Abstract
A 66-year-old man with a ramus chronic total occlusion had escalating angina and a high-risk stress test. Coronary angiography the day of his planned ramus chronic total occlusion percutaneous coronary intervention demonstrated a large left main aneurysm. He underwent bypass with left internal mammary artery left anterior descending and failed saphenous vein graft ramus, followed by successful covered stent placement from left main into left circumflex and ramus chronic total occlusion percutaneous coronary intervention. (Level of Difficulty: Advanced.)
Key Words: computed tomography, coronary artery aneurysm, coronary artery bypass, high-risk, hybrid, percutaneous coronary intervention, stents
Abbreviations and Acronyms: CAA, coronary artery aneurysm; CTO, chronic total occlusion; LAD, left anterior descending; LCx, left circumflex; LIMA, left internal mammary artery; LM, left main; PCI, percutaneous coronary intervention
Graphical abstract
A 66-year-old man with a ramus chronic total occlusion had escalating angina and a high-risk stress test. Coronary angiography the day of his planned…
History of Presentation
A 66-year-old man with known coronary artery disease underwent a prior percutaneous coronary intervention (PCI) to his left circumflex (LCx) and diagonal arteries. Five months ago, he returned for a chronic total occlusion (CTO)-PCI of the right coronary artery and had a residual ramus CTO. He represented with worsening angina and nuclear perfusion imaging demonstrating severe ischemia in the ramus territory. At the time of presentation, his review of symptoms was now significant for rest angina despite optimal medical therapy. His physical examination was within normal limits. Angiography revealed patent prior stents; previously identified ramus CTO; and new, large, saccular aneurysm of the left main (LM) coronary artery (Video 1).
Learning Objectives
-
•
To gain an understanding for the incidence, etiology, and management options of a left main coronary artery aneurysm.
-
•
To appreciate a novel treatment strategy for a left main coronary artery aneurysm involving a hybrid surgical and percutaneous approach.
Online Video 1.
Coronary angiography revealed patent stents, previously identified Ramus CTO, and a new, large, left main coronary artery saccular aneurysm.
Past Medical History
The patient has a history of ischemic cardiomyopathy with left ventricular ejection fraction of 40%, hypertension, hyperlipidemia, peripheral arterial disease, insulin-dependent diabetes mellitus (hemoglobin A1C of 7.5%), and previously treated throat cancer for which he received radiation and chemotherapy.
Differential Diagnosis
The pathogenesis of coronary artery aneurysm (CAA) is poorly understood. Because trauma is believed to be a provoking factor, we hypothesized that his recent prior PCIs in combination with his various coronary artery disease risk factors may have contributed to the development of his newly discovered LM CAA.
Investigations
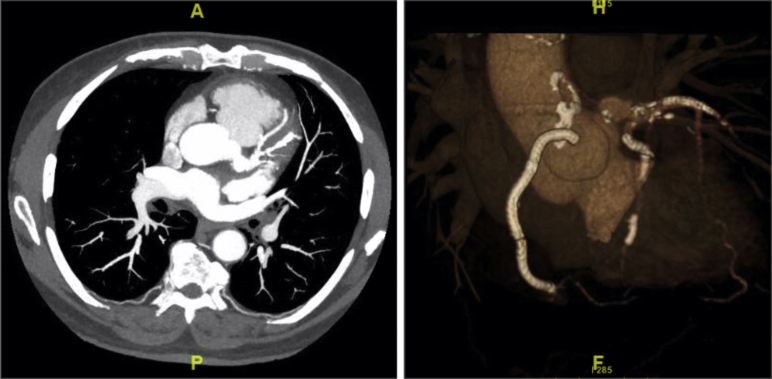
On discovery of the patient’s large LM CAA, intravascular ultrasound (Philips Healthcare, Amsterdam, Netherlands) of the LM was performed. It revealed a maximal aneurysmal dimension of at least 10 × 10 mm beginning approximately 2 mm from the aortic wall. Decision was made for the patient to undergo a cardiac computed tomography scan. This computed tomography confirmed a saccular aneurysm measuring 13 × 13 mm (Figure 1).
Figure 1.
Cardiac Computed Tomography and 3-Dimensional Reconstruction
Cardiac computed tomography and 3-dimensional reconstruction demonstrating the 13 × 13 mm left main coronary artery saccular aneurysm.
Management
The patient was placed on anticoagulation with warfarin, with goal international normalized ratio of 2 to 3. The case was discussed in detail with our cardiothoracic surgery colleagues. The decision was made for him to undergo coronary artery bypass surgery with the plan for him to receive a left internal mammary artery (LIMA) to his left anterior descending (LAD) artery and vein grafts to his ramus and LCx. A percutaneous strategy would only be instituted if the bypass grafts were unsuccessful. Ultimately, he received a LIMA to LAD and a single saphenous vein graft was presumably anastomosed to his ramus.
Post-bypass, the patient continued to experience his rest angina. He remained afebrile, with a heart rate of 65 beats/min and a blood pressure of 130/80 mm Hg. We elected to repeat his angiography 3 days after his surgery to evaluate his graft patency and insert a covered stent extending from the LM to the LCx to completely exclude the aneurysm. Angiography demonstrated a patent LIMA-LAD, but an occluded saphenous vein graft. Therefore, the following novel percutaneous strategy was used. An 8-F 45-cm sheath (Terumo, Tokyo, Japan) was advanced in the left femoral artery and an 8-F EBU4 guiding catheter (Medtronic, Minneapolis, Minnesota) was used to engage the LM. The LM into LCx was wired for safety and the ramus CTO was then attempted via antegrade approach, which failed. Collaterals were evaluated and a faint left to left epicardial collateral from the diagonal was successfully attempted. Retrograde wire escalation was performed with a Sion (Asahi Intecc, Seto-shi, Japan), followed by a Gladius Mongo (Asahi Intecc) and then a Gladius (Asahi Intecc), which was successfully advanced into the aorta (Video 2).
Online Video 2.
A left to left epicardial collateral from the diagonal was wired and the wire was advanced in retrograde fashion through the Ramus until it reached the distal cap. At this point, retrograde wire escalation was performed, and the wire was advanced into the aorta.
At this point, the retrograde wire was externalized and the ramus was predilated. Intravascular ultrasound demonstrated the ramus to be 3.5 mm in diameter and the LCx to be 5.0 mm. The externalized ramus wire was exchanged for an antegrade wire with the aid of a microcatheter. A 3.5 × 38 mm drug-eluting stent was placed in the ramus, post-dilated with a 4.0 × 30 mm noncompliant balloon (Video 3). While the ramus antegrade wire remained in place, the LM into LCx was stented with a 5.0 × 26 mm PK Papyrus covered stent (Biotronik, Berlin, Germany) and post-dilated with a 5.0 × 15 mm noncompliant balloon. An Astato 20 (Asahi Intecc) within a 135 Corsair (Asahi Intecc) was used to puncture across the LM covered stent into the ramus using the previously placed ramus antegrade wire as a landmark (Video 4). The Corsair was advanced through the Papyrus to enlarge this opening and the Astato 20 was de-escalated to a workhorse wire and the original antegrade wire was removed. Laser atherectomy with a 1.4-mm Excimer laser (Philips Healthcare) was then performed into the ramus to ablate the polyurethane membrane of the Papyrus covered stent. The Papyrus stent strut into the ramus was then dilated with a 3.0 × 15 mm balloon. A final kissing balloon inflation of the LM-LCx and LM-ramus was performed with simultaneous inflation of 5.0 × 15 mm and 3.5 × 15 mm noncompliant balloons, respectively (Video 5).
Online Video 3.
A 3.5x38mm drug eluting stent was inserted into the Ramus, post-dilated with a 4.0x30mm noncompliant balloon.
Online Video 4.
An Astato 20 (Asahi, Tustin, CA) within a 135 Corsair (Asahi, Tustin, CA) was used to puncture across the Left Main covered stent into the Ramus using the previously placed Ramus antegrade wire as a landmark.
Online Video 5.
A final kissing balloon inflation of the LM-LCx and LM-Ramus was performed with simultaneous inflation of 5.0x15mm and 3.5x15mm noncompliant balloons, respectively.
Final angiography revealed TIMI (Thrombolysis In Myocardial Infarction) flow grade III into all 3 coronary vessels and their branches in addition to retrograde filling of the LIMA. There was no evidence of stenosis, dissection, perforation, or thrombosis (Video 6).
Online Video 6.
Final angiography revealed excellent flow into all three coronary arteries, in addition to retrograde filling of the left internal mammary artery bypass.
Discussion
CAAs are defined as a dilation of the coronary to more than 1.5 to 2 times that of the diameter of adjacent normal coronary segments (1). The true prevalence of coronary aneurysms is unclear but ranges from 0.2% to 10% (2). There are a variety of hypothesized culprits in the development of CAA including atherosclerosis, trauma, angioplasty, congenital, inflammatory arterial diseases (polyarteritis nodosa, Takayasu arteritis, Behçet disease, syphilis), connective tissue disorders (systemic lupus erythematosus, rheumatoid arthritis, ankylosing spondylitis, progressive systemic sclerosis), and hereditary collagen defects (Marfan syndrome, Ehler-Danlos syndrome) (1,3).
In light of an era of efficient coronary angiography, an LM aneurysm remains rare. In a study conducted by Topaz et al. (4), there was an incidence for LM aneurysms of 0.1% among 20,332 adult patients who underwent routine coronary angiography. It is because of the paucity of these cases in literature, that treatment options are unclear. However, most would agree that the principal concern when dealing with aneurysms is the development of thrombus and subsequent embolization. Rath et al. (5) published a case series of 5 patients with coronary aneurysms who were asymptomatic without other critical lesions who went on to suffer from acute myocardial infarctions secondary to complete occlusion of their aneurysmal vessels.
There are currently no guidelines for the management of CAA. Therefore, because our patient had an LM CAA and was unable to receive 3 grafts, we elected to proceed with this novel hybrid approach. Our initial plan was to seal the CAA with the covered stent extending from LM into the LCx because this vessel was unable to be grafted. Unfortunately, in discovering that his saphenous vein graft to ramus was not patent, we had to change our strategy. We were able to extrapolate the idea of fenestrating through a covered stent, to allow flow into another territory from other case reports (6).
Before his PCI, we did discuss the need for left ventricular support. However, because our patient had a patent LIMA to his LAD with an ejection fraction of 45%, we did not believe it was necessary to place support upfront. Fortunately, throughout the intervention, he remained hemodynamically stable.
Follow-Up
Post-PCI, the patient had TIMI flow grade III into his LAD, ramus, and LCx with complete relief of his angina. He was discharged 2 days later and is currently underway with cardiac rehabilitation.
Conclusions
CAAs can result from a variety of factors and our patient likely developed his from trauma induced during prior coronary interventions. Treatment options typically involve anticoagulation to prevent complication from thrombus burden and surgical intervention. However, if the LM is involved, a hybrid approach may be a viable option especially if all vessels are unable to receive a bypass graft.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
References
- 1.Robinson F.C. Aneurysms of the coronary arteries. Am Heart J. 1985;109:129–135. doi: 10.1016/0002-8703(85)90425-9. [DOI] [PubMed] [Google Scholar]
- 2.Lakshmanadoss U., editor. Novel Strategies in Ischemic Heart Disease. Intech; Rijeka, Croatia: 2012. pp. 381–404. [Google Scholar]
- 3.ElGuindy M.S., ElGuindy A.M. Aneurysmal coronary artery disease: an overview. Glob Cardiol Sci Pract. 2017;2017 doi: 10.21542/gcsp.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topaz O., DiSciascio G., Cowley M.J. Angiographic features of left main coronary artery aneurysms. Am J Cardiol. 1991;67:1139–1142. doi: 10.1016/0002-9149(91)90881-k. [DOI] [PubMed] [Google Scholar]
- 5.Rath S., Har-Zahav Y., Battler A. Fate of nonobstructive aneurysmatic coronary artery disease: angiographic and clinical follow-up report. Am Heart J. 1985;109:785–791. doi: 10.1016/0002-8703(85)90639-8. [DOI] [PubMed] [Google Scholar]
- 6.Adusumalli S., Gaikwad N., Raffel C. Treatment of rotablation-induced ostial left circumflex perforation by papyrus covered stent and its fenestration to recover the left anterior descending artery during CHIP procedure. Catheter Cardiovasc Interv [Internet] 2019;93:E331–E336. doi: 10.1002/ccd.28114. [DOI] [PubMed] [Google Scholar]