Abstract
Drugs account for 2% of all the causes of acute pancreatitis. To date, there are approximately 26 reported cases of acute pancreatitis associated with the use of cannabis. We report the case of a 20-year-old male who presented with intractable nausea, vomiting, and epigastric pain and a lipase level of 1541 with reportedly no alcohol use, and no evidence of medication, biliary, or autoimmune etiology. However, the patient did endorse heavily smoking cannabis prior to symptom onset. He was instructed to abstain from cannabis use on discharge and has not presented to the hospital since this episode. The reporting of this case aims to increase awareness of cannabis as a differential diagnosis in cases of pancreatitis that is not due to typical etiologies such as gallstones, medications, and alcohol use. There has yet to be definitive evidence as to how cannabis can cause pancreatitis. Further studies must be conducted to better understand the association between cannabis use and acute pancreatitis and the mechanism by which cannabis affects the pancreas.
Keywords: pancreatitis, cannabis
Introduction
Acute pancreatitis (AP) is an acute inflammatory disorder of the pancreas commonly presenting as severe upper abdominal pain. 1 Diagnosis requires 2 out of 3 of the criteria that are acute onset of persistent, severe, epigastric pain that may in some cases radiate to the back, elevation in serum lipase or amylase to 3 times or greater than the upper limit of normal and findings of AP on ultrasound, computed tomography, or magnetic resonance imaging. 1 The 2 most common causes of pancreatitis are obstruction of the bile duct by stones (38%) and alcohol abuse (36%). 2 Rarer etiologies include medications (<5%), biliary sludge, hypercalcemia, infections, and toxins. 1 We present a case of pancreatitis in a 20-year-old man with no identifiable cause.
Case
A 20-year-old male with past medical history of nephrolithiasis presented to the emergency department for abdominal pain and intractable nausea and vomiting. The patient was in his normal state of health until approximately 7 pm when he became nauseous and had 5 to 6 episodes of bilious vomiting. Prior to 7 pm, the patient endorsed smoking 3 blunts and overeating several pieces of pizza and multiple hamburgers. He endorsed chills and severe epigastric abdominal pain rated 8 out of 10 that radiated to his back. Physical examination findings were significant for nondistended abdomen, epigastric and RUQ (right upper quadrant) tenderness with negative Murphy’s sign, and normal bowel sounds. On presentation to the emergency department, blood pressure was 143/76 mm Hg, pulse was 66 beats per minute, and temperature was 36.4 °C. Significant blood test results included the following: lipase 1541 IU/L, lactic acid 3.6 mg/dL, white blood cells 18.6 × 109/L with absolute neutrophil count of 17.3, lactate dehydrogenase 251 U/L, alkaline phosphatase 122 IU/L, calcium 8.4 mg/dL, magnesium 1.6 mEq/L, and phosphorus 2.4 mg/dL (see Table 2). Aspartate aminotransferase, alanine aminotransferase, direct and total bilirubin were all within normal limits (see Table 3). Body mass index was 20.52 kg/m2. Ranson criteria score was 1 due to leukocytosis of 21.5 × 109/L on admission. Urine toxicology was positive for cannabinoids (see Table 1). The patient denied any recent abdominal trauma or infections. He denied any family history of pancreatitis and had no previous history of similar symptoms. He denied taking any medications but endorsed taking a single dose of NyQuil to help him sleep but he could not due to the severe abdominal pain. He stated that he last had alcohol over 1 year ago and denied any illicit drug use. He endorsed vaping concentrated cannabis wax at least 2 times per day, each day of the week.
Table 2.
General Chemistry.
| Laboratory findings | Level | Reference ranges |
|---|---|---|
| Sodium level | 141 | 136-145 mmol/L |
| Potassium level | 3.8 | 3.5-5.1 mmol/L |
| Chloride level | 108 (High) | 98-107 mmol/L |
| CO2 | 25 | 21-32 mmol/L |
| Calcium level | 9.4 | 8.5-10.1 mg/dL |
| Magnesium level | 1.7 (Low) | 1.8-2.4 mg/dL |
| Phosphorus level | 2.7 | 2.5-4.9 mg/dL |
| Blood urea nitrogen | 24 (High) | 7.0-18 mg/dL |
| Creatine level | 0.85 | 0.67-1.17 mg/dL |
| Glucose level | 134 | 70-140 mg/dL |
| Anion gap | 8 | 6.0-14 mmol/L |
Table 3.
Complete Blood Count With Differential.
| Values | Reference ranges | |
|---|---|---|
| WBC | 18.6 (High) | 4.5 to 11 × 103/µL |
| RBC | 5.31 | 4.26 to 5.80 × 106/µL |
| Hgb | 14.7 | 13.2 to 17.4 g/dL |
| Hct | 44.3 | 38.9% to 51.0% |
| MCV | 83.4 | 80.0 to 98.0 fL |
| MCH | 27.6 | 27.1 to 34.0 pg |
| MCHC | 33.1 | 32.4 to 35.9 g/dL |
| RDW | 13.3 | 11.4% to 14.1% |
| Platelets | 243 | 150 to 450 × 103/µL |
| MPV | 8.7 | 6.5 to 10.5 fL |
| Neutrophils auto% | 93.2 (High) | 50.0% to 75.0% |
| Lymphocytes auto% | 3.8 (Low) | 20.0% to 45.0% |
| Monocytes auto% | 2.4 | 2.0% to 12.0% |
| Eosinophils auto% | 0.1 | ≤6.0% |
| Basophils auto% | 0.5 | ≤2.0% |
| Neutro absolute | 17.3 (High) | 1.8 to 7.7 × 103/µL |
| Lymph absolute | 0.7 (Low) | 1.2 to 4.5 × 103/µL |
| Mono absolute | 0.4 | 0.1 to 1.0 × 103/µL |
| Eos absolute | 0 | ≤0.7 × 103/µL |
| Baso absolute | 0.1 | ≤2.0 × 103/µL |
| NRBC auto | 0 | ≤1.0/100 (WBCs) |
| ALP | 122 (High) | <117 unit/L |
| ALT | 27 | <61 unit/L |
| AST | 19 | <37 unit/L |
| Bilirubin direct | <0.1 | <0.2 mg/dL |
| Bilirubin total | 0.3 | <1.0 mg/dL |
| LDH | 251 (High) | <241 unit/L |
| Lipase level | 1541 (High) | <288 unit/L |
| IgG subclass 4 | 16.9 | 4-86 mg/dL |
Abbreviations: WBC, white blood cell; RBC, red blood cells; Hgb, hemoglobin; Hct, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width; MPV, mean platelet volume; NRBC, nucleated red blood cells; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; IgG, immunoglobulin G.
Table 1.
Toxicology.
| Toxin | Results |
|---|---|
| Ethanol level | <3 |
| Amphetamine screen Ur | Negative (<1000) |
| Barbiturate screen Ur | Negative (<200) |
| Benzodiazepines Ur | Negative (<200) |
| Cocaine screen Ur | Negative (<300) |
| Opiate screen Ur | Negative (<300) |
| Phencyclidine screen Ur | Negative (<25) |
| Cannabinoid screen Ur | Positive (≥50) (A) |
Abbreviation: Ur, urine.
Computed tomography of the abdomen and pelvis with contrast demonstrated distal necrotizing pancreatitis with adjacent moderate complex free fluid compatible with an acute necrotic collection (see Figures 1 and 2). Imaging also showed dilated stomach and duodenum suggesting ileus. Limited ultrasound of the abdomen demonstrated hepatic steatosis but no cholelithiasis or gallbladder sludge, and the common bile duct measured a normal size of 3 mm. Magnetic resonance imaging of the abdomen with contrast demonstrated acute distal necrotizing pancreatitis with moderate amount of peripancreatic complex inflammatory fluid and a partially loculated fluid collection inferior to the pancreatic tail measuring at least 4.1 × 3.9 × 3.0 cm. There was no magnetic resonance cholangiopancreatography evidence of cholelithiasis or choledocholithiasis, or biliary or pancreatic ductal dilatation.
Figure 1.
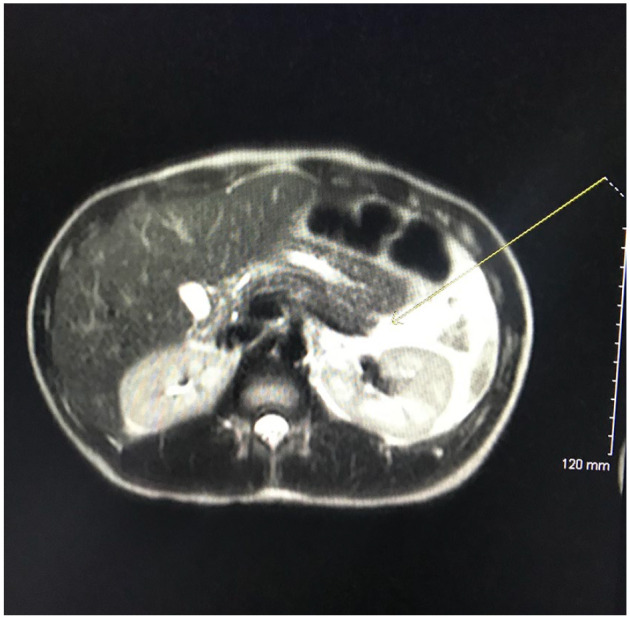
T2-weighted computed tomography scan of the pancreas demonstrates a large amount of peripancreatic (marked with yellow arrow) and left upper quadrant fluid that can be correlated clinically with acute pancreatitis.
Figure 2.
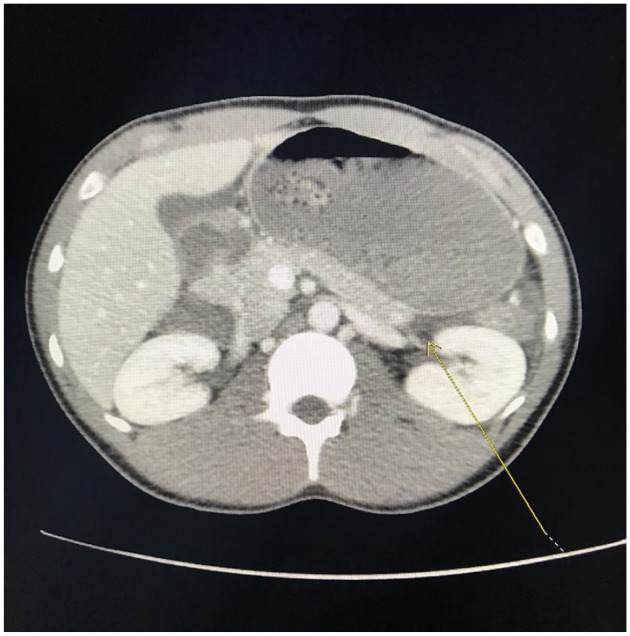
T1-weighted computed tomography images demonstrate distal necrotizing pancreatitis with adjacent moderate free fluid (marked with yellow arrow) compatible with an acute necrotic collection.
The diagnosis of pancreatitis secondary to cannabis ingestion was made based on clinical, laboratory (Tables 3, 4 and 5), and imaging findings (Figure 1 and 2). Biliary etiology of the pancreatitis was ruled out by blood tests and ultrasound that demonstrated no evidence of cholelithiasis. Immunoglobulin G4 levels were normal (see Table 3), which excluded autoimmune etiology. The patient reported no medication use and thus medication-induced pancreatitis was excluded from the differential diagnosis. The patient was given intravenous fluids and made NPO (nothing by mouth). Morphine was given as needed for pain management. Due to the patient’s persistent nausea and vomiting, Reglan and Zofran were given. The patient’s pain and PO (per os) intake improved within 2 days, and he was discharged with pain medication and recommendations on cessation of cannabis use.
Table 4.
Lipid Panel.
| Lipid study | Values | Reference ranges |
|---|---|---|
| Cholesterol total | 166 | ≤200 mg/dL |
| HDL | 65 | ≥40 mg/dL |
| Triglycerides | 57 | ≤150 mg/dL |
| LDL | 90 | ≤100 mg/dL |
| Cholesterol/HDL | 2.6 | ≤4.4 mg/dL |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Table 5.
Inflammatory Markers.
| Inflammatory marker | Value | Reference ranges |
|---|---|---|
| C-reactive protein | 6.67 (High) | ≤0.30 mg/dL |
| Lactic acid plasma | 3.6 (High) | <2.0 mmol/L |
Discussion
In recent years, cannabis has been either legalized or decriminalized in more than half of the states within the United States. 3 It has been estimated that approximately 4% of the world’s population uses marijuana per year. 4 The growing popularity of cannabis use begs for further understanding as to how it affects the human body and specifically its individual organs.
Acute pancreatitis is the most common gastrointestinal diagnosis in the United States on discharge from the hospital. Its diagnosis and treatment drastically consume health care resources and accounts for significant patient morbidity. 3 Currently, approximately 20% of cases of AP are classified as idiopathic in origin. 3 The first published case of pancreatitis secondary to cannabis use was by Grant et al. in 2004. 5 Since then, approximately 26 cases have been published in the literature.6,7 These documented cases were mainly in males between the age of 16 and 44 years who had heavily consumed cannabis from 5 days to 2 weeks before being diagnosed with AP.6,7 In all cases, the Naranjo scores ranged from 6 to 9 meaning that there was a probable causality between the use of cannabis and onset of AP.6,7 The only documented prospective study analyzing cannabis use and AP development found that cannabis accounted for the etiology of 13% of all cases of AP in patients younger than the age of 35 years. 8 Furthermore, a systematic review by Sagaram et al. found that males had a higher proportion of cannabis-induced pancreatitis compared with females. 9 It also documented that there was an increase in the proportion of males and the proportion of patients younger than the age of 35 years with more severe cases of pancreatitis. 9 These findings suggest that AP induced by cannabis use is associated with being male and younger than the age of 45 years. This may be because men younger than the age of 46 contributes to the majority of the population who is heavily using cannabis as opposed to an underlying predisposition of young males to AP from cannabis ingestion.
In order to consider cannabis-induced AP as a differential diagnosis, it is important to ask specific questions regarding illicit drug use. This may be difficult in that it is still considered an illicit drug in some states and parts of the world, so patients may be more hesitant to reveal their use of this substance. A study in France reported that in 50% of patients with acute pancreatitis, it took questioning on cannabis use one or more times until the patient confessed to recent cannabis consumption.3,8,10 In a systematic review by Barkin et al. more than half of the 26 reported cases of cannabis-induced AP had increased use of cannabis before being diagnosed with AP, suggesting a possible dose-dependent relationship.6,11
Although the definitive mechanism by which marijuana ingestion causes AP is not known, there is evidence of cannabinoid 1 and cannabinoid 2 receptors expressed in the islets of Langerhans within the pancreas. 7 Recent studies have found contradictory results when trying to discern the link between cannabis use and AP. 7 It has been shown that the expression and activation of these receptors increases when the pancreas is inflamed. 7 A study by Dembinski et al. induced AP in mice using cerulein. 12 They then demonstrated that an endogenous CB1 receptor ligand increased the severity of AP by increasing pancreatic edema and inflammatory infiltration. 12 Moreover, administration of an exogenous CB1 receptor antagonist resulted in a reduction of pancreatic inflammation. 12 Contrarily, Michalski et al. conducted a study showing that AP patients demonstrated an upregulation of cannabinoid receptors and increased levels of endogenous cannabinoids within the pancreas. 13 Administration of CB1 and CB1 receptor agonists in mice with AP resulted in decreased abdominal pain and pancreatic tissue inflammation. 13 Due to the conflicting evidence, it is apparent that more studies need to be done to better understand how cannabinoids affect the pancreas.
Due to the lack of knowledge surrounding the association between cannabis use and onset of AP, there is not a definitive treatment for this specific etiology. In our case, the patient was treated as a typical case of AP by being made NPO, given fluids, and administered morphine for pain control. A systematic review by Barkin et al. highlighted that in 7 studies the cessation of cannabis use resulted in no further episodes of AP suggesting that patients should be informed that resuming cannabis use may increase their chance of developing recurrent AP.6,7
Conclusion
In the past 10 years, the number of cases of reported cannabis-induced pancreatitis has increased. 3 This may be in part due to its increased use due to legalization in several parts of the world. The increase in popularity of this drug suggests that it is important to specifically delve into cannabis use when taking a patient’s history in order to form a better differential diagnosis or suspicion of etiologic source of the patient’s presenting symptoms. The case we present adds to the scarce literature that suggests heavy cannabis use is a causative factor of AP and highlights the importance of considering cannabis use as an etiology behind pancreatitis in patients who do not have classical risk factors such as gallstones, alcohol use, or medication use. More studies must be done in order to better understand the association between cannabis use and the development of AP and the definitive mechanism by which cannabinoids affect the pancreas.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Ethical approval to report this case was obtained from Kern Medical Institutional Review Board (21028).
Informed Consent: Informed consent for patient information to be published in this article was not obtained because patient was unable to be contacted.
References
- 1. Vege SS. Etiology of acute pancreatitis. Published 2021. Accessed July 13, 2021. https://www.uptodate.com/contents/etiology-of-acute-pancreatitis
- 2. Wang GJ, Gao CF, Wei D, Wang C, Ding SQ. Acute pancreatitis: etiology and common pathogenesis. World J Gastroenterol. 2009;15:1427-1430. doi: 10.3748/wjg.15.1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simons-Linares CR, Barkin JA, Wang Y, et al. Is there an effect of cannabis consumption on acute pancreatitis? Dig Dis Sci. 2018;63:2786-2791. doi: 10.1007/s10620-018-5169-2 [DOI] [PubMed] [Google Scholar]
- 4. Weissman S, Aziz M, Perumpail RB, et al. Ever-increasing diversity of drug-induced pancreatitis. World J Gastroenterol. 2020;26:2902-2915. doi: 10.3748/wjg.v26.i22.2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grant P, Gandhi P. A case of cannabis-induced pancreatitis. JOP. 2004;5:41-43. [PubMed] [Google Scholar]
- 6. Barkin J, Nemeth Z, Saluja A, Barkin JS. Cannabis-induced acute pancreatitis: a systematic review. Pancreas. 2017;46:1035-1038. doi: 10.1097/MPA.0000000000000873 [DOI] [PubMed] [Google Scholar]
- 7. Pagliari D, Saviano A, Brizi MG, et al. Cannabis-induced acute pancreatitis: a case report with comprehensive literature review. Eur Rev Med Pharmacol Sci. 2019;23:8625-8629. doi: 10.26355/eurrev_201910_19179 [DOI] [PubMed] [Google Scholar]
- 8. Culetto A, Bournet B, Haenning A, Alric L, Peron JM, Buscail L. Prospective evaluation of the aetiological profile of acute pancreatitis in young adult patients. Dig Liver Dis. 2015;47:584-589. [DOI] [PubMed] [Google Scholar]
- 9. Sagaram M, Sundar P, Reddy S. Cannabis-induced acute pancreatitis: a systematic review. Am J Gastroenterol. 2020;115:S10. doi: 10.14309/01.ajg.0000697968.52080.f4 [DOI] [Google Scholar]
- 10. Culetto A, Bournet B, Buscali L. Clinical profile of cannabis-associated acute pancreatitis. Dig Liver Dis. 2017;49:1284-1285. [DOI] [PubMed] [Google Scholar]
- 11. John J, Gandhi S, Nam D, Niakan L. A case of cannabis-induced acute pancreatitis. Cureus. 2019;11:e5754. doi: 10.7759/cureus.5754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dembiński A, Warzecha Z, Ceranowicz P, et al. Dual, time-dependent deleterious and protective effect of anandamide on the course of cerulein-induced acute pancreatitis. Eur J Pharmacol. 2008;591:284-292. [DOI] [PubMed] [Google Scholar]
- 13. Michalski C, Laukert T, Sauliunaite D, et al. Cannabinoids ameliorate pain and reduce disease pathology in cerulein-induced acute pancreatitis. Gastroenterology. 2007;132:1968-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


