Abstract
Ellis Type III cavity spilling coronary perforation is a rare complication. We report to our knowledge, the first case of rotational atherectomy induced Type III cavity spilling coronary perforation of right posterior descending artery draining into middle cardiac vein, successfully managed by covered stent deployment. (Level of Difficulty: Advanced.)
Key Words: coronary perforation, covered stent, Ellis Type III cavity spilling, middle cardiac vein, rotational atherectomy
Abbreviations and Acronyms: CP, coronary perforation; CS, cavity spilling; RCA, right coronary artery; RPDA, right posterior descending artery
Graphical abstract
Ellis Type III cavity spilling coronary perforation is a rare complication. We report to our knowledge, the first case of rotational atherectomy…
A 48-year-old man presented with progressively worsening exertional chest pain and dyspnea, which had been occurring predominantly at rest for the previous 2 weeks.
Learning Objectives
-
•
To recognize coronary perforation as potential complication associated with rotational atherectomy especially in small arteries.
-
•
To understanding the acute management of Type III cavity spilling perforation.
Past Medical History
The patient had a prior history of hypertension, myocardial infarction, and percutaneous coronary interventions in left anterior descending artery, left circumflex artery, and distal right coronary artery (RCA).
Differential Diagnosis
The differential diagnosis for chest pain includes acute coronary syndrome, aortic dissection, pulmonary embolism, panic disorder, and gastroesophageal reflux disease.
Investigations
Physical examination, electrocardiography, and laboratory results, including troponin, were unremarkable. Transthoracic echocardiogram revealed preserved ejection fraction of 60% along with hypokinesis of basal inferior and inferolateral wall. For further evaluation of the unstable angina, the patient underwent invasive coronary angiography via right radial artery. Angiography revealed patent stents in the left anterior descending artery and left circumflex artery, and 70% in-stent restenosis of distal RCA with severely calcified subtotal occlusion of right posterior descending artery (RPDA) (Figure 1A, Video 1).
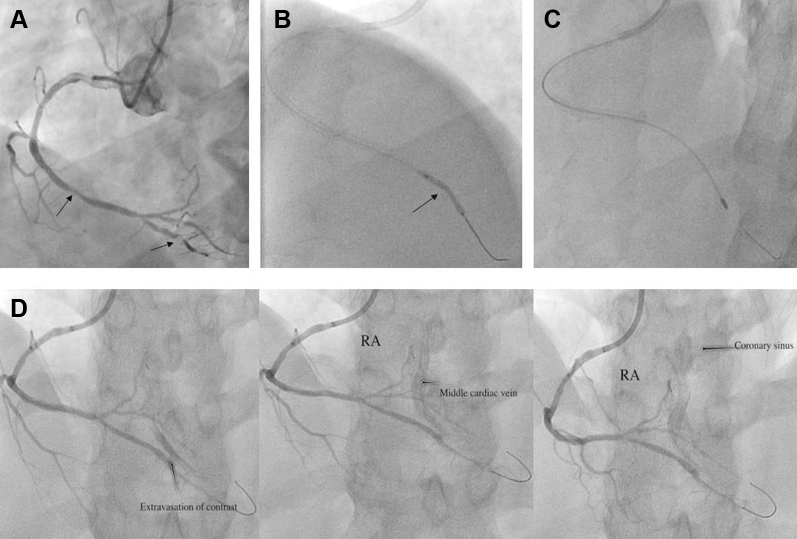
Figure 1.
Percutaneous Revascularization of RPDA Using Rotablation
(A) Significant in-stent restenosis in distal right coronary artery (RCA) and calcific lesion in right posterior descending artery (RPDA). (B) Undilatable lesion (arrow) in RPDA despite high pressure balloon inflation. (C) A 1.25-mm burr with RotaWire Floppy was used to cross the lesion. (D) RCA angiography showed development of Type III cavity spilling coronary perforation of RPDA draining into the middle cardiac vein and coronary sinus.
Online Video 1.
Significant in-stent restenosis in distal right coronary artery and calcific lesion in right posterior descending artery.
Management
Given the worsening chest pain, decision was made to proceed with percutaneous coronary intervention. A 6-F internal mammary guide catheter (Mach 1, Boston Scientific, Marlborough, Massachusetts) was used to engage RCA. After successful PTCA to distal RCA in-stent restenosis, a Gaia-2 wire (Asahi Intec, Tokyo, Japan) was used to cross the subtotal occlusion in RPDA with support of a Teleport microcatheter (Orbus Neich, Fort Lauderdale, Florida), which was used subsequently to exchange with a 0.014-inch Runthrough wire (Terumo, Tokyo, Japan) after dilation with a 1.0 × 5.0-mm Sapphire II Pro balloon (Orbus Neich). This was followed by serial dilatation with 2.25 × 20-mm noncompliant balloon as well as atherotomy with 2.25 × 6.0-mm Wolverine cutting balloon (Boston Scientific) at high pressure; however, there was incomplete balloon expansion in the RPDA lesion (Figure 1B, Video 2) and thus a decision was made to proceed with rotational atherectomy for further plaque modification. The workhorse wire was exchanged with a 0.009-inch RotaWire Floppy (Boston Scientific) using a Teleport microcatheter (Orbus Neich). The 1.25-mm burr was subsequently advanced over the wire to a position proximal to the lesion (Figure 1C). The rotational speed was set at the conventional range (160,000 rotations/min) with a total of 3 runs with each run time <20 s. Post atherectomy RCA angiography showed Type III cavity spilling (CS) coronary perforation of RPDA draining into the middle cardiac vein and coronary sinus (Figure 1D, Video 3). The patient remained hemodynamically stable, with no chest pain or electrocardiography changes. Emergent bedside transthoracic echocardiogram excluded any pericardial effusion or tamponade physiology. Anticoagulation (Heparin) was not reversed. The perforation persisted despite prolonged (>12 min) balloon inflation using 2.25 × 20.0-mm noncompliant balloon. Given the extent of perforation, the decision was made to implant a covered stent. A PK Papyrus (Biotronik AG, Bülach, Switzerland) covered stent 2.5 × 15.0 mm was deployed in the RPDA, but perforation persisted, as there were multiple jets. Thus, another 2.5 × 15-mm Papyrus stent graft was deployed in RPDA distal to the first covered stent in an overlapping fashion, which successfully sealed the perforation (Figure 2, Video 4).
Online Video 2.
Undilatable lesion in right posterior descending artery despite high-pressure balloon inflation.
Online Video 3.
Type III cavity spilling coronary perforation of right posterior descending artery draining into middle cardiac vein and coronary sinus.
Figure 2.
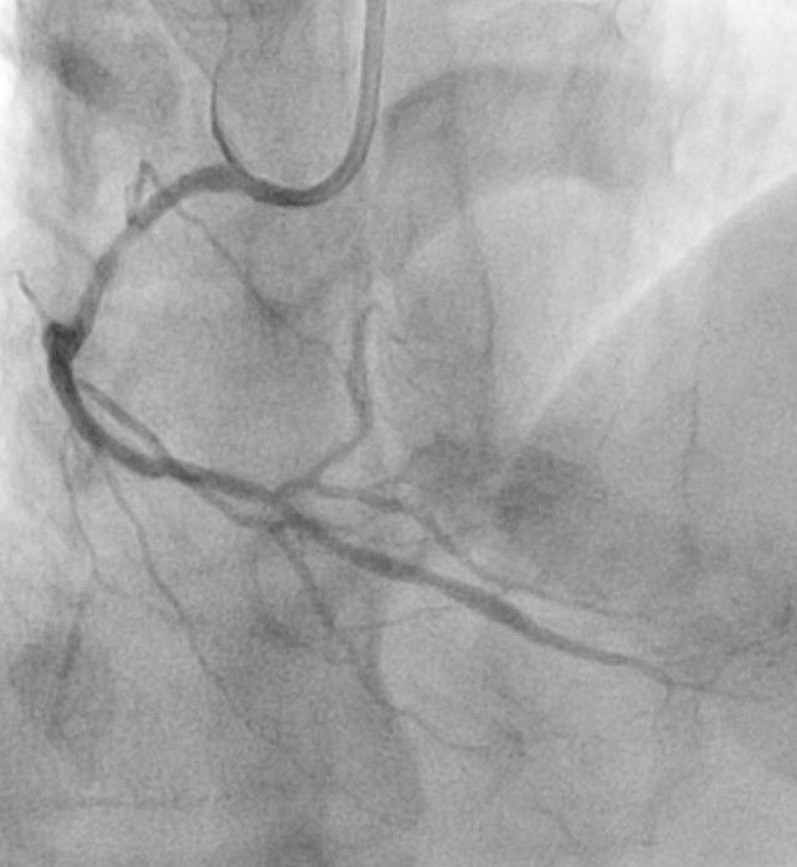
Final Angiogram of Right Posterior Descending Artery Post-Stent Graft Deployment
Final angiogram after stent graft deployment showing successful sealing of the perforation.
Online Video 4.
Final angiogram after stent graft deployment showing successful sealing of the perforation.
Discussion
We hereby describe a case of rotational atherectomy induced Type III CS coronary perforation (CP) and its acute management.
Ellis classification is the most commonly used classification method of CPs (1). Previous studies have reported combined incidence of all kinds of CPs to be 0.1% to 3.0% (2, 3, 4, 5) with reported incidence of Type III CS CP being 3.0% to 3.3% (1,3). Several patient, angiographic, and technical factors, including older age, female sex, Type C lesions, calcified arteries, tortuous and angulated vessels, previous coronary bypass grafts, balloon/stent oversizing, and use of atheroablative devices, have been identified as predictors of CPs (1, 2, 3, 4, 5).
Non-CS type III perforations frequently require pericardiocentesis, covered stenting, and/or emergent surgery. On the other hand, type III CS perforations usually have a favorable prognosis, as they seldom lead to acute hemodynamic compromise or ischemia (6,7). In our case, the posterior descending artery (PDA) was a small- to moderate-size vessel that was underfilled due to chronic subtotal occlusion. Considering the lesion under expansion after PTCA and cutting balloon, we decided to proceed with rotational atherectomy for optimal lesion preparation before stent placement. Therefore, a 1.25-mm burr (the smallest available burr) was chosen, and atherectomy was executed considering risks versus benefits involving this small-moderate size vessel. The Intravascular Lithotripsy System (Shockwave Medical, Santa Clara, California) could have been an alternative strategy but was unavailable in our laboratory, so it was not considered. Use of rotational atherectomy in PDA resulted in the dreaded complication of coronary perforation. Although rotational atherectomy in small- to moderate-size vessels can be safely performed in the hands of experienced and skilled operators, the operator should be well prepared and consider these risks. The perforation resulted in a coronary arteriovenous fistula from RPDA to middle cardiac vein draining into the coronary sinus, without hemodynamic instability, or ischemic changes. The middle cardiac vein is one of the coronary sinus tributary veins that arises in the left ventricle apex and travels in the posterior interventricular grove along with the RPDA and drains into the coronary sinus (8).
There is no consensus to manage type III CS perforations, and management of these coronary artery fistulas can be addressed with different approaches. Prolonged perfusion balloon inflation may treat some of them and previous reports have described spontaneous closure of some of these iatrogenic coronary artery fistulas. Patients with these fistulas can remain asymptomatic in 50% to 60% of cases, but can also lead to progressive development of dyspnea and heart failure symptoms when a significant amount of left to right shunt develops over time (8). Considering a large amount of shunt, we decided to proceed with covered stent implantation to seal the perforation after prolonged balloon inflation failed to do so. We used a PK Papyrus covered stent, which provides greater flexibility and smaller crossing profile and successfully sealed the perforation (9,10).
Follow-Up
The patient was discharged home the following day after a repeat transthoracic echocardiogram showed preserved ejection fraction and no pericardial effusion. Post–percutaneous coronary intervention peak troponin was 4 ng/ml and peak creatine kinase–MB was 31 IU/l.
Conclusions
We hereby describe a rare case of rotational atherectomy–associated Type III CS CP of RCA– posterior descending artery draining into the middle cardiac vein and its management.
Footnotes
Dr. Sharma has received speaker honoraria from Abbott Vascular, Boston Scientific, and Cardiovascular Systems. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
References
- 1.Ellis S.G., Ajluni S., Arnold A.Z. Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation. 1994;90:2725–2730. doi: 10.1161/01.cir.90.6.2725. [DOI] [PubMed] [Google Scholar]
- 2.Al-Lamee R., Ielasi A., Latib A. Incidence, predictors, management, immediate and long-term outcomes following grade III coronary perforation. J Am Coll Cardiol Intv. 2011;4:87–95. doi: 10.1016/j.jcin.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 3.Fasseas P., Orford J.L., Panetta C.J. Incidence, correlates, management, and clinical outcome of coronary perforation: analysis of 16,298 procedures. Am Heart J. 2004;147:140–145. doi: 10.1016/s0002-8703(03)00505-2. [DOI] [PubMed] [Google Scholar]
- 4.Parsh J., Seth M., Green J. Coronary artery perforations after contemporary percutaneous coronary interventions: evaluation of incidence, risk factors, outcomes, and predictors of mortality. Catheter Cardiovasc Interv. 2017;89:966–973. doi: 10.1002/ccd.26917. [DOI] [PubMed] [Google Scholar]
- 5.Shimony A., Joseph L., Mottillo S., Eisenberg M.J. Coronary artery perforation during percutaneous coronary intervention: a systematic review and meta-analysis. Can J Cardiol. 2011;27:843–850. doi: 10.1016/j.cjca.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Dippel E.J., Kereiakes D.J., Tramuta D.A. Coronary perforation during percutaneous coronary intervention in the era of abciximab platelet glycoprotein IIb/IIIa blockade: an algorithm for percutaneous management. Catheter Cardiovasc Interv. 2001;52:279–286. doi: 10.1002/ccd.1065. [DOI] [PubMed] [Google Scholar]
- 7.McCormick L.M., Ko B.S., Zaman S., Ahmar W., Meredith I.T. Persistent type III cavity-spilling coronary perforation due to covered stent malapposition. Cardiovasc Interv Ther. 2016;31:269–274. doi: 10.1007/s12928-015-0346-0. [DOI] [PubMed] [Google Scholar]
- 8.Sirajuddin A., Chen M.Y., White C.S., Arai A.E. Coronary venous anatomy and anomalies. J Cardiovasc Comput Tomogr. 2020;14:80–86. doi: 10.1016/j.jcct.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Nagaraja V., Schwarz K., Moss S., Kwok C.S., Gunning M. Outcomes of patients who undergo percutaneous coronary intervention with covered stents for coronary perforation: a systematic review and pooled analysis of data. Catheter Cardiovasc Interv. 2019 Dec 18 doi: 10.1002/ccd.28646. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Romaguera R., Waksman R. Covered stents for coronary perforations: is there enough evidence? Catheter Cardiovasc Interv. 2011;78:246–253. doi: 10.1002/ccd.23017. [DOI] [PubMed] [Google Scholar]