Abstract
Skin and Adnexal tumours are a group of benign and malignant tumours whose basic diagnosis relies on histopathology. A single tumour may show more than 1 appendageal differentiation. Morphologic distinction between benign and its malignant counterpart is of utmost importance as it affects the treatment and prognosis of patient. We have described 3 cases who presented in our university hospital, in which final resection pathological diagnosis differed from initial core biopsy interpretation. The authors have made an attempt to provide a brief overview of diagnostic overlap existing between nodular hidradenocarcinoma and tumours of clear cell histology. Salient morphologic features differentiating cylindroma or trichilemmal carcinoma from squamous cell carcinoma have also been discussed. Final diagnosis is paramount for adjuvant management and prognostication of the patient in a clinical setting.
Keywords: Adnexal tumours, core biopsy, cylindroma, hidradenocarcinoma, trichilemmal carcinoma
Introduction
Adnexal tumours are a group of benign and malignant neoplasms which exhibit largely similar histologic features. They arise from hair follicles, sebaceous gland, apocrine gland and eccrine gland.1,2 These tumour may express 1 or more lines of appendageal differentiation during neoplastic transformation as they arise from multipotent stem cells present within epidermis or its appendageal structures. 3 Carcinoma are rare with incidence around 0.05% reported in western literature.4,5 Precise categorisation of these benign tumours is purely academic and generally does not affect clinical outcome. However, some of these tumours can be a feature of a syndrome, for example, multiple trichilemmomas in Cowden syndrome, sebaceous adenomas in Muir–Torre syndrome. 6
As clinical presentation is very nonspecific, definitive diagnosis can be made on histopathology only and classification is according to predominant morphological component. Small biopsy and cytology may not prove to be accurate many a times for these lesions but at least it may differentiate between benign and its malignant counterpart and thus surgical resection can be determined. We herewith present a concise report of 3 cases with either prior cytology or biopsy diagnosis of adnexal neoplasm and how their diagnosis was modified on wide excision, the impact of discrepancy between biopsy and final excision diagnosis on patient’s management and prognosis.
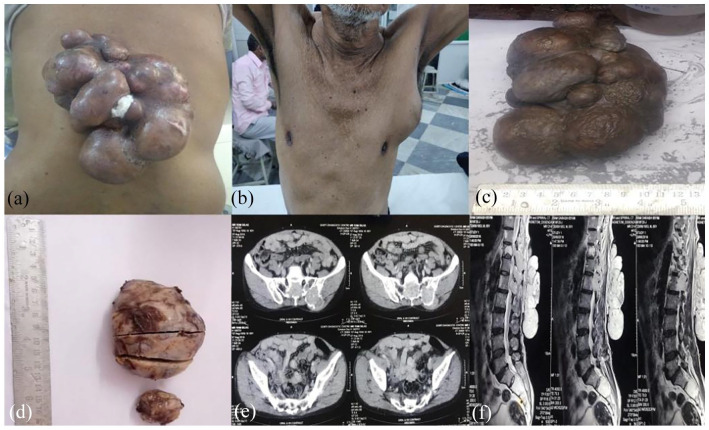
Case 1: A 60 years old male presented with multinodular gradually progressive growth over back measuring 16 × 11 cm (Figure 1a) for 7 years and a left axillary swelling (Figure 1b) measuring 6 × 6 cm for 4 years. Both swellings were firm with restricted mobility. CT imaging (Figure 1e) showed large heterogeneously enhancing multilobulated soft tissue lesion arising from the skin and subcutaneous tissue with axillary lymphadenopathy, pulmonary and bony metastases. MRI imaging also showed T2 hyper intense soft tissue lesion involving vertebral bodies (Figure 1f). 99mTc-MDP whole body bone scan revealed increased tracer uptake in right scapula and multiple foci in pelvis, suggesting bone metastasis. Biopsies were performed from both back swelling and axillary swelling. H&E sections from both biopsies revealed a malignant neoplasm disposed in sheets with ductal structures with areas of necrosis and tumour invasion (Figure 2a). Aspirate smears from back swelling were cellular and showed papillaroid fragments with plasmacytoid and basaloid cells and finely clumped chromatin (Figure 2b). Features of malignancy like tumour necrosis and infiltration were evident; hence morphological diagnosis of malignant adnexal neoplasm, probably ‘eccrine cylindroma’ was given. In view of malignant diagnosis, complete wide local excision was performed for back swelling and axillary lump. On gross examination, it was multinodular, well encapsulated with no capsular breech (Figure 1c) with close deep margins. Cut surface was yellowish white in colour with haemorrhagic areas. Largest axillary lump (Figure 1d) showed greyish white areas along with areas of haemorrhage and necrosis. H&E sections from both back and axillary lesion revealed an intradermal infiltrative tumour composed of polygonal cells with eosinophilic cytoplasm and small round to oval cells with clear cytoplasm showing moderate pleomorphism and nuclear atypia (Figure 2c–e). Cystic spaces filled with mucin were also seen (Figure 2f). Infrequent mitosis were present along with areas of necrosis and haemorrhage. All resection margins were microscopically free of tumour invasion. An IHC panel was applied which showed diffuse positivity for S100 and CK (Figure 3a and b) and negative for CEA and CD10 (Figure 3c and d). EMA expression was seen in the Ductular formations. Based on above findings final diagnosis was revised to ‘nodular hidradenocarcinoma’. Cytology identified the lesion to be malignant which led to wide local excision with clear margins in the above patient; hence providing adequate treatment. The patient was further advised radiotherapy by the tumour board. However patient was lost to follow up.
Figure 1.
The clinical and gross images of back (a and c) and axillary (b and d) swelling show proliferative tumour. CECT and MRI images (e and f) showing bony infiltration of tumour.
Figure 2.
Biopsy section show infiltrative tumour in (a) (H&E × 100). Cytology smear show papillaroid fragments in (b) (H&E × 200). Resection sections show intradermal tumour in (c) (H&E × 100), infiltration in (d and e) (H&E × 200) with clear cells and mucin filled spaces in (f) (H&E × 200).
Figure 3.
Immunohistochemistery sections (×200) showing: Cytokeratin in (a) and S 100 positivity in (b). Negative staining for CEA in (c) and CD 10 in (d).
Case 2: A 85 years old male presented with lesion over right posterior auricular area since 1 year and bleeding since 3 days. Past history of previous surgeries at peripheral hospitals with recurrence was present which was reported as ‘cylindroma’ as histopathology. The patient underwent local excision on basis of outside histopathology. Gross specimen showed an ulcero-proliferative growth measuring 10.5 × 7.0 × 1.6 cm (Figure 4a) with close (<0.1 cm) margins. Microscopy revealed an infiltrative tumour arranged in nests and sheets of atypical squamous cells displaying moderate pleomorphism with hyperchromatic nuclei, high nucleo cytoplasmic ratio, vesicular chromatin, prominent nucleoli and clear to eosinophilic cytoplasm with keratin pearl formation (Figure 4b). Deep/base margin was involved (Figure 4c) and focal perineural invasion was noted (Figure 4d). Overlying epithelium was ulcerated at places showing necrosis and fibrin deposition. Intervening stroma between tumour cells showed desmoplasia. Frequent mitosis was seen (10/10 hpf). IHC panel showed CK, EMA and p63 positivity (Figure 4e and f) and absence of CD10 and BerEP4 (Figure 4g and h) in tumour cells. Report was signed out as ‘squamous cell carcinoma, SCC’ with clear cell changes was made. Benign histological initial biopsy diagnosis had a grave consequence on this old gentleman, the margins of the excision performed were involved and post operative healing of the wound did not occur. The patient further deteriorated and succumbed to disease.
Figure 4.
Gross picture of specimen (a). H&E section (×200) showing focal keratin pearl formation in (b). H&E sections (×100) showing positive margin (c) and perineural invasion (d). Immunohistochemistery sections (×200) showing positivity for p63 (e) and Cytokeratin (f). Negative staining is seen for CD 10 seen in (g) and BerEP4 in (h).
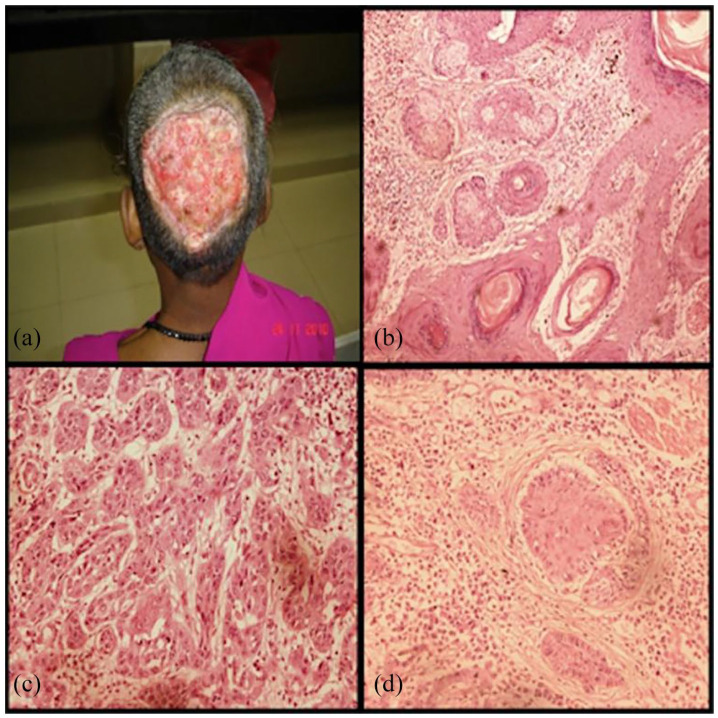
Case 3: Forty years old female presented with previously operated case of an ulcerated lesion 10 months back. A 20 × 20 cm raw area present over occipital region involving skin, underlying tissue, bone and portion of dura. The edges were rolled out; floor was covered with pale granulation tissue. Base was formed by dura in centre and calvarium in the periphery. Granulation tissue over defect was present with flakes (Figure 5a). The histopathology of H&E stained sections revealed a large, asymmetric, poorly circumscribed, infiltrative tumour. The tumour was composed of basophilic basaloid islands and horn cysts (Figure 5b), arranged in a lace-like pattern with solid aggregates. The malignant tumour consisted of nests and cords of neoplastic cells, and the peripheral tumour cells showed frequent cytological atypia, mitotic figures and central transitions toward squamoid or occasionally clear appearing cells, which resembled cells of the follicular outer root sheath. Individual tumour nuclei displayed moderate nuclear pleomorphism, prominent nucleoli (Figure 5c and d), and areas of necrosis with focal area in continuity with the epidermis. The cells were PAS positive and diastase resistant. A diagnosis of trichilemmal carcinoma was made. This female was further managed with radiotherapy and transposition flap reconstruction.
Figure 5.
Scalp lesion of the patient (a). H&E section (×4) with pilar type keratinisation and area of clear cells (b). H&E section (×20) Areas of nucleomegaly, nucleolar prominence and mitosis (c). H&E section (×20) with area of pilar differentiation (d).
Discussion
Diagnosis of adnexal tumours is difficult as 1 lesion can mimic histologic features of 2 or more adenexal lesions. The diagnosis of malignancy may be evident but the recognition of adnexal origin and precise subclassification may be problematic. 7 There are a large number of defined entities but a debatable nomenclature or classification schemes from various authors seem to be conflicting till date. 8 A practical approach to the diagnosis of cutaneous adnexal tumours begins with – (a) derivation of the structure of origin (which may be difficult sometimes in view of multi-differentiation cells in the lesion), (b) evidence of malignancy and finally (c) determination of primary or secondary metastatic nature or a part of syndrome.
The correct diagnosis rendered taking above considerations has got utmost clinical and prognostic significance for a clinician and patient. Most skin adnexal tumours are benign, and therefore local complete surgical excision is curative. Morphological typing may be partially discounted still identification and differentiation of benign lesion from malignant lesion is of utmost clinical significance. A malignant counterpart of almost each of these tumours is rare, locally aggressive, and has a potential for nodal involvement and distant metastasis and thus has poor clinical outcome. The histological examination remains the gold standard in the diagnosis of skin adnexal tumours besides detailed clinical information with immuno-histochemical (IHC) studies aiding in confirmation of diagnosis. Most adnexal tumours are benign in nature. Malignant cutaneous adnexal tumour arises de novo or occurs in association with a benign component with propensity for local recurrence or distant metastases and thus overall poor outcomes. 9 Pinpointing the pathology is of paramount significance in determining the patient’s long-term survival and follow-up as 5-years overall survival for cutaneous adnexal tumours is between 70% and 80%. 10
Assessment of cytological and architectural patterns is necessary in categorising cutaneous adnexal tumours. 11 Adnexal neoplasms can be categorised according to their association to overlying epidermis (Figure 6) or the prominent cell type (Table 1). The overlaps in histologic features pose an immense challenge for differentiating between benign and malignant lesions. For instance, numerous mitotic figures are often seen in benign tumours with follicular origin, such as pilomatricoma. Cytological atypia does not always entail malignancy and benign-appearing cutaneous adnexal tumours have been known to metastasise. The prominent morphological differentiating features between benign and malignant lesions are summarised in Table 2. All these histological features are relatively easily assessed in a complete excision; the challenges in the diagnosis are often related to a limited, superficial biopsy/cytology. A simplified immunohistochemical panel that may help in diagnosis of adnexal neoplasms is shown in Table 3.3,12,13 Clinical correlation is of paramount significance. A long-standing lesion that recently and suddenly increased in size may indicate a malignant transformation of an otherwise benign cutaneous adnexal tumour. Further the authors have discussed the major differentials of the pathologies of the above cases.
Figure 6.

Relationship of adnexal tumour with epidermis.
Table 1.
Approach to adnexal neoplasm on basis of cell type.
| Tumour character | Differential diagnosis |
|---|---|
| Tumour with clear cell change | Nodular hidradenoma |
| Syringoma | |
| Poroma | |
| Spiradenoma | |
| Tumour with ductal/tubuloglandular formation | Syringoma poroma and porocarcinoma |
| Syringoma | |
| Cylindroma | |
| Hidradenoma | |
| Microcystic adnexal carcinoma | |
| Tumour with small keratin cyst | Desmoplastic trichoepithelioma |
| Trichoblastoma | |
| Trichoadenoma | |
| Microcystic adnexal carcinoma | |
| Tumour with follicular differentiation | Trichoepithelioma |
| Trichoblastoma | |
| Trichilemmoma | |
| Trichofolliculoma pilomatricoma |
Table 2.
Morphological differences between benign and malignant adnexal lesions.
| Tumour character | Benign | Malignant |
|---|---|---|
| Circumscription | Proper – Usually symmetrical, vertical orientation with uniform collection of epithelial cells | Poorly circumscribed – Asymmetrical, horizontal orientation, irregular arrangement of cells |
| Borders | Smooth, pushing borders | Irregular and infiltrative borders |
| Cytological and nuclear atypia | No/Minimal | Usually present (There may be areas with no atypia as well) |
| Necrosis | Not seen | Present (may be focal) |
| Mitosis | Usually not seen | Present (Atypical mitosis also seen) |
| Surrounding stroma | Dense fibrotic stromal reaction | Less sclerotic stroma |
Table 3.
Simplified and summarised – immunohistochemical approach to broad categories of adnexal neoplasms.
| IHC marker | Squamous cell carcinoma | Basal cell carcinoma | Sebaceous cell carcinoma | Sweat gland tumour (Eccrine/Apocrine) | Hair follicle tumour |
|---|---|---|---|---|---|
| Pan CK | + | + | + | + In HAC, PC, CS and AC |
− |
| EMA | + | − | + | + In PEA, HAC, PC, CS and CYLIN |
− |
| BerEP4 | − | + | + | + In Poroma |
− |
| CD 10 | Stromal staining + | + | − | − | Stromal Staining + In Trichoblastoma |
| CK 20 | − | − | − | − | + In Merkel cells |
| CK 17 | − | − | − | − | + In Trichillemal Ca |
| PHLDA1 | − | Negative/Variable (<25% cases) | − | − | + In Trichoepithelioma and Trichoblastoma |
| AR | Negative/Variable (0%-10%) | +60% | + | + In HAC |
− |
| GCDFP- 15 | − | − | − | + In HAC, SP, AA/AC |
− |
| CEA | Negative/Variable (0%-30%) | Negative/Variable (0%-20%) | − | + In PEA, HAC, PC, CS, SP, CYLIN |
− |
| S 100 | − | − | − | + In PEA, HAC, CS |
− |
Abbreviations: AA, apocrine adenoma; AC, apocrine carcinoma; CS, chondroid syringoma; CYLIN, cylindroma; HAC, hidradenocarcinoma; PC, porocarcinoma; PEA, papillary eccrine adenoma; SP, syringocystadenoma papilleferum.
Hidradenocarcinoma (HAC) versus other tumours with clear cell histology
Hidradenocarcinoma (HAC), a malignant intradermal sweat gland tumour usually presents in fifth to seventh decade of life with no sexual and racial preferences. 14 The first case of hidradenocarcinoma was reported in 1954 by Keasbey and Hadley. 15 This tumour represents malignant equivalent of hidradenoma and amounts 6% of malignant eccrine tumour.4,5 Occasionally occurring in trunk, this tumour has a predilection for face and extremities.5,16 With pathogenesis still unknown, literature states that HAC has both eccrine and apocrine variants.16,17 Hall et al 18 has proposed that HAC arises only from ductal part of eccrine sweat glands only to contradict his own theory later. Clinically it presents as solitary asymptomatic, slow growing nodular skin lesion measuring approximately 1 to 5 cm.4,16 Metastasis is usually to regional lymph nodes, lung and bones5,19,20 On gross pathology, HACs are well-circumscribed nodules on the superficial skin. Morphologically, definitive histologic features that distinguish HAC from the benign counterpart or other related malignant tumours are lacking. Broad differentials may include adenoid cystic eccrine carcinomas, aggressive digital papillary adenocarcinomas, eccrine adenocarcinomas, and mucinous eccrine carcinomas. Primary eecrine tumours presenting similar features of benign adenexal tumours pose a great challenge to differ from HAC and metastatic visceral adenocarcinomas. 21 The morphological features supporting HAC are – no connection to overlying epidermis, nodular pattern, presence of 2 cell types and degenerating cells forming cyst like structures which were all seen in first case resection sections. However these features were not pronounced in biopsy as seen in Figure 1e though features of malignancy were seen. Immunohistochemical marker which may be of help from differentiating HAC from its eccrine counterparts is androgen receptor (AR) as seen in Table 3, rest others have overlapping results.
In summary, a lesion may be classified as malignant HAC if 3 or more of the following features are met viz. loss of circumscription, infiltrative growth pattern, deep extension, necrosis, perineural and lymphovascular invasion, nuclear pleomorphism and 4 or more mitoses per 10 high power fields. 22 Loss of circumscription, infiltrative growth pattern and necrosis were seen in our case with variable IHC expression – diffuse strong expression of CK, EMA and S-100 with no expression for CEA and CD10 (to rule out metastatic renal cell carcinoma). Genetically t(11; 19) translocation due to fusion of MECT1 and MAML2 gene and wild type TP53 mutation has been mentioned in anecdotal reports. 23
Cylindromas versus SCC
Benign tumour arising from eccrine gland is known as cylindroma. It has rare incidence of malignant transformation which may be clinically seen as recent increase in growth size, ulceration or reddish discoloration of tumour nodules. 24 Histomorphologically it is characterised by sheets of high grade undifferentiated tumour cells with abundant eosinophilic cytoplasm. Tumour necrosis and mitosis may also be seen. Morphologic features which are overlapping with SCC or its variants may be present in malignant cylindromas. 25 Though various published reports stress on the presence of benign adnexal tumour component to consider for malignant transformation; it may not be seen in a long-standing tumour. Immunohistochemically CEA may be useful in some scenarios as it is consistently seen in cylindromas but has variable to no expression in SCC (Table 3).
Increased risk of recurrence along with tumour metastasis and death is usually seen in cylindromas in cases where the following morphological features are evident. These include tumour location (ear, lip, anogenital, scars), size (>2 cm in diameter), depth of invasion (>4 mm or beyond subcutaneous fat), perineural invasion, poorly differentiated morphology, infiltrative or desmoplastic growth pattern and history of local recurrence. 26
Trichilemmoma (TA)/Trichilemmal carcinoma (TC) versus SCC with clear cell features
TA and its rare malignant counterparts are derived from the follicular outer sheath and have characteristic clear cytoplasm due to increased glycogen content and occasional presence of trichohylaine granules.8,27,28 It usually occurs on the sun-exposed areas of older individuals or may be a part of syndrome viz. Cowden’s syndrome. 29 The lesion is usually solitary and may present as an exophytic or polypoid nodule that may be hyperkeratotic with ulceration. TC was first described in 1976 as ‘a histologically invasive, cytologically atypical, clear cell neoplasm of adnexal keratinocytes which is in continuity with the epidermis and/or follicular epithelium’. 30 Histopathologically, TC demonstrates a lobular proliferation centred on pilosebaceous structures composed of clear cells with prominent nucleoli. Focal necrosis, haemorrhage or areas of squamous differentiation may also be present. The histological distinction between TC and SCC with clear features is challenging but also holds importance with the fact that chances of metastasis in TC are very low unlike SCC. Defined as the abrupt transition of nucleated squamous epithelial cells to enucleate keratinisation without the formation of a granular layer, it is a consistent feature of TC along with the presence (at least focally) of pagetoid interface, dermal invasion, trichohyalin granules and areas of peripheral nuclear palisading (Figure 5b and d). Moreover, in cases of SCC with clear cell features, an in-situ component is usually present which was not seen in our case. Clear cells of TC were stained positive with PAS as they contain glycogen but areas of trichohyalin granules are PAD positive. Both express CK5/6; however, there are reports that CD34, CK14 and CK17 are specific for TC and p63 and p40 are explicit for SCC (Table 3).
Conclusion
We present a simplified approach to the morphological diagnosis of adnexal neoplasms with summarised IHC. During a pathological evaluation of specimens for skin adnexal tumours, the pathologist should be provided with sufficient clinical data that may prove to be useful in achieving accurate diagnosis considering the fact that they show heterogeneous differentiation from the common folliculo-sebaceous apocrine unit. The gross examination should be done meticulously and status of the surgical resection margins should be recorded. The specimen should be thoroughly sampled. Sections from tumour and uninvolved areas are important to evaluate the growth pattern of the tumour. Small or superficial biopsy should be best avoided and complete specimen should be examined in entirety. Careful morphological observation with clinical co-relation still stands to be pivotal in diagnosis and classification of adnexal lesions.
Acknowledgments
We are thankful to King George’s Medical University for providing the infrastructure to perform our work. We are also thankful to our Immunohistochemistry and histopathology laboratory staff for their support.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Conceptualization: Preeti Agarwal, Apoorva Agarwal.
Methodology: Preeti Agarwal, Apoorva Agarwal, Akshay Anand.
Software: Apoorva Agarwal, Preeti Agarwal.
Validation: Preeti Agarwal, Apoorva Agarwal, Akshay Anand, Manish Agarwal.
Formal analysis: Apoorva Agarwal, Preeti Agarwal.
Investigation: Apoorva Agarwal, Preeti Agarwal, Mala Sagar, Shalini Bhalla.
Resources: Madhu Mati Goel, Arun Abhinav Sonkar.
Data Curation: Apoorva Agarwal, Preeti Agarwal.
Writing - Original Draft: Apoorva Agarwal, Preeti Agarwal, Akshay Anand.
Writing - Review & Editing: Preeti Agarwal, Shalini Bhalla, Madhu Mati Goel.
Visualisation: Apoorva Agarwal, Preeti Agarwal.
Supervision: Madhu Mati Goel, Arun Abhinav Sonkar.
Project administration: Preeti Agarwal.
ORCID iD: Preeti Agarwal  https://orcid.org/0000-0001-8107-8501
https://orcid.org/0000-0001-8107-8501
References
- 1. Alsaad KO, Obaidat NA, Ghazarian D. Skin adnexal neoplasms—part 1: an approach to tumours of the pilosebaceous unit. J Clin Pathol. 2007;60:129-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Obaidat NA, Alsaad KO, Ghazarian D. Skin adnexal neoplasms—part 2: an approach to tumours of cutaneous sweat glands. J Clin Pathol. 2007;60:145-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ahmed TS, Priore JD, Seykora JT. Tumours of epidermal appendages. In: Elder DE, ed. Lever’s Histopathology of Skin. 11th ed. Lippincott Williams and Wilkins; 2015:851-909. [Google Scholar]
- 4. Gauerke S, Driscoll J. Hidradenocarcinomas: a brief review and future directions. Arch Pathol Lab Med. 2010;134:781-785. [DOI] [PubMed] [Google Scholar]
- 5. Berg J, McDivitt RW. Pathology of sweat gland carcinoma. Pathol Annu. 1968;3:123-144. [Google Scholar]
- 6. Lazar AJ, Murphy GF. The skin. In: Aster KA, ed. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Elsevier; 2014:1141-1178. [Google Scholar]
- 7. Ahmed TS, Priore JD, Seykora JT. Tumours of epidermal appendages. In: Elder DE, ed. Lever’s Histopathology of Skin. 10th ed. Lippincott Williams and Wilkins; 2009:892. [Google Scholar]
- 8. Danialan R, Mutyambizi K, Aung P, Prieto VG, Ivan D. Challenges in the diagnosis of cutaneous adnexal tumors. J Clin Pathol. 2015;68:992-1002. [DOI] [PubMed] [Google Scholar]
- 9. Calonje E, Brenn T, Lazar A, Mckee P. McKee’s Pathology of the Skin. 4th ed. Elsevier/Saunders; 2012. [Google Scholar]
- 10. Ho VH, Ross MI, Prieto VG, Khaleeq A, Kim S, Esmaeli B. Sentinel lymph node biopsy for sebaceous cell carcinoma and melanoma of the ocular adnexa. Arch Otolaryngol Head Neck Surg. 2007;133:820-826. [DOI] [PubMed] [Google Scholar]
- 11. Sirikanjanapong S, Seymour AW, Amin B. Cytologic features of microcystic adnexal carcinoma. Cytojournal. 2011;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Compton LA, Murphy GF, Lian CG. Diagnostic immunohistochemistry in cutaneous neoplasia: an update. Dermatopathology. 2015;2:15-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weedon D. Tumors of cutaneous appendages. In: Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone/Elsevier; 2010:758-807. [Google Scholar]
- 14. Cooper PH. Carcinoma of sweat glands. Pathol Annu. 1987;22:83-124. [PubMed] [Google Scholar]
- 15. Keasbey LE, Hadley GG. Clear cell hidradenoma; report of three cases with widespread metastases. Cancer. 1954;7:934-952. [DOI] [PubMed] [Google Scholar]
- 16. Souvatzidis P, Sbano P, Mandato F, Fimiani M, Castelli A. Malignant nodular hidradenoma of the skin: report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-554. [DOI] [PubMed] [Google Scholar]
- 17. Ko CJ, Cochran AJ, Eng W, Binder SW. Hidradenocarcinoma: a histological and immunohistochemical study. J Cutan Pathol. 2006; 33:726-730. [DOI] [PubMed] [Google Scholar]
- 18. Hall J, Knee G, A’Hern RP, et al. Sweat-gland tumours: a clinical review of case in one centre over 20 years. Clin Oncol. 2006;18:351-359. [DOI] [PubMed] [Google Scholar]
- 19. Junkins-Hopkins JM. Polypoid malignant acrospiroma: a clinical variant with aggressive behavior. J Cutan Pathol. 2000;27:561. [Google Scholar]
- 20. Waxtein L, Vega E, Cortes R, Hojyo T, Dominguez-Soto L. Malignant nodular hidradenoma. Int J Dermatol. 1998;37:225-228. [DOI] [PubMed] [Google Scholar]
- 21. Urso C, Bondi R, Paglierani M, Salvadori A, Anichini C, Giannini A. Carcinoma of sweat glands: report of 60 cases. Arch Pathol Lab Med. 2001;125:498-504. [DOI] [PubMed] [Google Scholar]
- 22. Nazarian RM, Kapur P, Rakheja D, et al. Atypical and malignant hidradenomas: a histological and immunohistochemical study. Mod Pathol. 2009;22:600-610. [DOI] [PubMed] [Google Scholar]
- 23. Kazakov DV, Ivan D, Kutzner H, et al. Cutaneous hidradenocarcinoma: a clinicopathological, immunohistochemical, and molecular biological study of 14 cases, including Her2/neu gene expression/amplification, TP53 gene mutation analysis, and t (11;19) translocation. Am J Dermatopathol. 2009;31:236-247. [DOI] [PubMed] [Google Scholar]
- 24. Taheri MS, Talebnejad S, Birang SH, Nahvi V. Malignant transformation of multiple dermal cylindromas. Iran J Radiol. 2005;3:99-101. [Google Scholar]
- 25. Crowson AN, Magro CM, Mihm MC. Malignant adnexal neoplasms. Mod Pathol. 2006;19(suppl 2):S93-S126. [DOI] [PubMed] [Google Scholar]
- 26. Jennings L, Schmults CD. Management of high-risk cutaneous squamous cell carcinoma. J clin Aesthet Dermatol. 2010;3:39-48. [PMC free article] [PubMed] [Google Scholar]
- 27. Al-Saleh K, Hooda HS, El-Wakiel H, Safwat R, Bedair A, Eskaf W. Trichilemmal pilar tumor of the scalp: a case report. Gulf J Oncolog. 2012;12:62-64. [PubMed] [Google Scholar]
- 28. Hammami H, Benmously R, Badri T, et al. Atypical clinical appearance and localization of trichilemmoma. A case report. Pathologica. 2009;101:133-134. [PubMed] [Google Scholar]
- 29. Brownstein MH, Mehregan AH, Bikowski JB, Lupulescu A, Patterson JC. The dermatopathology of Cowden’s syndrome. Br J Dermatol. 1979;100:667-673. [DOI] [PubMed] [Google Scholar]
- 30. Headington JT. Tumor of the hair follicle: a review. Am J Pathol. 1976; 85:479-514. [PMC free article] [PubMed] [Google Scholar]