untransduced and 5F11-28Z-transduced T cells were cultured in the same manner. Cells were stained with antibodies for CD3, CD4, CD8, and CD30. The plots are gated on live CD3+ lymphocytes and then gated on either CD4+ or CD8+ cells. Both CAR+ and CAR-negative cells are included on the plots. (B) The plot is gated on live CD3+ lymphocytes from the same LSIN-5F11-28Z-transduced culture as in A. The plot shows that residual CD30+ T cells were found predominantly among the CAR+ cells in the culture. CAR staining was by Protein L. This is 1 of 4 cultures with similar results. The percentages are shown of (C) CD4+CD3+ lymphocytes that were CD30+ and (D) CD3+CD8+ lymphocytes that were CD30+. Cells were cultured and stained as in A. Lines connect paired results of LSIN-5F11-28Z-tansduced or untransduced cultures from the same donor. Statistics were by two-tailed paired t-test; n = 4. p < 0.05 was considered statistically significant. (E) Accumulation of LSIN-5F11-28Z-transduced T cells was compared with accumulation of untransduced T cells in culture. This is one of three experiments that had similar results. For the LSIN-5F11-28Z-transduced culture, 94.4% of the T cells were CAR+ on day 7 of culture. Counts started on day 3, when lentiviral transductions were complete.
Specificity of 5F11-28Z T cells and the effect of soluble CD30 on 5F11-28Z T cell function
Compared with the amount of IFNγ released when 5F11-28Z T cells were cultured with CD30+ HH cells, the amount of IFNγ released when the 5F11-28Z T cells were cultured with primary CD34+ hematopoietic stem cells or each of five CD30-negative cell lines was low (Supplementary Table S1). Because serum or plasma CD30 has been reported in HL patients at concentrations up to 2 μg/mL,57 we evaluated the effects of soluble CD30 on 5F11-28Z CAR T cell cytokine release. Soluble CD30 protein caused only modest decreases in IFNγ release when added at concentrations up to 5 μg/mL to cultures of 5F11-28Z T cells plus target cells (Supplementary Table S2).
Comparison of the 5F11 scFv to the HRS3 scFv
HRS3 is an scFv derived from a murine antibody; HRS3 is included in a clinically used CAR.34 We constructed a CAR designated HRS3-28Z. HRS3-28Z was identical to 5F11-28Z, except for the different scFvs. We compared these two CARs in experiments that used the MSGV1 vector to encode both CARs. When stimulated with CD30+ target cells, the level of IFNγ release was higher for T cells expressing 5F11-28Z T cells versus HRS3-28Z T cells (Supplementary Table S3). We established HH tumors in NSG mice and infused either 5F11-28Z T cells or HRS3-28Z T cells. The antitumor activity of 5F11-28Z T cells was superior to the antitumor activity of HRS3-28Z T cells in this model (Supplementary Fig. S2).
All in all, the 5F11-28Z CAR encoded by the LSIN vector was selected for use in a clinical trial based on its favorable comparison with the other CARs in vitro and in mouse experiments. A formal comparison of 5F11-28Z encoded by lentiviral versus gamma-retroviral vectors was not performed, but obvious differences were not observed. A clinical trial of 5F11-28Z T cells for treatment of CD30+ lymphomas is currently underway.
Supplementary Material
Acknowledgments
We thank the staff of the flow cytometry facilities of the Experimental Transplantation and Immunotherapy Branch and the Surgery Branch of the National Cancer Institute. We also thank the staff of the NIH 10/CRC animal facility for facilitating mouse experiments.
Author Disclosure
J.N.K. is an inventor on a patent covering the 5F11 CARs reported in this publication, and he is also Principal Investigator of a research agreement with Kite, a Gilead Company dealing with anti-CD30 CARs. The other authors have no relevant disclosures.
Funding Information
This work was supported, in part, by intramural funding of the Center for Cancer Research, National Cancer Institute, NIH. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Supplementary Material
References
- 1. Eshhar Z, Waks T, Gross G, et al. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A 1993;90:720–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov 2013;3:388–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dotti G, Gottschalk S, Savoldo B, et al. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev 2014;257:107–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jensen MC, Riddell SR. Designing chimeric antigen receptors to effectively and safely target tumors. Curr Opin Immunol 2015;33:9–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boyiadzis M, Bishop MR, Abonour R, et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of hematologic malignancies: multiple myeloma, lymphoma, and acute leukemia. J Immunother Cancer 2016;4:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol 2018;15:31–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alabanza L, Pegues M, Geldres C, et al. Function of novel anti-CD19 chimeric antigen receptors with human variable regions is affected by hinge and transmembrane domains. Mol Ther 2017;25:2452–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brudno JN, Lam N, Vanasse D, et al. Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma. Nat Med 2020;26:270–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012;119:2709–2720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-Cell lymphoma. N Engl J Med 2017;377:2531–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turtle CJ, Hanafi LA, Berger C, et al. Immunotherapy of non-Hodgkin's lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med 2016;8:355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019;380:45–56 [DOI] [PubMed] [Google Scholar]
- 14. Boyiadzis MM, Dhodapkar MV, Brentjens RJ, et al. Chimeric antigen receptor (CAR) T therapies for the treatment of hematologic malignancies: clinical perspective and significance. J Immunother Cancer 2018;6:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Falini B, Pileri S, Pizzolo G, et al. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood 1995;85:1–14 [PubMed] [Google Scholar]
- 16. Horie R, Watanabe T. CD30: expression and function in health and disease. Semin Immunol 1998;10:457–470 [DOI] [PubMed] [Google Scholar]
- 17. Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol 2003;3:609–620 [DOI] [PubMed] [Google Scholar]
- 18. Duckett CS, Gedrich RW, Gilfillan MC, et al. Induction of nuclear factor kappaB by the CD30 receptor is mediated by TRAF1 and TRAF2. Mol Cell Biol 1997;17:1535–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee SY, Lee SY, Kandala G, et al. CD30/TNF receptor-associated factor interaction: NF-kappa B activation and binding specificity. Proc Natl Acad Sci U S A 1996;93:9699–9703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blazar BR, Levy RB, Mak TW, et al. CD30/CD30 ligand (CD153) interaction regulates CD4+ T cell-mediated graft-versus-host disease. J Immunol 2004;173:2933–2941 [DOI] [PubMed] [Google Scholar]
- 21. Kurts C, Carbone FR, Krummel MF, et al. Signalling through CD30 protects against autoimmune diabetes mediated by CD8 T cells. Nature 1999;398:341–344 [DOI] [PubMed] [Google Scholar]
- 22. Podack ER, Strbo N, Sotosec V, et al. CD30-governor of memory T cells? Ann N Y Acad Sci 2002;975:101–113 [DOI] [PubMed] [Google Scholar]
- 23. Mir SS, Richter BW, Duckett CS. Differential effects of CD30 activation in anaplastic large cell lymphoma and Hodgkin disease cells. Blood 2000;96:4307–4312 [PubMed] [Google Scholar]
- 24. Schirrmann T, Steinwand M, Wezler X, et al. CD30 as a therapeutic target for lymphoma. BioDrugs 2014;28:181–209 [DOI] [PubMed] [Google Scholar]
- 25. Stein H, Mason DY, Gerdes J, et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood 1985;66:848–858 [PubMed] [Google Scholar]
- 26. van der Weyden CA, Pileri SA, Feldman AL, et al. Understanding CD30 biology and therapeutic targeting: a historical perspective providing insight into future directions. Blood Cancer J 2017;7:e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wasik MA, Jimenez GS, Weisenburger DD. Targeting CD30 in malignant tissues: challenges in detection and clinical applications. Pathobiology 2013;80:252–258 [DOI] [PubMed] [Google Scholar]
- 28. Schwarting R, Gerdes J, Durkop H, et al. Ber-H2: a new anti-Ki-1 (CD30) monoclonal antibody directed at a formol-resistant epitope. Blood 1989;74:1678–1689 [PubMed] [Google Scholar]
- 29. Croft M, Duan W, Choi H, et al. TNF superfamily in inflammatory disease: translating basic insights. Trends Immunol 2012;33:144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moskowitz CH, Nademanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015;385:1853–1862 [DOI] [PubMed] [Google Scholar]
- 31. Deng C, Pan B, O'Connor OA. Brentuximab vedotin. Clin Cancer Res 2013;19:22–27 [DOI] [PubMed] [Google Scholar]
- 32. Oki Y, Younes A. Brentuximab vedotin in systemic T-cell lymphoma. Exp Opin Biol Ther 2012;12:623–632 [DOI] [PubMed] [Google Scholar]
- 33. Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 2010;363:1812–1821 [DOI] [PubMed] [Google Scholar]
- 34. Savoldo B, Rooney CM, Di Stasi A, et al. Epstein Barr virus-specific cytotoxic T lymphocytes expressing the anti-CD30ζ artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood 2007;110:2620–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Di Stasi A, De Angelis B, Rooney CM, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood 2009;113:6392–6402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hombach A, Heuser C, Sircar R, et al. An anti-CD30 chimeric receptor that mediates CD3-ɛ-independent T-cell activation against Hodgkin's lymphoma cells in the presence of soluble CD30. Cancer Res 1998;58:1116–1119 [PubMed] [Google Scholar]
- 37. Ramos CA, Ballard B, Zhang H, et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor-redirected lymphocytes. J Clin Invest 2017;127:3462–3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ramos CA, Grover NS, Beaven AW, et al. Anti-CD30 CAR-T cell therapy in relapsed and refractory Hodgkin lymphoma. J Clin Oncol 2020;38:3794–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang CM, Wu ZQ, Wang Y, et al. Autologous T cells expressing CD30 chimeric antigen receptors for relapsed or refractory Hodgkin lymphoma: an open-label phase I trial. Clin Cancer Res 2017;23:1156–1166 [DOI] [PubMed] [Google Scholar]
- 40. Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016;126:2123–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lam N, Trinklein ND, Buelow B, et al. Anti-BCMA chimeric antigen receptors with fully human heavy-chain-only antigen recognition domains. Nature Communications 2020;11:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ying Z, Huang XF, Xiang X, et al. A safe and potent anti-CD19 CAR T cell therapy. Nat Med 2019;25:947–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Keler T, Graziano R, Treml J, et al. Human Monoclonal Antibodies Against CD30. Medarex, Inc. 2008. US 7,387,776 B2 Patent
- 44. Kochenderfer JN, Feldman SA, Zhao Y, et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother 2009;32:689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carpenter RO, Evbuomwan MO, Pittaluga S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res 2013;19:2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang S, Dudley ME, Rosenberg SA, et al. A simplified method for the clinical-scale generation of central memory-like CD8+ T cells after transduction with lentiviral vectors encoding antitumor antigen T-cell receptors. J Immunother 2010;33:648–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hughes MS, Yu YY, Dudley ME, et al. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther 2005;16:457–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chmielewski M, Hombach A, Abken H. Anti-CD30 Chimeric Antigen Receptor and Its Use. International Publication Number WO 2015/028444 A1 2014; World Intellectual Property Organization Patent [Google Scholar]
- 49. Hermans IF, Silk JD, Yang J, et al. The VITAL assay: a versatile fluorometric technique for assessing CTL- and NKT-mediated cytotoxicity against multiple targets in vitro and in vivo. J Immunol Methods 2004;285:25–40 [DOI] [PubMed] [Google Scholar]
- 50. Ito K, Watanabe T, Horie R, et al. High expression of the CD30 molecule in human decidual cells. Am J Pathol 1994;145:276–280 [PMC free article] [PubMed] [Google Scholar]
- 51. Hombach AA, Gorgens A, Chmielewski M, et al. Superior therapeutic index in lymphoma therapy: CD30(+) CD34(+) hematopoietic stem cells resist a chimeric antigen receptor T-cell attack. Mol Ther 2016;24:1423–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther 2009;17:1453–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Drent E, Poels R, Ruiter R, et al. Combined CD28 and 4-1BB costimulation potentiates affinity-tuned chimeric antigen receptor-engineered t cells. Clin Cancer Res 2019;25:4014–4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhao Z, Condomines M, van der Stegen SJC, et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 2015;28:415–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Amatya C, Pegues MA, Lam N, et al. Development of CAR T cells expressing a suicide gene plus a chimeric antigen receptor targeting signaling lymphocytic-activation molecule F7. Mol Ther 2020. [Epub ahead of print]; doi: 10.1016/j.ymthe.2020.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chan KW, Hopke CD, Krams SM, et al. CD30 expression identifies the predominant proliferating T lymphocyte population in human alloimmune responses. J Immunol 2002;169:1784–1791 [DOI] [PubMed] [Google Scholar]
- 57. Plattel WJ, Alsada ZND, van Imhoff GW, et al. Biomarkers for evaluation of treatment response in classical Hodgkin lymphoma: comparison of sGalectin-1, sCD163 and sCD30 with TARC. Br J Haematol 2016;175:868–875 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.