Abstract
A 39-year-old man presented with recurrent syncope. A 12-lead electrocardiogram and a 24-h Holter recording demonstrated atypical persistent Mobitz type I and high-degree atrioventricular block, respectively. The functional nature of the atrioventricular block was confirmed by atropine challenge, exercise testing, and electrophysiological study. The patient was successfully treated with a cardioneuroablation procedure. (Level of Difficulty: Intermediate.)
Key Words: atrioventricular block, cardioneuroablation, catheter ablation, syncope, vagal denervation
Abbreviations and Acronyms: AV, atrioventricular; AVB, atrioventricular block; CNA, cardioneuroablation; GP, ganglionated plexus; RSGP, right superior ganglionated plexus
Graphical abstract
A 39-year-old man presented with recurrent syncope. A 12-lead electrocardiogram and a 24-h Holter recording demonstrated atypical persistent Mobitz…
History of Presentation
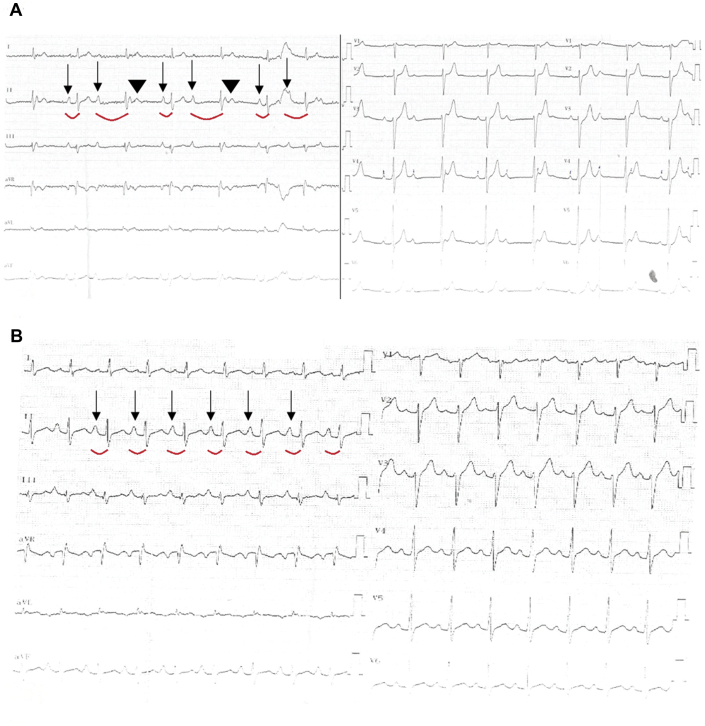
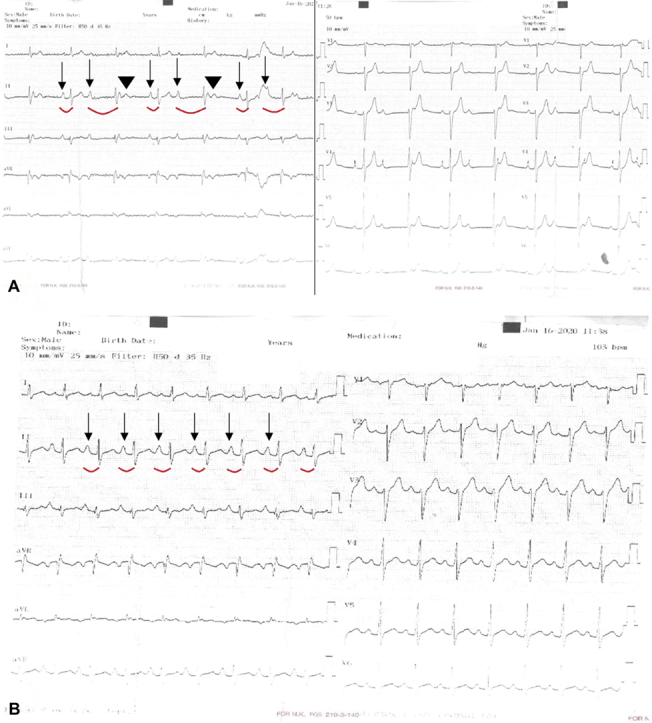
A 39-year-old man with recurrent dizziness and syncope, but no other medical issues, was referred to the University of Health Sciences, Kocaeli Derince Training and Research Hospital, for permanent pacemaker implantation. Physical examination was completely normal except for an irregular heart rate. A baseline electrocardiogram (ECG) showed an atypical persistent Mobitz type I atrioventricular (AV) block (AVB) and a narrow QRS complex (Figure 1A).
Learning Objectives
-
•
To differentiate among subtypes of AVB by means of functional noninvasive and invasive testing.
-
•
To understand the role of CNA in the treatment of functional AVB.
Figure 1.
Baseline and Atropine Challenge Electrocardiograms
(A) A 12-lead electrocardiogram at baseline with atypical persistent Mobitz type I atrioventricular block and a narrow QRS complex. (B) Response to 2 g intravenous atropine: normal 1:1 atrioventricular conduction is seen. Arrows show conducted P waves, curved red lines indicate PR intervals in conducted beats, and arrowheads point to blocked P waves.
Past Medical History
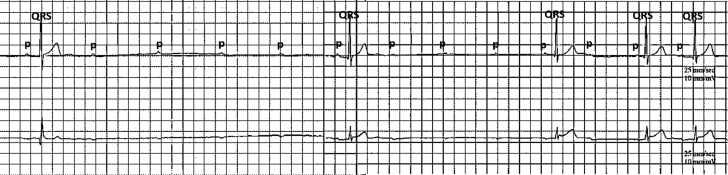
The patient had dyspnea with onset of exercise that resolved after a few minutes. He reported frequent syncopal episodes with prodromal symptoms of nausea, a sensation of warmth followed by clammy skin, blurry vision and lightheadedness since adolescence that occurred with upright posture and light activity. Holter recordings revealed frequent intermittent high-degree AVB, reaching up to 4 consecutive blocked P waves (Figure 2).
Figure 2.
Holter Recording Showing High-Degree Atrioventricular Block During Sleep
There are up to 4 consecutive blocked P waves resulting in a 4-s pause. A total of 9 pauses lasting >3 s occurred during 24-h Holter monitoring. Sinus slowing is evident at the time of atrioventricular block.
Differential Diagnosis
The differential diagnosis included functional (vagal) AVB, intrinsic (structural) AVB, and extrinsic idiopathic AVB with accompanying vasovagal syncope.
Investigations
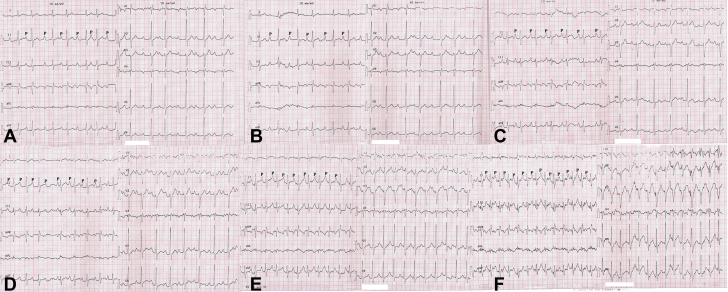
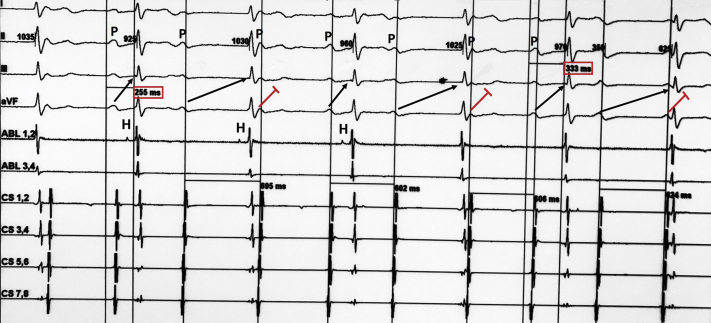
To determine the nature of the AVB, an atropine response test was conducted. During the test, a stepwise increase in sinus rate from 75 to 103 beats/min was noted, and partial resolution of AVB with a 240 ms PR interval was seen after administration of 2 mg of intravenous atropine (Figure 1B). Complete resolution of AVB was also noted during exercise stress testing (Figures 3A to 3F). At 20 min into a head-up tilt-table test, a typical cardioinhibitory response with an asystolic pause of 22 s was seen, resulting in loss of consciousness. An electrophysiological study revealed an HV interval of 46 ms and supra-Hisian atypical Mobitz type I AVB (Figure 4). Restoration of 1:1 AV conduction was achieved during overdrive atrial pacing (Figures 5A to 5C). On the basis of these findings, we considered his AVB to be functional. Pacemaker implantation and cardioneuroablation (CNA) were discussed with the patient as treatment options. Out of concern for a very likely high percentage of right ventricular pacing with a pacemaker implantation strategy given his prolonged PR interval at baseline, and despite the investigational nature of a CNA strategy, the benefits of preserving physiological ventricular stimulation with a CNA procedure were explained. The patient chose to proceed with CNA and gave informed consent for the procedure.
Figure 3.
Serial Electrocardiograms Obtained During Exercise Treadmill Testing
Note the gradual improvement in atrioventricular conduction with 1:1 atrioventricular conduction during stage 3 of the exercise protocol. (A and B) Atypical Mobitz type I atrioventricular block with irregular PR intervals. (C) 1:1 atrioventricular conduction. Note the constant PR intervals. (D) Atypical Mobitz type I atrioventricular block with irregular PR intervals. (E and F) Normal 1:1 atrioventricular conduction with constant PR intervals.
Figure 4.
Electrophysiological Study
Baseline measurements at 100 mm/s paper speed using 3 surface electrocardiographic leads (I, II, and III) and 6 intracardiac electrograms positioned in the His bundle (ABL) and the coronary sinus (CS). The surface electrocardiogram shows atypical Mobitz type I atrioventricular block with irregular PR intervals. The intracardiac electrograms show supra-His block with a progressive increase in the AH interval (interval between the activation potential of the right atrium and the activation potential of the His bundle [H]) until the A potential is not followed by an H potential. Black arrows point to conducted P waves (P), and red lines indicate nonconducted beats. Note the irregular PR intervals before the nonconducted P-wave and prolongation of the PR interval after the blocked beat (from 255 to 333 ms).
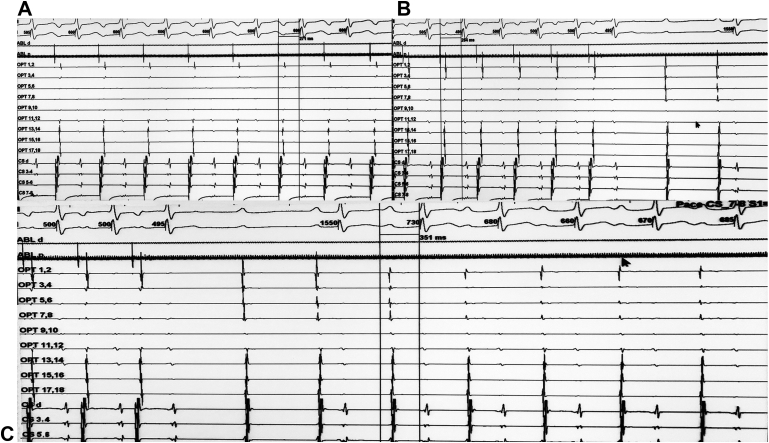
Figure 5.
Simultaneous Surface and Intracardiac EGMs at 100 mm/s Paper Speed With 3 Surface Electrocardiographic Leads (I, II, and III) and 15 Intracardiac EGMs During Atrial Pacing
The intracardiac electrograms (EGMs) were recorded in the right atrium (ABL), the coronary sinus (CS), and the left atrium (OPT). (A) Atrial pacing through the coronary sinus catheter at a cycle length of 600 ms resulting in 1:1 atrioventricular conduction with a 271-ms PR interval. (B) Atrial pacing at a cycle length of 500 ms causes a prolongation of the PR interval with continuation of 1:1 atrioventricular conduction. (C) 1:1 atrioventricular conduction with a 351-ms PR interval continued after cessation of pacing.
Management
The procedure was performed while the patient was under conscious sedation with midazolam and fentanyl. After a 3-dimensional electroanatomic map of both atria was obtained, bipolar endocardial atrial electrograms demonstrating 4 or more deflections in the anatomic regions consistent with the location of ganglionated plexuses (GPs) were tagged as ablation targets. After all GP targets were identified, radiofrequency ablation was first delivered on the posteromedial left GP, which in the majority of cases provides vagal innervation to the AV node. Because 1:1 AV conduction was not achieved at this site, other GPs were subsequently ablated according to our order (the Marshall tract GP, the left superior GP, the right superior GP [RSGP], and the right inferior GP through the left atrium) (Figure 6). During ablation on the RSGP, 1:1 AV conduction was achieved (Video 1). At the end of the procedure, final intervals were as follows: PR interval, 198 ms; sinus node cycle length, 560 ms (Figures 7A and 7B); AH interval, 130 ms; and Wenckebach cycle length, 330 ms. When comparing the post-ablation ECG values (PR interval and sinus rate) with these values during the pre-operative atropine response, the results were consistent with complete vagal denervation.
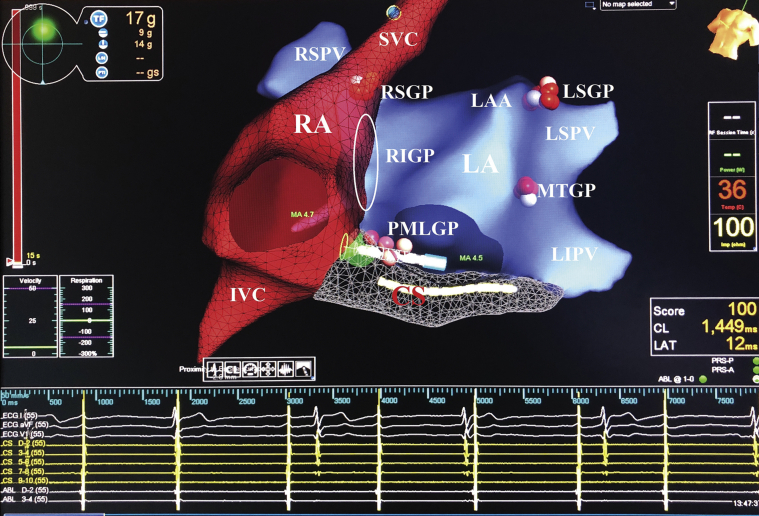
Figure 6.
Biatrial Electroanatomic Maps Obtained Using the EnSite Precision Mapping System (Abbott Laboratories, Abbott Park, Illinois) During Cardioneuroablation
Ablation points show location of ganglionated plexuses on the basis of fragmented electrograms. The right superior ganglionated plexus (RSGP) is located between the superior vena cava (SVC) and the right superior pulmonary vein (RSPV). The right inferior ganglionated plexus (RIGP) is located between the right pulmonary veins and the right atrium (RA) in the interatrial septum (indicated by a white oval). The posteromedial left ganglionated plexus (PMLGP) is bounded by the posterior wall of the left atrium (LA), the inferior vena cava (IVC), and the coronary sinus (CS) ostium. The left superior ganglionated plexus (LSGP) is located between the left atrial appendage (LAA) and the left superior pulmonary vein (LSPV). The Marshall tract ganglionated plexus (MTGP) is located in the lateral wall of the left atrium. The left inferior ganglionated plexus is located in the posterior wall of the left inferior pulmonary vein (LIPV) (not seen in the anteroposterior view). Intracardiac electrograms are shown at the bottom. Ablation lesions were tagged according to time as follows: white (<5 s); pink (5 to 15 s); and red (>15 s). ABL = ablation catheter; CL = cycle length; ECG = electrocardiogram; LAT = local activation time; MA = mitral annulus; PRS-A = patient reference sensor placed on the front of the patient; PRS-P = patient reference sensor placed on the back of the patient; TF = total force.
Online Video 1.
Achievement of 1:1 AV Conduction During Ablation on the Right Superior Ganglionated Plexus
The video shows biatrial electroanatomic maps obtained using the EnSite Precision mapping system (Abbott Laboratories, Abbott Park, Illinois) during cardioneuroablation. The intracardiac electrograms are shown below. At the beginning of the tracing, atrioventricular (AV) block is seen despite the presence of sinus tachycardia. After 11 seconds of ablation, 1:1 atrioventricular conduction is achieved. For detailed discussion of anatomic structures, please see Figures 6 and 7.
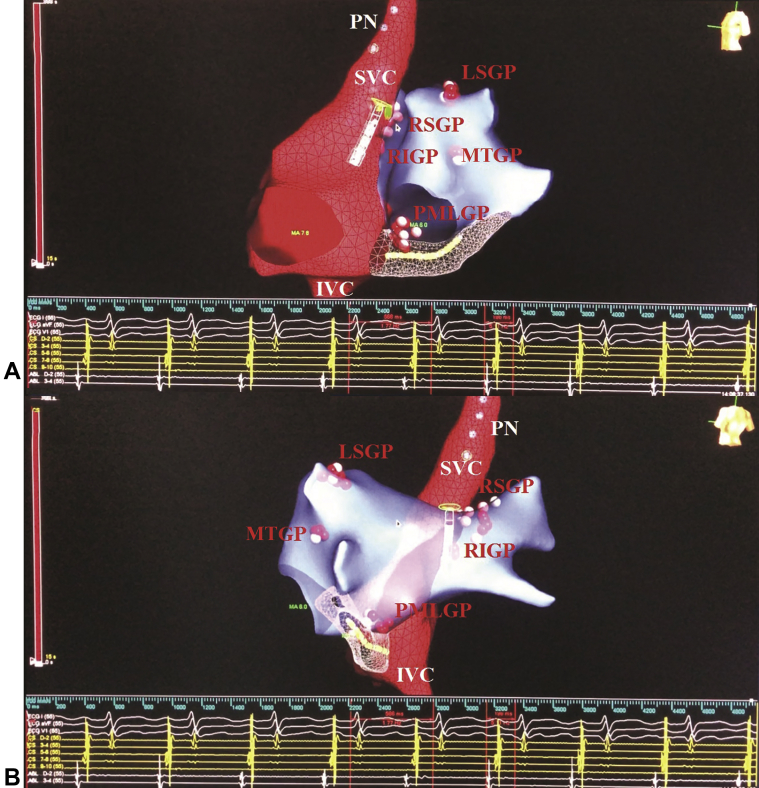
Figure 7.
Biatrial Electroanatomic Maps Obtained Using the EnSite Precision Mapping System (Abbott Laboratories, Abbott Park, Illinois) During Cardioneuroablation
(A and B) Electroanatomic maps. The intracardiac electrograms are shown at the bottom. The final PR interval and sinus node cycle length were 198 and 560 ms, respectively. Ablation lesions were tagged according to time as follows: white (<5 s); pink (5 to 15 s); and red (>15 s). PN = phrenic nerve; other abbreviations as in Figure 6.
Discussion
Syncope secondary to AVB accounts for more than one-half of all arrhythmia-related syncopal episodes (1). Functional or vagal AVB is usually characterized by a sudden change from seemingly normal AV conduction to transient second- or third-degree AVB in response to vagal overactivity (2). Differentiation of vagal from intrinsic and extrinsic idiopathic AVB is important because no studies to date have shown a benefit of prophylactic pacemaker implantation in patients with vagal AVB (3).
Intrinsic AVB is usually initiated by premature extrasystoles, and sinus rate acceleration is observed during the block (4). Conversely, AVB is often accompanied by slowing of the sinus rate in functional AVB, and progressive PR interval prolongation preceding the AVB may also be seen. To differentiate intrinsic from functional types in cases of persistent AVB, atropine response testing may be used (5,6). In these studies, resolution of AVB during exercise, following atropine administration and during atrial pacing, were highly suggestive of functional AVB, as in the present case. Moreover, our patient had a normal HV interval at baseline and during atrial pacing, a finding supporting a functional mechanism. Extrinsic idiopathic AVB is characterized by recurrent syncopal episodes without prodromal symptoms (7). In the present case, the prodromal symptoms were consistent with an enhanced vagal response before the syncope.
The intrinsic cardiac autonomic nervous system forms a complex network composed of GPs, following relatively stable anatomic distribution. Experimental studies using a canine model have shown that the sinoatrial and AV nodes may be innervated by different GPs. AV nodal innervation is usually provided by GPs in the vicinity of the AV nodal region, whereas the RSGP predominantly affects the sinoatrial node. This association has not been confirmed in human subjects yet (8,9). In a recently published study, Bulava et al. (10) described a new technique for CNA to treat functional AVB, thereby avoiding ablation on the RSGP to prevent an increase in the heart rate. In the present case, however, despite sequential ablation of GPs on the left side, correction of functional AVB was not achieved until ablation was conducted on the RSGP. This case supports the theory than in some patients, a much wider ablation may be necessary to eliminate the vagal effect on the AV node. Although the clinical utility of a pre-procedural atropine challenge to detect vagal denervation was recently presented by our group, a more objective assessment of the GP ablation denervation effect may be performed using extracardiac vagal stimulation techniques (11,12).
CNA for intermittent high-degree AVB was first reported by Pachon et al. (13) in 2006. The clinical efficacy and reproducibility of this technique were later confirmed by several groups worldwide (5,6,13, 14, 15). According to the current bradyarrhythmia guidelines, the presence of symptoms is a major determinant of whether permanent pacing will be required in the setting of bradycardia associated with AVB (1). Functional AVB is usually transient and generally does not require cardiac pacing. However, in symptomatic patients, treatment may be warranted. CNA could be postulated as a better option for young patients with symptomatic functional AVB because the current evidence shows excellent long-term outcomes and safe and reproducible results with this technique. Most importantly, CNA can eliminate the need for permanent pacing in this group of patients, thus avoiding a potential lifetime risk of pacemaker-related complications such as pace-mediated cardiomyopathy, lead malfunction, and device-related infections.
Follow-Up
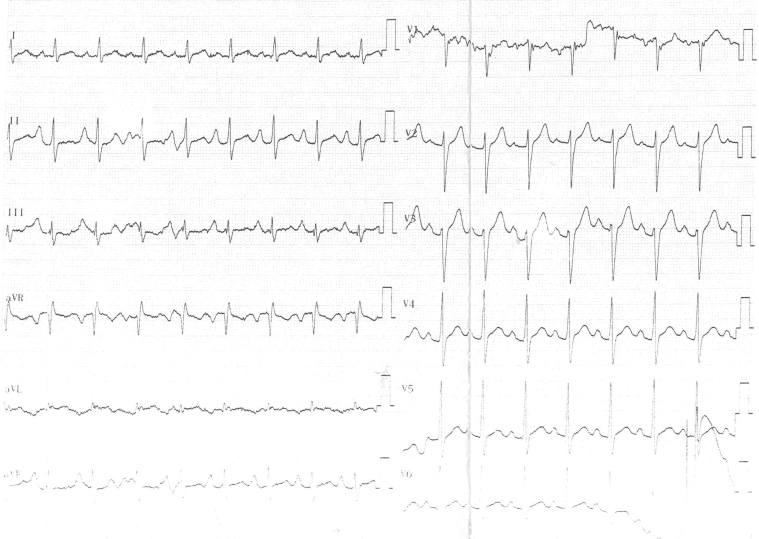
Repeated Holter recordings 1, 2, and 3 months after CNA revealed no further AVB episodes. Results of follow-up head-up tilt-table testing were completely normal at 1 and 3 months. No recurrent syncope was noted after a 3-month follow-up. Follow-up resting ECGs confirmed normal AV conduction (Figure 8).
Figure 8.
Follow-Up 12-Lead Electrocardiogram
Sinus rhythm; heart rate, 100 beats/min; P-wave duration, 120 ms; and PR interval, 200 ms; 1:1 atrioventricular conduction is seen.
Conclusions
CNA may be a valuable adjunctive therapy in patients with vagal AVB who cannot be adequately treated by conventional modalities and who refuse pacemaker implantation.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For a supplemental video, please see the online version of this paper.
References
- 1.Kusumoto F.M., Schoenfeld M.H., Barrett C. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:1016–1018. doi: 10.1016/j.jacc.2018.10.043. [DOI] [PubMed] [Google Scholar]
- 2.Alboni P., Holz A., Brignole M. Vagally mediated atrioventricular block: pathophysiology and diagnosis. Heart. 2013;99:904–908. doi: 10.1136/heartjnl-2012-303220. [DOI] [PubMed] [Google Scholar]
- 3.Raviele A., Giada F., Menozzi C. A randomized, double-blind, placebo-controlled study of permanent cardiac pacing for the treatment of recurrent tilt-induced vasovagal syncope. The Vasovagal Syncope and Pacing Trial (SYNPACE) Eur Heart J. 2004;25:1741–1748. doi: 10.1016/j.ehj.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 4.Aksu T., Guler T.E., Bozyel S., Yalin K. Potential usage of cardioneuroablation in vagally mediated functional atrioventricular block. SAGE Open Med. 2019;7 doi: 10.1177/2050312119836308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pachon J.C., Pachon E.I., Pachon J.C. “Cardioneuroablation”—new treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. Europace. 2005;7:1–13. doi: 10.1016/j.eupc.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Aksu T., Golcuk E., Yalin K., Guler T.E., Erden I. Simplified cardioneuroablation in the treatment of reflex syncope, functional AV block, and sinus node dysfunction. Pacing Clin Electrophysiol. 2016;39:42–53. doi: 10.1111/pace.12756. [DOI] [PubMed] [Google Scholar]
- 7.Brignole M., Deharo J.C., Guieu R. Syncope and ıdiopathic (paroxysmal) AV block. Cardiol Clin. 2015;33:441–447. doi: 10.1016/j.ccl.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Armour J.A., Murphy D.A., Yuan B.X., Macdonald S., Hopkins D.A. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247:289–298. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 9.Chiou C.W., Eble J.N., Zipes D.P. Efferent vagal innervation of the canine atria and sinus and atrioventricular nodes. The third fat pad. Circulation. 1997;95:2573–2584. doi: 10.1161/01.cir.95.11.2573. [DOI] [PubMed] [Google Scholar]
- 10.Bulava A., Osório T.G., Hanis J. Cardioneuroablation instead of pacemaker implantation in a young patient suffering from permanent 2:1 atrioventricular block after a slow pathway ablation. HeartRhythm Case Rep. 2020;6:261–264. doi: 10.1016/j.hrcr.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aksu T., Guler T.E., Bozyel S., Yalin K. Vagal responses during cardioneuroablation on different ganglionated plexi: is there any role of ablation strategy? Int J Cardiol. 2020;304:50–55. doi: 10.1016/j.ijcard.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Pachon M.J.C., Pachon M.E.I., Santillana P.T.G. Simplified method for vagal effect evaluation in cardiac ablation and electrophysiological procedures. J Am Coll Cardiol EP. 2015;1:451–460. doi: 10.1016/j.jacep.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Pachon M.J.C., Pachon M.E.I., Lobo T.J. Syncopal high-degree AV block treated with catheter RF ablation without pacemaker implantation. Pacing Clin Electrophysiol. 2006;29:318–322. doi: 10.1111/j.1540-8159.2006.00340.x. [DOI] [PubMed] [Google Scholar]
- 14.Rivarola E.W., Hachul D., Wu T. Targets and end points in cardiac autonomic denervation procedures. Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.116.004638. [DOI] [PubMed] [Google Scholar]
- 15.Piotrowski R., Baran J., Kułakowski P. Cardioneuroablation using an anatomical approach: a new and promising method for the treatment of cardioinhibitory neurocardiogenic syncope. Kardiol Pol. 2018;76:1736–1738. doi: 10.5603/KP.a2018.0200. [DOI] [PubMed] [Google Scholar]