Abstract
Objective
To investigate the predictive value of hyperhomocysteinaemia (HHcy) for obstructive coronary artery disease (CAD) in an Asian population in northern China.
Methods
This retrospective study enrolled patients at their first cardiac assessment and assigned them to an obstructive CAD group or a non-obstructive CAD group according to the coronary angiography results. HHcy was defined as a homocysteine (Hcy) level > 15 µmol/l.
Results
This study enrolled 2987 participants: 1172 in the non-obstructive CAD group and 1815 in the obstructive CAD group. Hcy level in the obstructive CAD group was significantly higher than in the non-obstructive CAD group. The proportion of patients with HHcy in the obstructive CAD group was significantly greater than in the non-obstructive CAD group. Multivariate logistic regression analysis demonstrated that HHcy was independently correlated with obstructive CAD in both young (aged ≤ 55 years) and old patients (aged > 55 years). HHcy showed a higher sensitivity (93.1%), specificity (86.1%) and accuracy (90.0%) for obstructive CAD. The odds ratio for HHcy was 84.2. The Kappa value (0.8) showed substantial agreement between obstructive CAD and HHcy.
Conclusions
HHcy was associated with obstructive CAD and may be a potentially independent risk factor for obstructive CAD with good predictive value.
Keywords: Coronary artery disease, homocysteine, hyperhomocysteinaemia, northern China, risk prediction
Introduction
Coronary artery disease (CAD) is a cardiovascular disease that has become the leading cause of deaths worldwide. 1 In 2010, the prevalence of CAD in the US was 19.8% in those aged >65 years, 7.1% for those aged 45–64 years and 1.2% in those aged 18–44 years. 1 In 2016, there were 15.5 million people over 20 years of age with CAD in the US. 1 CAD is the main cause of sudden cardiac arrest, accounting for about 80%. 2 More than 4 million people die of CAD every year in the 49 countries in Europe and North Asia. 2 CAD is an atherosclerotic disease that involves vascular smooth cell activation, vascular remodelling, endothelial dysfunction, oxidative stress, lipid metabolism disorder, inflammation, thrombosis, platelet activation, matrix metabolism changes and genetic factors. 2 Risk factors for CAD include disorders of lipid and lipoprotein metabolism, diabetes mellitus, hypertension, chronic kidney disease, smoking, diet, obesity, sex, lifestyle, age and family history.1,3
It is estimated that the annual cost of CAD medical services in the US exceeds $200 billion and it results in a substantial economic burden. 4 Therefore, the early prediction of CAD is very important. The Framingham risk scoring system includes sex, age, smoking, dyslipidaemia and blood pressure to accurately predict CAD. 5 The PROCAM simple scoring system uses the following eight independent risk variables: age, family history of premature myocardial infarction, blood pressure, smoking, diabetes mellitus, and levels of triglycerides (TG), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C). 6 PROCAM is limited to predicting the risk of CAD based on conventional risk factors for specific individuals. 6 Although a consensus has not been reached, researchers must continue to focus on newer risk factors, which may improve the ability to predict CAD and promptly intervene when combined with conventional risk factors. 7 The newer risk factors include homocysteine (Hcy) levels.8–11
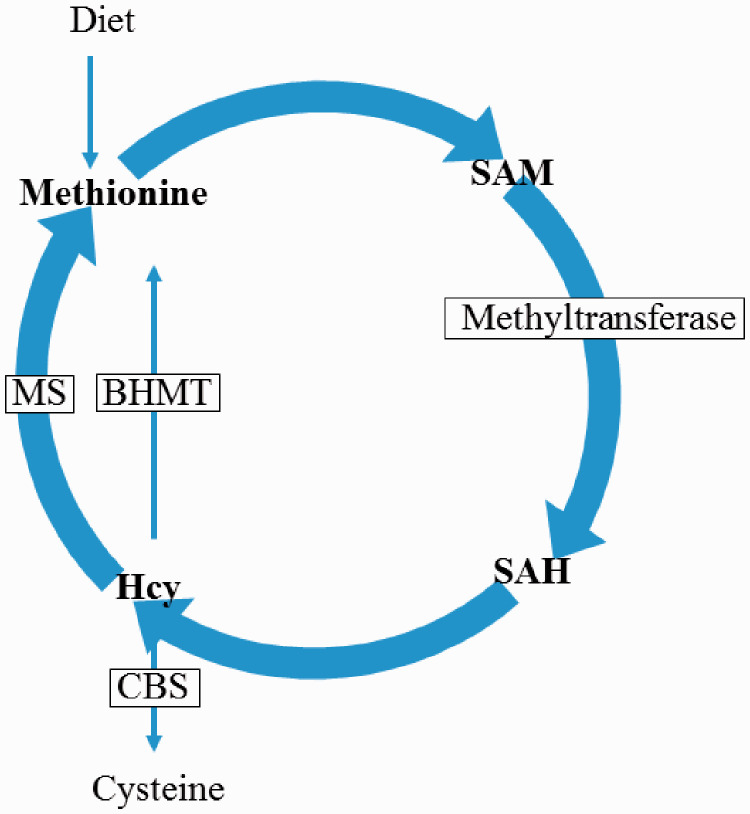
Homocysteine is a sulphur-containing amino acid, which is an intermediate product of methionine metabolism. 12 Hcy can be transformed into cystathionine by cystathionine ß-synthase. Hcy can also be re-methylated back to methionine by methionine synthase or betaine Hcy methyltransferase (Figure 1). 13 Folic acid plays an important role in the remethylation of Hcy by providing 5-methyltetrahydrofolate. Disruption of any key step in Hcy metabolism may lead to a change in Hcy concentration, including mutations in methionine synthase, methyleneterahydrofolate reductase, cystathionine ß-synthase and methionine synthase reductase, which lead to inherited disorders.14,15
Figure 1.
The biogenesis pathway of homocysteine (Hcy). CBS, cystathionine β-synthase; MS, methionine synthase; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; BHMT, betaine Hcy methyltransferase.
Homocysteine is considered to be a risk factor for CAD, but the exact molecular mechanism remains elusive. 15 Endothelial damage, as a key initial event of atherosclerosis, has been observed in hyperhomocysteinaemia (HHcy). For example, Hcy inhibits the production of nitric oxide, which is a major regulator of endothelial homeostasis. In addition, Hcy blocks the signalling pathway related to another important endothelial gasotransmitter (hydrogen sulfide). 16 Hcy also causes the loss of the endothelial antioxidant system, increases the concentration of intracellular reactive oxygen species, which interferes with lipoprotein metabolism and promotes atherosclerosis. 16 Moreover, excessive Hcy may be indirectly bound to proteins. This process is called protein N-homocysteination, which induces vascular damage. Finally, S-adenosylhomocysteine (SAH), as the metabolic precursor of Hcy, accumulates in the HHcy environment and is a negative regulator of methyltransferases in most cells. 17 SAH-induced cellular hypomethylation is also considered to be the molecular basis of Hcy's vascular toxicity. 18
Although some studies have reported an association between circulating Hcy levels and the occurrence of CAD, only a few reports have assessed whether Hcy levels are conducive to the reclassification of risk beyond the Framingham Risk Score study.19–22 However, the results of studies on Hcy levels in patients with CAD have always been contradictory because some studies have failed to prove the link between Hcy levels and CAD, especially in Asian populations. 20 , 21 One possible reason is that these studies included a small sample size. This current study was undertaken in a large community in northern China to explore the relationship between Hcy and obstructive CAD. The predictive value of HHcy for obstructive CAD after adjusting for conventional risk factors was also investigated.
Patients and methods
Study population
This retrospective study enrolled consecutive patients in the Department of Cardiology, Shaanxi Provincial People's Hospital, Xi’an, Shaanxi Province, China for their first cardiac assessment between May 2017 and May 2020. The inclusion criteria were as follows: (i) patients that underwent coronary angiography at their first cardiac assessment; (ii) patients with obstructive CAD or those patients with suspected CAD but no coronary disease diagnosed by coronary angiography. The exclusion criteria were as follows: (i) patients with folic acid or vitamin B complex supplementation; (ii) patients receiving oral hormone replacement therapy; (iii) patients with renal insufficiency (creatinine ≥1.5 mg/dl); (iv) patients with folic acid or vitamin B complex deficiency; (v) presence of malignancy; (vi) patients with a known history of liver disease or those that were taking any of the known drugs that affect liver functions, such as isoniazid, phenytoin and chlorpromazine. Patients with suspected CAD but no coronary disease confirmed by coronary angiography were assigned to the non-obstructive CAD group; and patients with obstructive CAD diagnosed by coronary angiography were assigned to the obstructive CAD group.
This study complied with the Declaration of Helsinki and was approved by the Medical Ethical Review Board of Shaanxi Provincial People's Hospital, Xi’an, Shaanxi Province, China (no. 20170008). Written informed consent was obtained from the patients and all patient details were de-identified. The reporting of this study complied with the STROBE statement. 23
Coronary angiography and diagnosis of obstructive CAD
Coronary angiography was conducted using the standard Judkins technique with a Philips digital subtraction angiography machine (UNIQ FD20; Philips, Amsterdam, the Netherlands). Two independent interventional cardiologists (H.Y.W. & X.W.Y.) analysed the degree of coronary artery stenosis. One or more major coronary arteries with a diameter stenosis of 50% or more were defined as obstructive CAD.
Laboratory measurements
Venous blood (5 ml) was collected in ethylenediaminetetraacetic acid tubes (2.0 mg/ml) within 24 h after admission in the fasting state. Within 30 min of collection, samples were centrifuged at 1006 g for 20 min at room temperature (Eppendorf 5414S centrifuge; Eppendorf, Hamburg, Germany ) and then stored at –70°C until batch analysis. Fasting blood glucose (FBG), total cholesterol (TC), TG, HDL-C and LDL-C levels were measured with an automated biochemical analyser (7180; Hitachi, Tokyo, Japan). Plasma Hcy levels were measured by enzyme-linked immunosorbent assay (ELISA) as previously described. 24 The minimum detectable concentration of Hcy was 0.5 µmol/l. The intra-assay and interassay coefficients of variation for the ELISA were 4.8% and 6.6%, respectively.
Definition of risk factors
Risk factors for obstructive CAD were as follows: (i) an active smoker was defined as a person that actively smoked and had a history of regular smoking in the past year before enrolment; 25 (ii) a family history of premature obstructive CAD was defined as a diagnosis of obstructive CAD in a first degree relative aged less <55 years in men or <65 years in women; 26 (iii) hypertension was defined as having received medication and/or having a blood pressure ≥140/90 mmHg; 27 (iv) diabetes mellitus was defined as a previous medical history and/or postprandial glucose level > 150 mg/dl or FBG level > 126 mg/dl; 28 (v) hypertriglyceridaemia was defined as TG levels ≥1.7 mmol/l; (vi) hypercholesterolaemia was defined as TC levels ≥5.2 mmol/l; (vii) a low HDL-C level was defined as an HDL-C level <1.0 mmol/l; (viii) a high LDL-C level was defined as an LDL-C level ≥3.4 mmol/l; (ix) HHcy was defined as an Hcy level > 15 µmol/l.
Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY, USA). Continuous variables are presented as mean ± SD and Student’s t-test was used to compare the differences between the two groups. Categorical variables are presented as numbers (%) and χ2-test was used to compare the differences between the two groups. A multivariate logistic regression analysis was used to evaluate the predictive ability of risk factors for obstructive CAD. Diagnostic statistics were used to evaluate the diagnostic value of risk factors for obstructive CAD. A receiver operating characteristic (ROC) curve analysis was performed to determine the best predictive value of Hcy levels. Youden’s index was employed to evaluate the ideal cut-off of Hcy levels for screening obstructive CAD in this patient population. A P-value <0.05 was considered statistically significant.
Results
This retrospective study included 2987 participants, of which 1172 were included in the non-obstructive CAD group and 1815 patients were included in the obstructive CAD group. The obstructive CAD group included a significantly higher proportion of males and a higher proportion of patients with a family history of premature obstructive CAD, diabetes mellitus, hypertension and active smoking status compared with the non-obstructive CAD group (P < 0.001 for all comparisons) (Table 1). Compared with the non-obstructive CAD group, patients with obstructive CAD had significantly higher levels of FBG, TC, TG and LDL-C and significantly lower levels of HDL-C (P < 0.001 for all comparisons). The Hcy level in the obstructive CAD group was significantly higher than that in the non-obstructive CAD group (P < 0.001). Moreover, a higher proportion of patients were diagnosed with HHcy in the obstructive CAD group than in the non-obstructive CAD group (1314 of 1815 [72.40%] versus 405 of 1172 [34.56%]; P < 0.001).
Table 1.
Baseline demographic and clinical characteristics of patients (n = 2987) that were included in a study to investigate the ability of hyperhomocysteinaemia (HHcy) to predict obstructive coronary artery disease (CAD).
| Characteristic | Obstructive CAD group n = 1815 | Non-obstructive CAD group n = 1172 | Statistical analysisa |
|---|---|---|---|
| Age, years | 52.14 ± 8.02 | 51.61 ± 7.12 | NS |
| Male | 1368 (75.37) | 709 (60.49) | P < 0.001 |
| BMI, kg/m2 | 25.32 ± 4.64 | 24.98 ± 4.75 | NS |
| SBP, mmHg | 136.35 ± 15.90 | 135.28 ± 16.92 | NS |
| DBP, mmHg | 89.62 ± 10.42 | 88.93 ± 10.21 | NS |
| Family history of premature obstructive CAD | 386 (21.27) | 175 (14.93) | P < 0.001 |
| Diabetes mellitus | 635 (34.99) | 199 (16.98) | P < 0.001 |
| Hypertension | 1281 (70.58) | 416 (35.49) | P < 0.001 |
| Active smoker | 1089 (60.00) | 410 (34.98) | P < 0.001 |
| FBG, mmol/l | 7.16 ± 2.08 | 6.74 ± 1.78 | P < 0.001 |
| TC, mmol/l | 4.61 ± 1.28 | 4.31 ± 1.16 | P < 0.001 |
| TG, mmol/l | 1.85 ± 0.51 | 1.41 ± 0.59 | P < 0.001 |
| LDL-C, mmol/l | 3.43 ± 1.09 | 2.76 ± 1.01 | P < 0.001 |
| HDL-C, mmol/l | 1.01 ± 0.23 | 1.21 ± 0.26 | P < 0.001 |
| Creatinine, µmol/l | 80.54 ± 16.67 | 79.39 ± 16.08 | NS |
| Urea nitrogen, mmol/l | 5.64 ± 1.42 | 5.58 ± 1.51 | NS |
| Hcy, µmol/l | 20.71 ± 6.32 | 13.22 ± 5.74 | P < 0.001 |
| HHcy | 1314 (72.40) | 405 (34.56) | P < 0.001 |
Data presented as mean ± SD or n of patients (%).
Continuous variables were compared using Student’s t-test ; categorical variables were compared using χ2-test; NS, no significant between-group difference (P ≥ 0.05).
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; Hcy, homocysteine.
Hyperhomocysteinaemia was independently correlated with obstructive CAD in both young (aged ≤55 years [n = 1277], P < 0.001) (Table 2) and old patients (aged > 55 years [n = 538], P < 0.001) (Table 3). Male sex, diabetes mellitus, hypertension, active smoking, hypertriglyceridaemia, high LDL-C levels and low HDL-C levels were also independently correlated with obstructive CAD in young and old patients.
Table 2.
Multivariate logistic regression analysis of the risk factors for obstructive coronary artery disease in patients aged ≤55 years.
| Risk factor | Odds ratio | 95% confidence interval | Statistical analysis |
|---|---|---|---|
| Male | 1.22 | 1.09, 1.88 | P = 0.008 |
| Diabetes mellitus | 2.67 | 1.42, 4.87 | P < 0.001 |
| Hypertension | 1.32 | 1.02, 2.27 | P = 0.004 |
| Active smoker | 1.21 | 1.01, 2.64 | P = 0.003 |
| Hypertriglyceridaemia | 1.90 | 1.24, 3.02 | P < 0.001 |
| High LDL-C level | 2.12 | 1.47, 3.85 | P < 0.001 |
| Low HDL-C level | 2.21 | 1.45, 3.97 | P < 0.001 |
| HHcy | 3.03 | 2.10, 5.76 | P < 0.001 |
LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; HHcy, hyperhomocysteinaemia.
Table 3.
Multivariate logistic regression analysis of the risk factors for obstructive coronary artery disease in patients aged > 55 years.
| Risk factor | Odds ratio | 95% confidence interval | Statistical analysis |
|---|---|---|---|
| Male | 1.34 | 1.19, 2.88 | P = 0.003 |
| Diabetes mellitus | 2.97 | 1.64, 5.21 | P < 0.001 |
| Hypertension | 1.52 | 1.22, 3.21 | P = 0.004 |
| Active smoker | 1.26 | 1.21, 2.95 | P = 0.004 |
| Hypertriglyceridaemia | 1.81 | 1.32, 3.67 | P < 0.001 |
| High LDL-C level | 2.04 | 1.37, 3.98 | P < 0.001 |
| Low HDL-C level | 2.42 | 1.56, 4.07 | P < 0.001 |
| HHcy | 3.56 | 2.06, 5.96 | P < 0.001 |
LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; HHcy, hyperhomocysteinaemia.
Hyperhomocysteinaemia showed a higher sensitivity (93.1%), specificity (86.1%), positive predictive value (PPV, 89.9%), negative predictive value (NPV, 90.7%) and accuracy (90.0%) compared with diabetes mellitus, hypertension, active smoker, hypertriglyceridaemia and low HDL-C level (Table 4). The odds ratio for HHcy was 84.2 and the Kappa value (0.8) showed substantial agreement between obstructive CAD and HHcy, which was inferior only to a high LDL-C level.
Table 4.
Comparison of diagnostic values for obstructive coronary artery disease in patients with different risk factors.
| Diagnostic values | Diabetes mellitus | Hypertension | Active smoker | Hypertriglyceridaemia | High LDL-C level | Low HDL-C level | HHcy |
|---|---|---|---|---|---|---|---|
| Sensitivity, % | 59.0 | 70.0 | 60.3 | 74.8 | 93.1 | 75.7 | 93.1 |
| Specificity, % | 70.6 | 77.1 | 69.0 | 67.8 | 86.1 | 72.4 | 86.1 |
| PPV, % | 69.9 | 74.8 | 72.2 | 74.6 | 91.2 | 74.9 | 89.9 |
| NPV, % | 55.1 | 65.5 | 50.1 | 70.9 | 92.2 | 76.6 | 90.7 |
| Accuracy, % | 50.0 | 69.2 | 60.2 | 74.6 | 91.3 | 73.7 | 90.0 |
| Odds ratio | 2.1 | 7.0 | 2.8 | 76.8 | 86.3 | 70.2 | 84.2 |
| Kappa value | 0.2 | 0.5 | 0.4 | 0.7 | 0.8 | 0.7 | 0.8 |
| P-value | P = 0.002 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; HHcy, hyperhomocysteinaemia; PPV, positive predictive value; NPV, negative predictive value.
Figure 2 shows the ROC curve analysis of HHcy for predicting obstructive CAD (area under the curve: 0.912; 95% confidence interval 0.871, 0.932; P < 0.001). The highest value for Youden’s index was observed at the 15.13 µmol/l Hcy cut-off value in the patient population (index = 0.815; sensitivity = 91.9%; specificity = 89.6%).
Figure 2.
The receiver operating characteristic curve analysis of hyperhomocysteinaemia for predicting obstructive coronary artery disease.
Discussion
Although most patients with CAD have more than one conventional cardiovascular risk factor, 25% of patients with CAD are not diagnosed with conventional risk factors. 29 Therefore, clinicians must identify other risk factors. This current retrospective study identified HHcy as an independent risk factor for CAD in both young and old patients based on a large community study conducted in northern China after adjusting for other conventional risk factors, including male sex, smoking, diabetes mellitus, hypertension, hypertriglyceridaemia, high LDL-C and low HDL-C levels. According to the present study, HHcy had a high diagnostic value for CAD, which was only inferior to a high LDL-C level.
Homocysteine is a sulphur-containing amino acid extracted from the diet that is associated with cardiovascular events. 30 The mechanisms responsible for endothelial damage and subsequent atherosclerosis and thrombosis are currently unknown. The mechanisms may be related to epithelial cell injury, changes in platelet function, alterations in coagulation proteins and increased pro-coagulant activity, changes in LDL-C levels and the stimulation of smooth muscle cell proliferation. 30 A previous study reported the relationship between elevated Hcy levels and thrombosis, along with thrombovascular abnormalities in patients with homocysteinuria. 31 In the past four decades, some studies have reported an association between Hcy levels and CAD.19–22 However, the results for Hcy levels in patients with CAD have been conflicting, especially in Asian populations. For example, two previous studies did not find a link between Hcy levels and CAD in Indian populations.20,21 However, another study showed that Hcy levels were increased significantly in patients with CAD, with a very high sensitivity, specificity and accuracy with a PPV greater than 90%. 19 These studies had some limitations, including relatively small study populations.19–21 Thus, the evidence for the role of plasma Hcy level as an independent risk factor for CAD in the Indian population remains unclear.
A previous study conducted in southern China reported the association of Hcy levels in young patients with CAD. 24 HHcy was a risk factor for CAD in young patients. 24 However, the study had several limitations. First, coronary angiography was not performed on all controls to exclude CAD. 24 Secondly, the study population was relatively small. 24 Thirdly, the enrolled population was aged ≤55 years. 24 In contrast, this current study had the following characteristics. First, the sample size was relatively large. Secondly, patients were enrolled for the first cardiac assessment. Thirdly, coronary angiography was performed on all patients. Finally, the patients with obstructive CAD were stratified into young patients (aged ≤55 years) and old patients (aged > 55 years).
In the present study, 1172 patients with non-obstructive CAD and 1815 patients with obstructive CAD were enrolled. Patients were grouped according to the results of coronary angiography. The conventional risk factors and Hcy levels were compared between the obstructive CAD and non-obstructive CAD groups. Hcy levels in patients with obstructive CAD were significantly higher than those in non-obstructive CAD patients. When comparing the sensitivity, specificity and accuracy of these risk factors to be able to diagnose obstructive CAD, HHcy had a higher sensitivity and accuracy and was considered a better predictor of obstructive CAD compared with diabetes mellitus, hypertension, active smoker, hypertriglyceridaemia and low HDL-C level. Youden’s index is used to assess the authenticity of screening tests. A higher index indicates a better effect of screening tests with greater authenticity. In this study, Youden’s index was used to evaluate the ideal cut-off level of Hcy for screening obstructive CAD in this patient population. The cut-off value of 15.13 µmol/l for Hcy resulted in the highest Youden’s index (index = 0.815). Genetic factors, B vitamins and folic acid intake may determine plasma Hcy levels. 32 A report suggested that a polymorphism of the methylene tetrahydrofolate reductase (MTHFR) gene in individuals with the TT genotype led to reduced enzyme activity and increased Hcy levels. 33 Another report demonstrated that approximately 12% of Asians are homozygous for the T allele (T/T) and more than 50% are heterozygous. 34 In China, dietary intake of folic acid is insufficient. 33 These factors may explain why more patients with HHcy were included in this current study. These factors may also explain why the cut-off value in the population was slightly higher than the traditional definition of HHcy of >15 µmol/l. 19
Research has demonstrated close correlations between plasma Hcy levels and age, sex and smoking. 35 This current study grouped the patients with obstructive CAD into young patients (aged ≤55 years) and old patients (aged > 55 years). Multivariate logistic regression analysis identified HHcy as an independent risk factor not only in young patients with obstructive CAD but also in old patients with obstructive CAD after adjusting for other risk factors.
This study had several limitations. First, this study was conducted at a single centre, although it did include a relatively large sample. Secondly, the design of this study did not clearly assess the potential effects of all risk factors as in a prospective study. Thirdly, the MTHFR gene polymorphism was not measured, although a previous meta-analysis showed that the MTHFR C677T polymorphism did not significantly affect CAD. 36
In conclusion, this current retrospective study demonstrated that the Hcy level in patients with obstructive CAD was significantly higher than that in non-obstructive CAD patients. HHcy was associated with obstructive CAD and may be a potentially independent risk factor for obstructive CAD with good predictive value. Prospective studies with large sample sizes are needed to further clarify this association.
Footnotes
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: This study received support from the Natural Science Basic Research Programme of Shaanxi Province (no. 2020JQ-939) and the Science and Technology Development Incubation Fund Project of Shaanxi Provincial People's Hospital (no. 2019YXQ-08).
ORCID iD: Yi-Wei Cao https://orcid.org/0000-0002-3195-5489
References
- 1.Malakar AK, Choudhury D, Halder B, et al. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol 2019; 234: 16812–16823. [DOI] [PubMed] [Google Scholar]
- 2.Shao C, Wang J, Tian J, et al. Coronary Artery Disease: From Mechanism to Clinical Practice. Adv Exp Med Biol 2020; 1177: 1–36. [DOI] [PubMed] [Google Scholar]
- 3.Bai MF, Wang X. Risk factors associated with coronary heart disease in women: a systematic review. Herz 2020; 45: 52–57. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chock B, Lin TC, Li CS, et al. Plasma testosterone is associated with Framingham risk score. Aging Male 2012; 15: 134–139. [DOI] [PubMed] [Google Scholar]
- 6.Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Münster (PROCAM) study. Circulation 2002; 105: 310–315. [DOI] [PubMed] [Google Scholar]
- 7.Sharma H, Kapur P, Jalali RK, et al. Atherosclerosis risk assessment in patients with chronic obstructive pulmonary disease: a case-control study. Ther Clin Risk Manag 2019; 15: 1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonseca FA, Izar MC. High-sensitivity C-reactive protein and cardiovascular disease across countries and ethnicities. Clinics (Sao Paulo ) 2016; 71: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steen DL, O’Donoghue ML. Lp-PLA2 inhibitors for the reduction of cardiovascular events. Cardiol Ther 2013; 2: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaric BL, Obradovic M, Bajic V, et al. Homocysteine and hyperhomocysteinaemia. Curr Med Chem 2019; 26: 2948–2961. [DOI] [PubMed] [Google Scholar]
- 11.Bahulikar A, Tickoo V, Phalgune D. Association of non-HDL cholesterol, homocysteine and vitamin D in acute coronary syndrome. J Assoc Physicians India 2018; 66: 22–25. [PubMed] [Google Scholar]
- 12.Jakubowski H. Homocysteine Modification in Protein Structure/Function and Human Disease. Physiol Rev 2019; 99: 555–604. [DOI] [PubMed] [Google Scholar]
- 13.McCully KS. Homocysteine Metabolism, Atherosclerosis, and Diseases of Aging. Compr Physiol 2015; 6: 471–505. [DOI] [PubMed] [Google Scholar]
- 14.McCully KS. Homocysteine, vitamins, and prevention of vascular disease. Mil Med 2004; 169: 325–329. [DOI] [PubMed] [Google Scholar]
- 15.Rehman T, Shabbir MA, Inam-Ur-Raheem M, et al. Cysteine and homocysteine as biomarker of various diseases. Food Sci Nutr 2020; 8: 4696–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chrysant SG, Chrysant GS. The current status of homocysteine as a risk factor for cardiovascular disease: a mini review. Expert Rev Cardiovasc Ther 2018; 16: 559–565. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Kim H, Roh H, et al. Causes of hyperhomocysteinemia and its pathological significance. Arch Pharm Res 2018; 41: 372–383. [DOI] [PubMed] [Google Scholar]
- 18.Esse R, Barroso M, Tavares De Almeida I, et al. The contribution of homocysteine metabolism disruption to endothelial dysfunction: state-of-the-art. Int J Mol Sci 2019; 20: 867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harish RB, Govindaraju V, Manjunath CN. Risk prediction-homocysteine in coronary heart disease. Indian J Clin Biochem 2007; 22: 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snehalatha C, Ramachandran A, Satyavani K, et al . Plasma homocysteine concentration and coronary artery disease in Asian Indians. J Assoc Physicians India 2002; 50: 1229–1231. [PubMed] [Google Scholar]
- 21.Deepa R, Velmurugan K, Saravanan G, et al . Absence of association between serum homocysteine levels and coronary artery disease in south Indian males. Indian Heart J 2001; 53: 44–47. [PubMed] [Google Scholar]
- 22.Bickel C, Schnabel RB, Zengin E, et al. Homocysteine concentration in coronary artery disease: Influence of three common single nucleotide polymorphisms. Nutr Metab Cardiovasc Dis 2017; 27: 168–175. [DOI] [PubMed] [Google Scholar]
- 23.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, Huang Y, Hu Y, et al. Hyperhomocysteinemia is an independent risk factor in young patients with coronary artery disease in southern China. Herz 2013; 38: 779–784. [DOI] [PubMed] [Google Scholar]
- 25.Dudum R, Dzaye O, Mirbolouk M, et al. Coronary artery calcium scoring in low risk patients with family history of coronary heart disease: Validation of the SCCT guideline approach in the coronary artery calcium consortium. J Cardiovasc Comput Tomogr 2019; 13: 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y, Hu Y, Mai W, et al. Plasma oxidized low-density lipoprotein is an independent risk factor in young patients with coronary artery disease. Dis Markers 2011; 31: 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flack JM, Calhoun D, Schiffrin EL. The new ACC/AHA hypertension guidelines for the prevention, detection, evaluation, and management of high blood pressure in adults. Am J Hypertens 2018; 31: 133–135. [DOI] [PubMed] [Google Scholar]
- 28.Petersmann A, Müller-Wieland D, Müller UA, et al. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes 2019; 127: S1–S7. [DOI] [PubMed] [Google Scholar]
- 29.Knopp RH. Risk factors for coronary artery disease in women. Am J Cardiol 2002; 89: 28E–34E; discussion 34E–35E. [DOI] [PubMed] [Google Scholar]
- 30.Shenoy V, Mehendale V, Prabhu K, et al. Correlation of serum homocysteine levels with the severity of coronary artery disease. Indian J Clin Biochem 2014; 29: 339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol 1969; 56: 111–128. [PMC free article] [PubMed] [Google Scholar]
- 32.Nagele P, Meissner K, Francis A, et al. Genetic and environmental determinants of plasma total homocysteine levels: impact of population-wide folate fortification. Pharmacogenet Genomics 2011; 21: 426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang F, Sui X, Xu N, et al. The relationship between plasma homocysteine levels and MTHFR gene variation, age, and sex in Northeast China. Niger J Clin Pract 2019; 22: 380–385. [DOI] [PubMed] [Google Scholar]
- 34.Schneider JA, Rees DC, Liu YT, et al. Worldwide distribution of a common methylenetetrahydrofolate reductase mutation. Am J Hum Genet 1998; 62: 1258–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Y, Lv T, Xie M, et al. Blood homocysteine and folic acid levels may provide reference value for the treatment of sudden total frequency deafness. Ann Palliat Med 2019; 8: 604–610. [DOI] [PubMed] [Google Scholar]
- 36.Clarke R, Bennett DA, Parish S, et al. Homocysteine and coronary heart disease: meta-analysis of MTHFR case-control studies, avoiding publication bias. PLoS Med 2012; 9: e1001177. [DOI] [PMC free article] [PubMed] [Google Scholar]