Abstract
This case report describes a 46-year-old female with a history of multiple endocrine neoplasia type 1 syndrome status post-parathyroidectomy, thymectomy via robotic video-assisted thoracoscopic surgery, and pituitary adenoma resection presenting with parasympathetic hyperactivity and Parry–Romberg syndrome. Parry–Romberg syndrome is a rare disorder that manifests as facial hemiatrophy. Reported symptoms include cognitive dysfunction, olfactory hallucinations, and parasympathetic hyperactivity: miosis of the right eye, anhidrosis, diarrhea, urinary incontinence, bowel incontinence, and orthostatic hypotension. Previous reports have noted associations between Parry–Romberg syndrome and sympathetic hyperactivity and dysregulation. This case report focuses on an association between Parry–Romberg syndrome and unopposed parasympathetic activity.
Keywords: Parry–Romberg syndrome, hemifacial atrophy, parasympathetic hyperactivity, dysautonomia, autonomic dysfunction
Introduction
Parry–Romberg syndrome (PRS) is a rare disease, manifesting as facial hemiatrophy, affecting the skin, subcutaneous tissues, bone, and muscle.1,2 It has a predilection for females.1,2 PRS is gradually progressive and a self-limiting disease where the process usually resolves within 2–10 years. 2 The etiology of PRS is still unknown. Several theories have been proposed: trauma, infection, sympathetic dysfunction, vasculitis, and autoimmune.2,3 Linear scleroderma en coup de sabre (LSCS) is described as a unilateral linear depression typically located between the crown and forehead. 4 The association of PRS with LSCS has been well described in the literature.1–5 In a 2007 retrospective review of 54 patients with LSCS, 28% of patients also had PRS while a 1956 review reported 42% of patients having both PRS and LSCS.1,6 Some authors advocate that PRS and LSCS are on a spectrum of the same pathology.2–4
A wide array of neurological symptoms in PRS has been noted in the literature. In a 2015 review, the authors reviewed neurological symptoms in PRS and noted epilepsy as the most common. 7 Sympathetic dysregulation in PRS has also been noted in the literature. A 2006 case report noted sympathetic hyperactivity in a patient with PRS. The authors described increased pulse amplitude and diaphoresis on one side of the forehead ipsilateral to facial atrophy. 8 Another case report found changes in temperature and pain thresholds in the contralateral lower limb as well as an absence of a sympathetic skin response in the contralateral foot in a patient with PRS. 9 Some authors have even suggested sympathetic hyperactivity and dysregulation as a possible mechanism in the development of PRS.10,11 In 1960, cervical sympathectomy in rat models was used to induce facial hemiatrophy. 11 A case report in 2012 suggested cervical sympathetic hyperactivity as a possible etiology of facial hemiatrophy after linear polarized near-infrared light therapy to the stellate ganglion helped relieve pain in the temporomandibular joint in a patient with PRS. 10 Here, we present a case of parasympathetic hyperactivity, cognitive dysfunction, and olfactory hallucinations in PRS.
Case presentation
Here, we describe a 46-year-old female who came into the office reporting olfactory hallucinations. History included multiple endocrine neoplasia type 1 (MEN 1) syndrome status post-parathyroidectomy, thymectomy via robotic video-assisted thoracoscopic surgery (VATS), and pituitary adenoma resection. She had cognitive slowing and small fiber neuropathy. Her symptoms of parasympathetic hyperactivity include urinary incontinence, bowel incontinence, diarrhea, orthostatic hypotension, anhidrosis, and miosis of the right eye. Often these symptoms present as mild flares several times per month. During more severe flares, which occur about once a month, her symptoms progress to include severe blurry vision of the right eye, miosis of the right eye, severe difficulty with verbal cognition, short-term memory loss, and severe fatigue. She subsequently noticed a tender linear depression on the right center of her forehead that eventually widened and extended along the top and back of her skull, leading to her diagnosis of scleroderma en coup de sabre. She also noticed an expanding indent on her right temple. She eventually reported progression of tissue loss extending to the involvement of her right cheekbones and the right side of her nose. Wearing glasses became difficult due to the significant size difference of her face and head from right to left. Right-sided facial atrophy of the cheek and nose can be seen in Figure 1. In Figure 2, en coup de sabre is noted on her forehead. A magnetic resonance imaging (MRI) in March of 2019 demonstrated punctate T2 hyperintensities in the right corona radiata and left frontal periventricular white matter. Her symptoms were found to be consistent with facial hemiatrophy also known as PRS.
Figure 1.

Right-sided facial atrophy of the cheek and nose.
Figure 2.
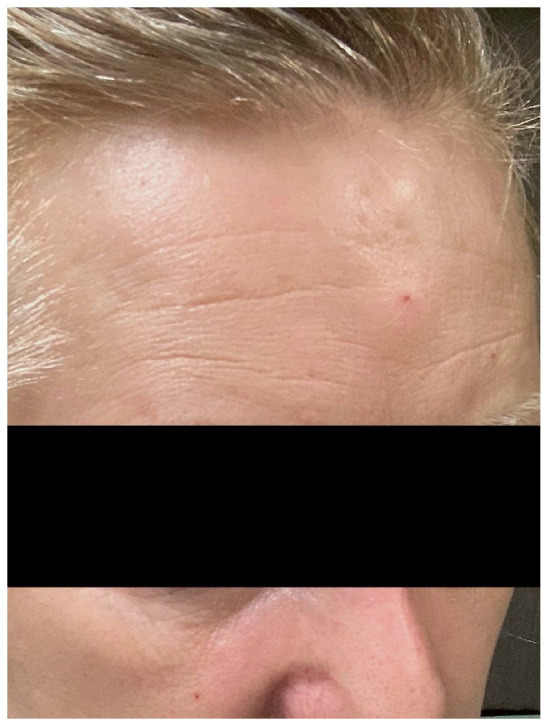
Coup de sabre noted on the forehead.
Discussion
PRS remains a clinical diagnosis with no universal diagnostic criteria. 2 The main characteristic feature of PRS is hemifacial atrophy within the dermatome of the trigeminal nerve branches. 2 Consistent with this description, our patient experienced slow progression of unilateral tissue loss of the cheek and nose. The linear depression in this case appears to be consistent with LSCS given its physical features, but other possible diagnoses still exist. One case report described linear cutaneous lupus erythematous on the forehead mimicking linear scleroderma. 12 Corticosteroid-induced atrophy, panniculitis, progressive partial lipodystrophy, and focal dermal hypoplasia have also been noted in the literature as part of the differential diagnosis for LSCS. 5
Typical MRI findings on T2-weighted imaging in PRS can demonstrate hyperintense white matter lesions usually ipsilateral to skin involvement but can be found bilaterally as well.2,3 Other MRI findings include ipsilateral cerebral hemiatrophy and intraparenchymal calcifications. 3 Consistent with the literature, our patient’s MRI demonstrated hyperintensities in the white matter on T2-weighted imaging.
There are no guidelines on the treatment of PRS.2,3 A 2015 literature review describes methotrexate as standard therapy for active disease and is sometimes combined with oral prednisone for the first 3 months. 2 A 2020 literature review includes the following additional treatment modalities for PRS: anticonvulsants for seizures and cosmetic procedures, such as autologous fat grafts, after stabilization of disease. 3
Our patient’s autonomic symptoms present intermittently as flares of parasympathetic hyperactivity: unilateral miosis, urinary incontinence, anhidrosis, diarrhea, fecal incontinence, and orthostatic hypotension. The etiology of parasympathetic hyperactivity includes multiple pathologies. Horner’s syndrome, which is characterized by ipsilateral ptosis, miosis, and anhidrosis, is caused by disruption of sympathetic input to the head allowing for unopposed parasympathetic activity.13,14 It can occur with lesions to the lateral medulla, such as stroke and multiple sclerosis. 13 Other causes of Horner’s syndrome include damage to the peripheral sympathetic system, such as from thoracic or thyroid surgery. 14 Our patient’s recent surgical history included parathyroidectomy, thymectomy via robotic VATS, and pituitary adenoma resection. Given the location of these surgical sites, it is possible that traumatic disruption of sympathetic fibers of the cervical plexus or thoracic sympathetic trunk could have led to unopposed parasympathetic activity. With absent or minimal sympathetic input, the parasympathetic system is unopposed, leading to the symptoms of parasympathetic hyperactivity: unilateral miosis, urinary incontinence, anhidrosis, diarrhea, fecal incontinence, and orthostatic hypotension. It is also possible that the PRS and dysautonomia occur coincidentally as alternative etiologies for gastrointestinal dysautonomia exist. Pandysautonomia can affect both sympathetic and parasympathetic nerves, leading to gastrointestinal symptoms. 15 Selective cholinergic dysautonomia can result in gastrointestinal dysmotility and occurs after infection with Epstein–Barr Virus (EBV) or influenza. 15 Idiopathic orthostatic hypotension can be associated with gastrointestinal motor dysfunction, such as fecal incontinence. 15
Our findings are consistent with another reported association between dysautonomia and PRS. Dysautonomia may contribute to inflammation resulting in tissue destruction. Although the pathogenesis of PRS is unknown, there are several theories, including sympathetic dysregulation and trauma. 3 A 2012 review cited that trauma has been hypothesized to be the origin of PRS in 24%–34% of patients, including operative traumas, such as thyroidectomy. 16 In one 2019 review, the authors noted sympathetic nervous system dysfunction after traumatic disruption of cervical plexus or thoracic sympathetic trunk as one of the potential etiologies of PRS. 17 One review cited several case reports of ipsilateral Horner’s syndrome in PRS, which would be consistent with a sympathetic nerve dysfunction. 16 A 2004 case report noted PRS development after a thoracoscopic sympathectomy of the upper thoracic sympathetic nerve ganglions for palmar hyperhidrosis. 18 Here, we describe symptoms of parasympathetic hyperactivity and PRS in a patient with a history of MEN 1 syndrome who underwent parathyroidectomy, thymectomy via robotic VATS, and pituitary adenoma resection. Given our patient’s surgical history and symptoms of parasympathetic hyperactivity, it is possible that traumatic disruption of sympathetic fibers of the cervical plexus or thoracic sympathetic trunk could have led to the development of PRS.
Currently, there is a lack of research on managing dysautonomia in PRS. Management in dysautonomia in other neurological diseases has been previously reported. A 2015 review on autonomic failure in E-synucleinopathies describes treatment concentrating on orthostatic hypotension in order to reduce syncope and fall risk. The authors noted the following measures to manage orthostatic hypotension: wearing compression stockings, standing up slowly, consuming 2–2.5 L of water and 8 g NaCl daily, and avoiding heat and alcohol while fludrocortisone and the pressor midodrine are used when lifestyle measures fail. 19
Our report includes limitations. Precocious diagnosis of PRS is possible, given the lack of precise and universal diagnostic tools available for PRS. Another limitation is the inability to establish causality between dysautonomia and PRS, given the descriptive nature of this report. It is possible that the association between unopposed parasympathetic activity and PRS is merely coincidental and the two disease processes are separate from one another. Further research will help to clarify the link between sympathetic dysregulation and PRS and to optimize the treatment of dysautonomia in PRS.
Conclusion
PRS is a rare self-limiting disease characterized by hemifacial atrophy and is strongly associated with LSCS. The etiology remains unclear, but sympathetic dysregulation has been proposed as a possible etiology. Our case presents a patient with PRS who presented to the clinic with symptoms of parasympathetic hyperactivity. Here, we propose interruption of sympathetic fibers as a possible mechanism for the development of parasympathetic hyperactivity and development of PRS. Clinicians should be aware of the association between PRS and dysautonomia, and further research is required to better optimize the treatment of dysautonomia in PRS.
Footnotes
Author contributions: A.N.C. contributed to the drafting/revision of manuscript for content; acquisition of data; analysis and interpretation of data. A.D.P.H. contributed to the drafting/revision of manuscript for content; acquisition of data; analysis and interpretation of data.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: Andrea N Clapp  https://orcid.org/0000-0001-7498-440X
https://orcid.org/0000-0001-7498-440X
References
- 1. Tollefson MM, Witman PM. En coup de sabre morphea and Parry–Romberg syndrome: a retrospective review of 54 patients. J Am Acad Dermatol 2007; 56(2): 257–263. [DOI] [PubMed] [Google Scholar]
- 2. Tolkachjov SN, Patel NG, Tollefson MM. Progressive hemifacial atrophy: a review. Orphanet J Rare Dis 2015; 10: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ahmed S, Tiwari S, Yadav T, et al. Parry Romberg syndrome: imaging features in 4 consecutive cases and review of literature. J Clin Neurosci 2020; 76: 249–253. [DOI] [PubMed] [Google Scholar]
- 4. Asano Y, Fujimoto M, Ishikawa O, et al. Diagnostic criteria, severity classification and guidelines of localized scleroderma. J Dermatol 2018; 45(7): 755–780. [DOI] [PubMed] [Google Scholar]
- 5. Kreuter A, Krieg T, Worm M, et al. German guidelines for the diagnosis and therapy of localized scleroderma. J Dtsch Dermatol Ges 2016; 14(2): 199–216. [DOI] [PubMed] [Google Scholar]
- 6. Christianson HB, Dorsey CS, Kierland RR, et al. Localized scleroderma; a clinical study of two hundred thirty-five cases. AMA Arch Derm 1956; 74(6): 629–639. [DOI] [PubMed] [Google Scholar]
- 7. Vix J, Mathis S, Lacoste M, et al. Neurological manifestations in Parry–Romberg syndrome: 2 case reports. Medicine 2015; 94(28): e1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drummond PD, Hassard S, Finch PM. Trigeminal neuralgia, migraine and sympathetic hyperactivity in a patient with Parry–Romberg syndrome. Cephalalgia 2006; 26(9): 1146–1149. [DOI] [PubMed] [Google Scholar]
- 9. Budrewicz S, Koszewicz M, Koziorowska-Gawron E, et al. Parry–Romberg syndrome: clinical, electrophysiological and neuroimaging correlations. Neurol Sci 2012; 33(2): 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Monobe H, Miyano K, Kagoya R, et al. Case of progressive facial hemiatrophy with cervical sympathetic hyperactivity as underlying aetiology. J Laryngol Otol 2012; 126(7): 725–728. [DOI] [PubMed] [Google Scholar]
- 11. Moss ML, Crikelair GF. Progressive facial hemiatrophy following cervical sympathectomy in the rat. Arch Oral Biol 1960; 1: 254–258. [DOI] [PubMed] [Google Scholar]
- 12. Sindhusen S, Chanprapaph K, Rutnin S. Adult-onset linear discoid lupus erythematosus on the forehead mimicking en coup de sabre: a case report. J Med Case Reports 2019; 13: 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tindle J, Tadi P. Neuroanatomy, parasympathetic nervous system. StatPearls, 2020, https://www.statpearls.com/articlelibrary/viewarticle/26653/ [PubMed]
- 14. Waxenbaum JA, Reddy V, Varacallo M. Anatomy, autonomic nervous system. Treasure Island, FL: StatPearls, 2020. [PubMed] [Google Scholar]
- 15. Camilleri M. Gastrointestinal motility disorders in neurologic disease. J Clin Invest 2021; 131(4): e143771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El-Kehdy J, Abbas O, Rubeiz N. A review of Parry–Romberg syndrome. J Am Acad Dermatol 2012; 67(4): 769–784. [DOI] [PubMed] [Google Scholar]
- 17. Schultz KP, Dong E, Truong TA, et al. Parry Romberg syndrome. Clin Plast Surg 2019; 46(2): 231–237. [DOI] [PubMed] [Google Scholar]
- 18. Scope A, Barzilai A, Trau H, et al. Parry–Romberg syndrome and sympathectomy—a coincidence? Cutis 2004; 73(5): 343–344, 346. [PubMed] [Google Scholar]
- 19. Sambati L, Calandra-Buonaura G, Doria A, et al. Diagnosis and management of autonomic failure in neurodegenerative disorders. Eur Neurol 2015; 73(1–2): 126–133. [DOI] [PubMed] [Google Scholar]


