Corresponding Author

Key Words: AV block, cardioneuroablation, pacemaker, RF ablation, syncope, vasovagal syncope
The treatment of atrioventricular (AV) block by radiofrequency (RF) ablation without pacemaker implantation is intriguing. This is the challenge of a new procedure called cardioneuroablation (CNA) (1). It is obviously reserved for very well-selected cases; however, it is appealing that the ablation of some targets in the heart could treat bradyarrhythmias, eliminating symptoms and correcting the electrocardiogram, to the point of making pacemaker implantation unnecessary.
In this issue of JACC: Case Reports, Erkan et al. (2) present a case report in which CNA is used to treat symptomatic AV block. The authors obtained excellent results and achieved real benefit to the patient. It is noteworthy, however, that the AV block presented is more advanced than what is normally considered for CNA. In addition, this case had a mixed disorder, predominantly functional. Nevertheless, the clue to outcome was the partial response to atropine before the procedure, predicting that CNA could have a positive result and validating the indication. Due to the complexity of AV node innervation, the CNA technique for AV block is not yet fully defined, and many considerations and prudence are necessary. However, the current study is well founded in the literature. Nevertheless, the follow-up was short, and thus it is essential to continue to follow up this case to determine if a pacemaker was definitely avoided or postponed.
Technical Aspects
The CNA technique was created in the 1990s, aiming for vagal denervation by ablation to treat symptomatic functional bradyarrhythmias without pacemaker implantation, as well as vagal atrial fibrillation. The first cohort of 25 patients followed up post-CNA was published in 2005 (1), having neurocardiogenic syncope, sinus dysfunction, and 7 cases with symptomatic functional AV block. Soon after, in 2006, we published the first case report of high-grade AV block treated with CNA, gathering the first 8 cases, with a mean follow-up of 15.1 ± 4.9 months, in which CNA was used exclusively for treating functional AV block. In 4 patients, it was performed only by the right atrium. All patients became asymptomatic, but 1 case (12.5%) remained with occasional nocturnal Mobitz I AV block. In the others, CNA was performed by using a biatrial approach, and no AV block was observed.
CNA for the treatment of functional AV blocks is more challenging than that for other bradyarrhythmias and should be performed by using a biatrial approach; however, in addition to the technical details, it is essential to achieve total elimination of the AV block induced by left vagus nerve stimulation as the endpoint of the procedure (Figures 1 and 2).
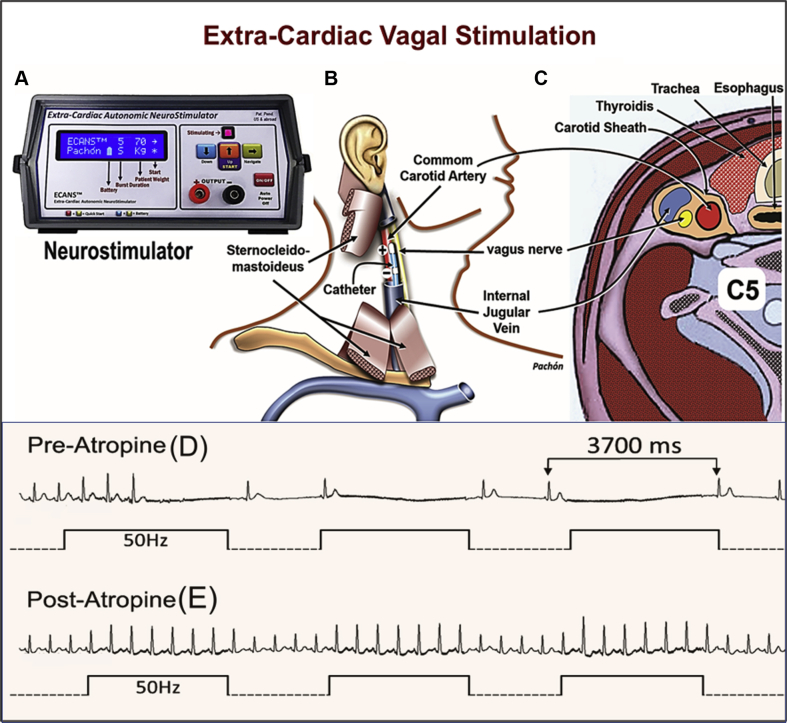
Figure 1.
Scheme of the Extracardiac Vagal Stimulation
(A) Customized neurostimulator (however, a general stimulator like Grass stimulator or neurological electromyograph could be used). (B) Relationship of the catheter inside of the internal jugular vein with the vagus nerve. (C) Transverse section of the neck showing the close relation of the vagus nerve with the internal jugular vein. (D) Immediate asystole caused by the vagal stimulation. (E) Disappearance of the vagal effect by atropine. ECVS = extracardiac vagal stimulation.
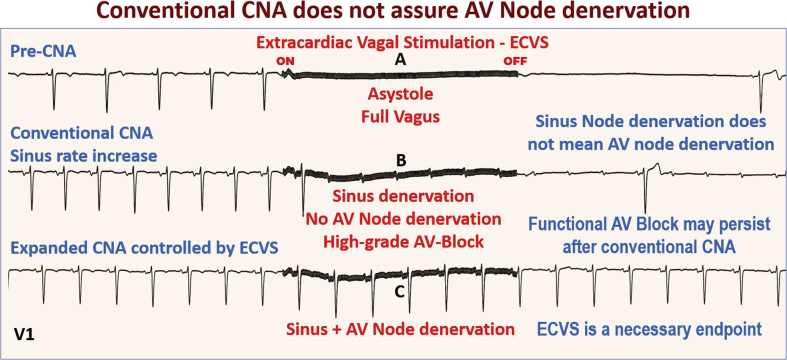
Figure 2.
Stepwise ECVS During CNA
Elimination of the vagal-induced atrioventricular (AV) block during extracardiac vagal stimulation (ECVS) of the left vagus comprises the hardest endpoint. AV = atrioventricular; CNA = cardioneuroablation.
Literature
There are currently 388 publications on Google Scholar about CNA, with 15 controlled, prospective studies totaling 501 patients and 1 systematic review (3). In addition, there are 9 case reports using CNA for treating AV block. In 41 cases, the primary indication was functional AV block. All the authors have been reporting high reproducibility, with very low complications and a mean recurrence of 9.2% with 19.8 months of follow-up. Nevertheless, CNA recurrences are a little more frequent than in neurocardiogenic syncope, ranging from 0% to 28.5%, depending on the author. This may be due to: 1) inadequate patient selection; 2) complexity of the anatomy and the innervation of the AV node; 3) different techniques and learning curves; and 4) lack of strict denervation control that can confirm the complete elimination of AV block induced by vagal effect at the end of the CNA (hard endpoint). Many cases with apparent immediate success may end with incomplete denervation, which can be completely reversed by natural reinnervation giving rise to recurrences.
Patient Selection
Before the procedure, we must be sure that the AV block is functional, that the conduction system is intact, and that there is an adequate response to atropine.
Complexity of AV Node Innervation
Due to the location, the AV node receives innervation from all ganglionic plexuses (GP), which makes it difficult to denervate. Furthermore, the procedure must be carefully performed to avoid AV node damage. Most of the innervation comes from GP2 located between the insertion of the right pulmonary veins (PVs), left atrial roof, and oval foramen, the so-called “P point” (4,5), from GP3, between the coronary sinus ostium, inferior vena cava, left atrium wall, and from the GP4 close to the insertion of the left PV. This innervation distribution may differ significantly between patients. This feature determines whether the technique for vagal denervation of the AV node is more extensive and depends on ablations through the right atrium, left atrium, coronary sinus, inferior vena cava, interatrial septum, and even at the left PV insertion and Waterston’s groove. Thus, the ablation extension differs among patients and should be controlled by vagal stimulation to determine the appropriate CNA endpoint. In the current case report, Erkan et al. (2) appropriately performed the biatrial approach. One of the common causes of recurrence is use of the simplified approach only through the right atrium, which can lead to insufficient denervation even with immediate apparent success.
Mapping the Neuro-myocardial Interface
This question is not yet fully defined. The spectral mapping of atrial fibrillation nests that gave origin to fractionation mapping in 2011 (Velocity-Precision, Abbott, Abbott Park, Illinois), associated with the anatomical approach, are highly appropriate (4). In 14 studies with 460 patients, spectral mapping was performed in 35.7%, high-frequency stimulation in 42.8%, and an anatomical approach in 92.9% of cases; in 28.6%, the approach was anatomical only. Erkan et al. (2) used atrial fibrillation nest ablation by mapping the fragmented potentials. As a rule, special care is needed, as the immediate result may not be sustained if enough denervation is not obtained in the initial procedure. Thus, stepwise functional verification during the procedure is essential.
Checking the Denervation Progress
In the original study, denervation was assessed by modification of the electrophysiological parameters, such as sinus node rate and the Wenckebach point increase, as well as by the loss of atropine response (1). However, due to the natural increase in sinus rate induced by CNA, the atropine test loss reliability. Furthermore, a sinus rate increase and 1:1 sinus AV conduction do not assure AV node denervation and the safety margin of AV conduction. Thus, we believe that CNA should be done under the strict control of vagal denervation by using extracardiac vagal stimulation (ECVS), which may be easily performed during the procedure (6) (Figures 1 and 2).
This approach consists of advancing a catheter through the internal jugular (Figure 1B) up to the jugular foramen at the cranium basis (6). In this location, due to the proximity to the vagus nerve (Figure 1C), ECVS is achieved even without direct contact with the nerve, using 50 Hz, 50 μs, with an amplitude of 1 V/kg of body weight up to a maximum of 70 V (Figure 1A). Typically, immediate asystole occurs due to the vagal effect (Figure 1D). However, considering that the AV node receives more innervation from the left vagus, while treating a functional AV block, we regularly perform stimulation of the left vagus during atrial pacing with 80 to 100 ppm, if necessary, to prevent bradycardia. This procedure aims to appropriately challenge the AV conduction (Figure 2B). The goal is to attain total disappearance of the vagal-induced AV block (Figure 2C) when complete AV node vagal denervation has been achieved.
Thus, the AV block induced by ECVS before or during CNA (Figure 2B) must be completely eliminated (Figure 2C). The hardest endpoint is the total elimination of vagal-induced AV block with or without atrial pacing. It is essential to achieve complete abolition of the vagal effect to guarantee a safety margin to counter the natural reinnervation that may affect the long-term result.
Conclusions
Several studies confirm that CNA can effectively treat functional AV blocks; however, a precise indication, a positive pre-CNA response to atropine, an accurate technique, and strict control of denervation during the procedure are essential.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Pachon J.C., Pachon E.I., Pachon J.C. “Cardioneuroablation”—new treatment for neurocardiogenic syncope, functional AV-block and sinus dysfunction using catheter RF-ablation. Europace. 2005;7:1–13. doi: 10.1016/j.eupc.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Baysal E., Guler T.M., Gopinathannair R. Catheter Ablation of Atrioventricular Block: From Diagnosis to Selection of Proper Treatment. J Am Coll Cardiol Case Rep. 2020;2:1793–1801. doi: 10.1016/j.jaccas.2020.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aksu T., Güler T.E., Bozyel S. Cardioneuroablation in the treatment of neurally mediated reflex syncope: a review of the current literature. Turk Kardiyol Dern Ars. 2017;45:33–41. doi: 10.5543/tkda.2016.55250. [DOI] [PubMed] [Google Scholar]
- 4.Pachon-M E.I., Pachon-Mateos J.C., Higuti C. Relation of fractionated atrial potentials with the vagal innervation evaluated by extracardiac vagal stimulation during cardioneuroablation. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.119.007900. [DOI] [PubMed] [Google Scholar]
- 5.Hu F., Zheng L., Liang E. Right anterior ganglionated plexus: the primary target of cardioneuroablation. Heart Rhythm. 2019;16:1545–1551. doi: 10.1016/j.hrthm.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Pachon M.J.C., Pachon M.E.I., Santillana P.T.G. Simplified method for vagal effect evaluation in cardiac ablation and electrophysiological procedures. J Am Coll Cardiol EP. 2015;1:451–460. doi: 10.1016/j.jacep.2015.06.008. [DOI] [PubMed] [Google Scholar]